Abstract
In recent years, bispecific antibodies (bsAb) have emerged as promising tools for a target-specific redirection of T cells in order to eliminate malignant cells. However, CD3-engaging constructs might also activate T regulatory cells (Tregs) present in the tumor microenvironment. Whether this has detrimental or beneficial effects for tumor therapy is still controversially discussed.
bsAb being able to engage T cells with malignant cells represent attractive tools for targeted tumor therapy.Citation1 First clinical trials proving the efficiency and feasibility of this approach have already been conducted or are currently on the way. As these bsAb trigger T cell activation via binding to the CD3 complex, not only T effector cells (Teff) but also Tregs should be affected.Citation2 While it is well accepted that Tregs enrich within tumors and, due to their immunosuppressive potential, play a pivotal role in tumor immune escape,Citation3 the killing capability of Tregs after engagement with bsAb is still under debate. Here, we summarize and discuss potential explanations and pitfalls that may help to explain the reported controversial data. In addition, we provide further experimental evidence that Tregs activated via bsAb should not be capable of killing their target cells at least not via the granzyme/perforin pathway.
Recently, we explored the cytotoxic potential of bsAb-activated Tregs in more detail.Citation4 We used highly pure, freshly isolated as well as expanded Tregs and found that neither of the different cell populations possess a considerable cytotoxic capacity upon cross-linkage to target cells via bsAb. To consolidate our data, we tested two bsAb targeting different antigens and included conventional αCD3/CD28-coated beads for T cell stimulation. Neither antigen-specific nor polyclonal activation via bsAb or beads, respectively, resulted in tumor cell elimination by expanded Tregs. We also substantiated our results in vivo using athymic nude mice and demonstrated that expanded Tregs were not capable of eliminating co-injected antigen-expressing tumor cells upon bsAb-mediated activation. On the contrary, inoculating Tregs completely abolished a Teff-mediated anti-tumor response.
However, these data are in contrast to results obtained by Choi and colleagues. They reported an efficient elimination of target antigen-expressing glioblastoma cells by anti-CD3-anti-EGFRvIII bsAb-redirected Tregs.Citation5,6 There are several potential technical explanations for the observed differences between Choi's and our results. First, we used sorted CD4+CD25+CD127lowCD45RA+ Tregs as starting population as it is well known that these cells have the highest capacity to maintain phenotypic and functional Treg properties after prolonged in vitro cultivation.Citation7 Indeed, after a 12-d expansion period, we obtained highly pure Tregs (95% ± 3% FOXP3+) and analyzed their cytotoxic potential after three additional days of cell resting in the absence of activator beads. Choi's group, however, used CD4+CD25+CD127dim/− Tregs isolated via magnetic beads that were expanded between 4 and 7 d. However, Tregs isolated by magnetic beads may not be as pure as sorted Tregs. Unfortunately, no information regarding the purity of the final Treg population after expansion was given by Choi and colleagues. However, purity is of great importance as only highly pure Tregs do not eliminate target cells while Treg fractions containing contaminating Teff elicit a remarkable killing capacity.Citation4 Vice versa, we cannot rule out that a subclass of Tregs was lost during our sorting process which is responsible for the killing capability of Tregs described by Choi and colleagues.
Furthermore, there might exist a close correlation between cultivation time and cytolytic potential of expanded Tregs. Comparing the protocols of Choi's and our lab, it could be assumed that Tregs are cytotoxic after one week of stimulation, but lose their cytotoxic activity during their expansion and long-term culture. Kinetic experiments testing the lytic capacity of Tregs over at least 2 weeks might shed some light into this issue. In addition, it might also be possible that the bsAb themselves account for the diverging results. Although the general format of the utilized bsAb, constructed as 2 single-chain variable fragments (scFvs) arranged in tandem, is comparable, the underlying recombinant Ab fragments differ with regard to the effector-binding anti-CD3 domain. While Choi and colleagues use the anti-CD3 clone OKT-3, our anti-CD3 domain is derived from an anti-CD3 mAb developed in our laboratory.Citation8,9 Whether or not these different anti-CD3 scFvs have distinct effects on T cell and in particular on Treg cell response after bsAb-mediated activation awaits further investigation. Currently, we have indeed first preliminary experimental evidence supporting such anti-CD3-dependent effects (Bachmann, unpublished).
