Abstract
Macroautophagy selectively degrades dysfunctional mitochondria by a process known as mitophagy. The purpose of the study published in Cell Death and Differentiation was to investigate the involvement of transglutaminase 2 (TG2) in the turnover and degradation of damaged mitochondria and its effects on cell metabolism.
Autophagy is an evolutionarily conserved and genetically programmed lysosomal degradation pathway in which cytoplasmic constituents are self-digested to maintain homeostasis in response to various metabolic stresses. Although it was originally considered to be a non-selective bulk degradative process, autophagy was recently found to selectively eliminate larger, longer-lived proteins and aggregates, as well as organelles such as mitochondria and peroxisomes and pathogenic microorganisms.Citation2 Several types of autophagy have been confirmed including mitophagy, the mitochondrial quality control mechanism responsible for the selective elimination of damaged or excess mitochondria.Citation3
Transglutaminase 2 (TG2), the ubiquitous multifunctional member of the transglutaminase family of proteins, is able to catalyze Ca2+-dependent post-translational modification of proteins. It may also act as a G protein in transmembrane signaling, as a kinase, as a cell surface adhesion mediator, and as a protein disulphide isomerase (PDI) to modify key mitochondrial proteins.Citation4 In fact, TG2 is implicated in the homeostasis of the mitochondrial respiratory chain and some of the characterized TG2 substrates play a key role in mitochondrial homeostasis.Citation5
In our previous publications we showed that TG2 plays a role in the autophagic process. Using both TG2 knockout mice and their derived embryonic fibroblasts (MEFs), we found an impairment in the maturation of autophagosomes in the absence of TG2.Citation6,7 Moreover, we demonstrated that TG2 transamidating activity plays an important role in the assembly of ubiquitinated protein aggregates and in their clearance by macroautophagy.Citation8 These data indicate that the enzyme plays a key role in the regulation of cellular proteostasis, particularly under stressful conditions.
The recent paper published in Cell Death and Differentiation extends these findings by focusing attention on the effect of TG2 on mitophagy and mitochondrial homeostasis.Citation1
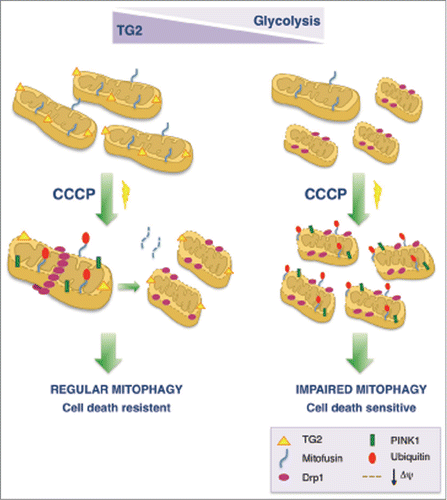
We observed that MEFs lacking TG2 display fragmented mitochondria with altered morphology and depolarization of the mitochondrial membrane. In line with our previous data indicating an impairment in the autophagic process, treatment of cells lacking TG2 with the uncoupler carbonyl cyanide m-chlorophenyl hydrazine (CCCP) leads to accumulation of undigested dysfunctional mitochondria (). Interestingly, after mitophagy induction by CCCP we observed a specific activation of TG2 transamidating activity on mitochondria, indicating a TG2-dependent modification of some mitochondrial proteins upon mitochondrial damage. Moreover Mdivi-1, an inhibitor of the dynamin-related protein (Drp1)-mediated mitochondrial fission and an indirect inhibitor of mitophagy, induces a marked decrease in TG2 intracellular cross-linking activity, further indicating involvement of the enzyme in regulation of the mitophagic process. These findings led us to investigate the cellular metabolic status of these cells. We discovered that cells lacking TG2 switch to a higher rate of aerobic glycolysis in an attempt to survive; in fact, they die upon treatment with the glycolytic inhibitor 2-deoxy-D-glucose (2-DG). In contrast, activation of TG2 in wild-type cells leads to inhibition/delay of 2-DG–induced apoptosis by crosslinking of caspase 3. Thus, these results indicate significant metabolic rearrangements in the absence of TG2 leading to a compensatory alteration of mitochondrial metabolism. In line with these results, it is important to keep in mind that a well-known property of primary and metastatic cancers is the upregulation of glycolysis, resulting in increased glucose consumption. In addition, mitophagy serves to remove dysfunctional mitochondria to alleviate oxidative stress and prevent carcinogenesis.Citation9 In this regard, the role of TG2 in cancer progression has been widely studied, and increased TG2 expression has been found in several tumors.Citation10 Understanding how TG2 protein influences carcinogenesis by regulating mitochondrial functionality and metabolism would be of great importance in the design of new therapeutic approaches for cancer therapy.
