Abstract
CD4+ T cells represent an entire arm of the immune system that has hitherto been incompletely understood, but their potential to act as both helper and effector may make them optimal protagonists in immunotherapeutic approaches to treat cancer. Cytokine therapy can activate this population in a manner that ensures maximal diversification of effector function for a robust immune response.
In the final issue for 2013, the editors of Science awarded the title of “Breakthrough of the Year” to Cancer Immunotherapy in light of advancements in the areas of adoptive T cell therapy (ACT), especially using chimeric antigen receptors (CAR), and immune checkpoint blockade with antibodies like α-CTLA4 (drug name: ipilimumab).Citation1 While most of the work on T cell therapies has been focused on inciting CD8+ T cell responses, by administering antigen (Ag)-loaded dendritic cells (DCs) or cytokine-producing autologous tumor cells, CD4+ T cells have become an increasingly interesting effector population. We describe in a recent publication how cellular interleukin-12 (IL-12) therapy results in development of CD4+ cytotoxic T lymphocytes (CTL).Citation2 CD4+ T cells are typically considered helper cells, important for licensing DCs and enabling activation of CD8+ T cells that ultimately perform the effector function. For some time, however, reports have been emerging about CD4+ T cells with cytotoxic function of their own and we previously published a murine model of IL-12 therapy that has a predominant CD4+ T cell response.Citation3 Furthermore, the response is diverse and robust as this CTL population constitutes only one of several effector mechanisms that we have observed to be responsible for leukemia cell killing in this model.
Last year, companion papers by Mucida et al.Citation4 and Reis et al.Citation5 characterized the unique gene signature responsible for the acquisition of Ag-dependent cytotoxic activity by CD4+ CTL. This adds another layer of phenotypic plasticity onto this population that is fundamentally different from the other subtypes, which remain “helpers”. CD4+ CTLs are likely a relevant effector population in a broader range of circumstances than is currently appreciated, but their “helper” label masks the range of their contribution and little is known about what drives them to become cytotoxic. We teased out the mechanism by which our cellular IL-12 therapy leads preferentially to a CD4+ response using an in vitro model to map out independent stages and determine the key players in each.Citation2
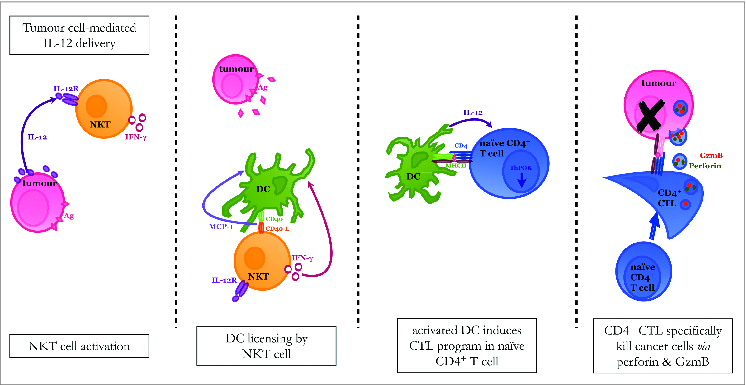
Natural killer T (NKT) cells, which constitutively express the IL-12 receptor and produce IFNγ, become activated in our experimental model by IL-12 delivered via the transduced leukemia cells. Once activated, the NKT cells interact with DCs through ligation of CD40/CD40-L, leading to production of MCP-1. This cytokine milieu licenses the DCs to mature CD4+ T cells into CTL. Acquisition of cytotoxic potential by the CD4+ T cells is marked by decreased expression of ThPOK, a transcription factor that generally suppresses the cytolytic program in helper T cells and is associated with the production of granzyme B (GzmB). While other cytolytic mechanisms may also be at play, specific eradication of the leukemia target cells is, at least in part, accomplished by way of GzmB and perforin (). Our in vitro system allowed us to systematically separate out the populations during different phases of the response and determine that while NKT cells are imperative for the activation of DC, they are not required during the effector phase. Experiments conducted in vivo demonstrated that our IL-12 therapeutic approach leads to a dominant CD4+ response where the CD4+ population can effectively cure mice in the absence of CD8+ T cellsCitation3 and effector cells derived from primed mice had the same ThPOKlow, GzmBhigh phenotype as the effector cells in our culture system.Citation2 Nonetheless, examination of the in vivo memory response revealed that memory resides in both the CD4+ and CD8+ T cell compartments. Interestingly, DCs are known to orchestrate different responses depending on whether they are licensed by NKT cells or CD4+ T cells.Citation6 This may in part explain the diverse nature of the response initiated in our system.
Figure 1. Proposed model for induction of CD4+ CTL in response to cytokine therapy. NKT cells are able to respond to IL-12 produced by the leukemia cell because they constitutively express IL-12 receptor. This signal induces IFNγ production by the NKT cell, which then acts on DCs to increase their expression of CD40. DCs and NKT cells reciprocally activate each other by interacting through CD40/CD40-L and MCP-1 is produced as a consequence of this interaction. The DC population matures and enhances its Ag-presentation capacity so that it can ultimately provide all of the necessary signals to induce a CD4+ T cell response. The CD4+ T cell reduces its expression of the transcription factor ThPOK, which normally suppresses the cytotoxic program, and becomes a CTL. The fully armed CD4+ CTL then kills leukemia target cells using the cytolytic granules perforin and GzmB as one mechanism of action.