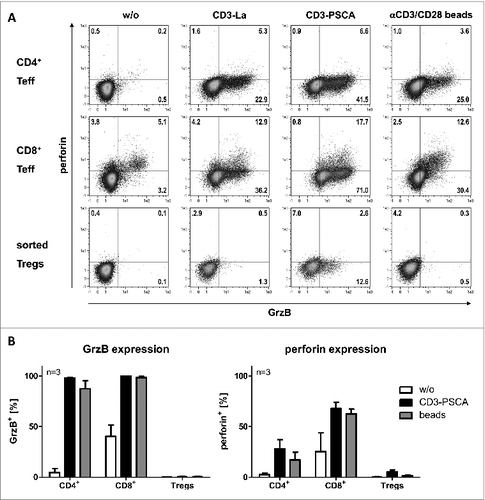
As a potential killing mechanism of bsAb-redirected Tregs, Choi et al. suggest granzyme/perforin expression. In order to support their assumption, they isolated whole CD4+ T cells, gated on CD25highFOXP3+ cells and observed a significant upregulation of granzyme A, granzyme B (GrzB), and perforin expression upon activation with an anti-EGFRvIII-anti-CD3 bsAb. Again these data are controversial to our findings as shown in . Freshly isolated CD8+ and CD4+CD25− Teff should upregulate GrzB expression following activation via bsAb or beads. However, GrzB could not or only marginally be detected in CD4+CD25+CD127low Tregs depending on the chosen bsAb (). Similar results could be obtained using expanded Teff and Treg cells (). Notably, Treg cell expansion was conducted in the absence of rapamycin in order to avoid suppression of putative GrzB expression.Citation10
Figure 1. Analysis of granzyme B and perforin expression of freshly isolated and expanded Teff and Tregs. T cells were incubated with antigen-positive PC3 cells at a 5:1 ratio in the presence or absence of 30 pmol/mL bsAb. Polyclonal stimulation with αCD3/CD28-coated beads was included as positive control. Purity of both freshly isolated as well as expanded Tregs was confirmed by flow cytometry analysis to be on average 95% FOXP3+. After 24 h, cells were harvested and stained for cell surface expression of CD69, CD4+, and CD8+ and intracellular expression of granzyme B (GrzB) and perforin. (A) CD4+ and CD8+ T cells were isolated by negative selection using MACS technology. Untouched CD4+ T cells were separated into CD25− Teff and CD25+ T cells using CD25 MicroBeads. CD4+CD25+ T cells were further sorted to obtain highly pure CD4+CD25+CD127low Tregs. Quadrant position was placed based on FMO control staining (not shown). Data shown are representative for one of three donors analyzed. (B) Isolated CD4+ and CD8+ Teff were expanded using conventional αCD3/CD28-coated beads at a ratio of 1:4 beads per cell in the presence of 200 U/mL IL‑2. After 4 d, beads were removed and cells were maintained in medium containing 50 U/mL IL‑2, 5 ng/mL IL-7 and 5 ng/mL IL‑15. For expansion of Tregs, the CD45RA+ subset as starting population was used. After sorting, the resulting population showed > 98% purity (CD4+CD25+CD127lowCD45RA+). Expansion was conducted as recently established.Citation2,4 After a 10-d expansion period, beads were removed and cells were cultured in medium supplemented with 300 U/mL IL-2. 13 d after T cell isolation, expression of cytotoxic molecules was analyzed. Data are represented as mean ± SD of three independent donors.
Taken together, there are still many unresolved issues related to the cytotoxic activity of bsAb-engaged Tregs. Even if it turns out that intratumoral Tregs retargeted via bsAb contribute to tumor cell lysis their activation may still result in profound Teff suppression. Whether or not the suppressive effect can outweigh the cytotoxic effect of bsAb-activated Teff, which might eventually impede the therapeutic success of the bsAb approach especially in case of solid tumor treatment, needs further analysis.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- Stamova S, Koristka S, Keil J, Arndt C, Feldmann A, Michalk I, Bartsch H, Bippes CC, Schmitz M, Cartellieri M, Bachmann M. Cancer immunotherapy by retargeting of immune effector cells via recombinant bispecific antibody constructs. Antibodies 2012; 1:172-98; PMID:NOT_FOUND; http://dx.doi.org/10.3390/antib1020172
- Koristka S, Cartellieri M, Theil A, Feldmann A, Arndt C, Stamova S, Michalk I, Töpfer K, Temme A, Kretschmer K, et al. Retargeting of human regulatory T cells by single-chain bispecific antibodies. J Immunol 2012; 188:1551-58; PMID:22184723; http://dx.doi.org/10.4049/jimmunol.1101760
- Nishikawa H, Sakaguchi S. Regulatory T cells in cancer immunotherapy. Curr Opin Immunol 2014; 27:1-7; PMID:24413387; http://dx.doi.org/10.1016/j.coi.2013.12.005
- Koristka S, Cartellieri M, Arndt C, Feldmann A, Töpfer K, Michalk I, Temme A, Ehninger G, Bachmann M. Cytotoxic response of human regulatory T cells upon T-cell receptor-mediated activation: a matter of purity. Blood Cancer J 2014; 4:e199; PMID:24727995
- Choi BD, Gedeon PC, Herndon JE 2nd, Archer GE, Reap EA, Sanchez-Perez L, Mitchell DA, Bigner DD, Sampson JH. Human regulatory T cells kill tumor cells through granzyme-dependent cytotoxicity upon retargeting with a bispecific antibody. Cancer Immunol Res 2013; 1(3):163; PMID:24570975; http://dx.doi.org/10.1158/2326-6066.CIR-13-0049
- Choi BD, Gedeon PC, Sanchez-Perez L, Bigner DD, Sampson JH. Regulatory T cells are redirected to kill glioblastoma by an EGFRvIII-targeted bispecific antibody. Oncoimmunology 2013; 2(12):e26757; PMID:AMBIGUOUS; http://dx.doi.org/10.4161/onci.26757
- Hoffmann P, Boeld TJ, Eder R, Huehn J, Floess S, Wieczorek G, Olek S, Dietmaier W, Andreesen R, Edinger M. Loss of FOXP3 expression in natural human CD4+CD25+ regulatory T cells upon repetitive in vitro stimulation. Eur J Immunol 2009; 39(4):1088-97; PMID:19283780; http://dx.doi.org/10.1002/eji.200838904
- Feldmann A, Stamova S, Bippes CC, Bartsch H, Wehner R, Schmitz M, Temme A, Cartellieri M, Bachmann M. Retargeting of T cells to prostate stem cell antigen expressing tumor cells: comparison of different antibody formats. Prostate 2011; 71(9):998-1011; PMID:21541976; http://dx.doi.org/10.1002/pros.21315
- Feldmann A, Arndt C, Töpfer K, Stamova S, Krone F, Cartellieri M, Koristka S, Michalk I, Lindemann D, Schmitz M et al. Novel humanized and highly efficient bispecific antibodies mediate killing of prostate stem cell antigen-expressing tumor cells by CD8+ and CD4+ T cells. J Immunol 2012; 189(6):3249-59; PMID:22875801; http://dx.doi.org/10.4049/jimmunol.1200341
- Efimova OV, Kelley TW. Induction of granzyme B expression in T-cell receptor/CD28-stimulated human regulatory T cells is suppressed by inhibitors of the PI3K-mTOR pathway. BMC Immunol 2009; 10:59; PMID:19930596; http://dx.doi.org/10.1186/1471-2172-10-59