An interesting phenomenon was observed in the clinic when a patient was successfully treated with an autologous CD4+ clone recognizing the NY-ESO-1 Ag expressed on a portion of his tumor cells; the entire tumor regressed in the wake of a CD8+ T cell response, recognizing multiple target molecules, initiated de novo by the adoptively transferred CD4+ T cell clone.Citation7 This begs the question, can a CD4+ T cell simultaneously behave as a helper cell and exhibit cytolytic activity? If so, how can we optimize treatment conditions to achieve this? Transferring CD4+ T cell clones might be one way, but another clinical report described cytotoxic CD4+ T cells arising in patients treated with ipilimumabCitation8; underlining that CD4+ CTL are likely important players in effective immune responses. An alternate possibility is that cytokine therapy may be optimal because it initiates a response further upstream, inducing a diverse set of effector populations, including CD4+ CTL, to maximize robustness.
The immune response downstream of cell-mediated IL-12 therapy is diverse and multi-pronged; consisting, at least, of CD4+ and CD8+ CTL. Despite limited clinical success to date, IL-12 was ranked as the 3rd most desirable therapeutic agent for its potential to successfully treat cancer precisely because of its ability to induce potent immune responses.Citation9 Notwithstanding, the greatest successes are likely to come from therapeutic regiments that combine synergistic approaches. Attempts to design such approaches that include cytokine therapy are beleaguered by a lack of clarity about the activity of specific cytokines under different conditions (see ref.Citation10 for discussion). Our system illustrates this point clearly as the mode of IL-12 delivery in vivo completely alters the dominant response; a classic CD8+ CTL response was observed when mice were injected with the recombinant protein, whereas CD4+ CTL dominated when the mice received a cellular vaccine of IL-12-producing syngeneic leukemia cells. This may be because other products of the inoculating cells alter the activity of IL-12, or because different delivery methods result in different amounts of IL-12 at the local site of interaction with the immune system, or it may have to do with the protein's source and attendant differences in post translational modifications. Whatever the case, an understanding of why this is may inform how treatment preparations can be manipulated to obtain the desired response.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- Couzin-Frankel J. Cancer immunotherapy. Science 2013; 342:1432-33; PMID:24357284; http://dx.doi.org/10.1126/science.342.6165.1432
- Nelles ME, Moreau JM, Furlonger CL, Berger A, Medin JA, Paige CJ. Murine splenic CD4⁺ T cells, induced by innate immune cell interactions and secreted factors, develop antileukemia cytotoxicity. Cancer Immunol Res 2014; 11:1113-24; http://dx.doi.org/10.1158/2326-6066.CIR-13-0208
- Labbe A, Nelles ME, Walia J, Jia L, Furlonger C, Nonaka T, Medin JA, Paige CJ. IL-12 immunotherapy of murine leukaemia: comparison of systemic versus gene modified cell therapy. J Cell Mol Med 2009; 13:1962-76; PMID:18624776; http://dx.doi.org/10.1111/j.1582-4934.2008.00412.x
- Mucida D, Husain MM, Muroi S, van Wijk F, Shinnakasu R, Naoe Y, Reis BS, Huang Y, Lambolez F, Docherty M et al. Transcriptional reprogramming of mature CD4⁺ helper T cells generates distinct MHC class II-restricted cytotoxic T lymphocytes. Nat Immunol 2013; 14:281-9; PMID:23334788; http://dx.doi.org/10.1038/ni.2523
- Reis BS, Rogoz A, Costa-Pinto FA, Taniuchi I, Mucida D. Mutual expression of the transcription factors Runx3 and ThPOK regulates intestinal CD4⁺ T cell immunity. Nat Immunol 2013; 14:271-80; PMID:23334789; http://dx.doi.org/10.1038/ni.2518
- Hunn MK, Hermans IF. Exploiting invariant NKT cells to promote T-cell responses to cancer vaccines. OncoImmunology 2013; 2:4; http://dx.doi.org/10.4161/onci.23789
- Hunder NN, Wallen H, Cao J, Hendricks DW, Reilly JZ, Rodmyre R, Jungbluth A, Gnjatic S, Thompson JA, Yee C. Treatment of metastatic melanoma with autologous CD4⁺ T cells against NY-ESO-1. N Engl J Med 2008; 358:2698-703; PMID:18565862; http://dx.doi.org/10.1056/NEJMoa0800251
- Kitano S, Tsuji T, Liu C, Hirschhorn-Cymerman D, Kyi C, Mu Z, Allison JP, Gnjatic S, Yuan JD, Wolchok JD. Enhancement of tumor-reactive cytotoxic CD4⁺ T-cell responses after ipilimumab treatment in four advanced melanoma patients. Cancer Immunol Res 2013; 1:235-44; PMID:24396833; http://dx.doi.org/10.1158/2326-6066.CIR-13-0068
- Cheever MA. Twelve immunotherapy drugs that could cure cancers. Immunol Rev 2008; 222:357-68; PMID:18364014; http://dx.doi.org/10.1111/j.1600-065X.2008.00604.x
- Dranoff G, Jaffee E, Lazenby A, Golumbek P, Levitsky H, Brose K, Jackson V, Hamada H, Pardoll D, Mulligan RC. Vaccination with irradiated tumor cells engineered to secrete murine franulocyte-macrophage colony-stimulating factor stimulates potent, specific, and long-lasting anti-tumor immunity. Proc Natl Acad Sci 1993; 90:3539-43; http://dx.doi.org/10.1073/pnas.90.8.3539