Figure 1. Involvement of transglutaminase 2 (TG2) in mitochondrial turnover and metabolism. TG2 and its transamidating activity are required for the proper degradation of dysfunctional mitochondria. Conversely, the accumulation of fragmented and depolarized mitochondria is correlated to the absence of TG2. In the absence of TG2, mitophagy induction by carbonyl cyanide m-chlorophenyl hydrazine (CCCP) leads to an impairment in the clearance of dysfunctional mitochondria as evidenced by the accumulation of mitochondrial proteins involved in the mitophagic process such as dynamin-related protein (Drp1) and PTEN induced putative kinase 1 (PINK1). As a consequence of the presence of undigested damaged mitochondria, cells lacking TG2 increase their rate of aerobic glycolysis and become more sensitive to cell death.
In conclusion, we believe that TG2 can be considered a specialized chaperone that is able to perform pleiotropic functions on many protein substrates as dictated by alterations of the proteostasis by various forms of cellular stresses. In fact, TG2 is barely expressed and/or its transamidating activity is silent in many cell types under normal homeostatic conditions, but is immediately turned on when there are changes in cellular conditions that alter the proteome. TG2 might be an ideal specialized chaperone that, through its 4 different enzymatic activities, can help to post-translationally modify unfolded, mutated, or superfluous proteins, leading either to their degradation by autophagy or their accumulation into aggresomes. In fact, TG2 has been shown to be involved in almost all human disease processes in which there are profound alterations of the protein profile. In keeping with this proposed function we showed that TG2 interacts with and helps to recruit ubiquitinated proteins into the autophagosomes by interacting with the scaffold protein sequestosome 1/p62 (SQSTM1) and the microtubule-associated protein light chain 3 β (LC3 II). In addition, TG2 has been shown to interact with many protein chaperones,Citation4 thus it can function either directly or indirectly as part of the protein machinery that acts during stressful conditions to re-establish normal cellular proteostasis. However, we believe that future studies should also clarify whether TG2 plays a role under physiological conditions independent from these stress-dependent events.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed
Funding
This work was supported by grants from MIUR (PRIN 2012 and FIRB), The Ministry of Health of Italy "Ricerca Corrente" and "Ricerca Finalizzata," and AIRC. The support of the EU grant “Transpath” Marie Curie project to MP is also acknowledged.
References
- Rossin F, D’Eletto M, Falasca L, Sepe S, Cocco S, Fimia GM, Campanella M, Mastroberardino PG, Farrace MG, Piacentini M. Transglutaminase 2 ablation leads to mitophagy impairment associated with a metabolic shift towards aerobic glycolysis. Cell Death Differ. 2014; http://dx.doi.org/10.1038/cdd.2014.106. [Epub ahead of print]
- Johansen T, Lamark T. Selective autophagy mediated by autophagic adapter proteins. Autophagy 2011; 7:279-296; PMID:21189453; http://dx.doi.org/10.4161/auto.7.3.14487
- Springer W, Kahle PJ. Regulation of PINK1-Parkin-mediated mitophagy. Autophagy 2011; 7:266-278; PMID:21187721; http://dx.doi.org/10.4161/auto.7.3.14348
- Fesus L, Piacentini M. Transglutaminase 2:an enigmatic enzyme with diverse functions. Trends Biochem Sci 2002; 27:534-539; PMID:12368090; http://dx.doi.org/10.1016/S0968-0004(02)02182-5
- Mastroberardino PG, Farrace MG, Viti I, Pavone F, Fimia GM, Melino G, Rodolfo C, Piacentini M. “Tissue” transglutaminase contributes to the formation of disulphide bridges in proteins of mitochondrial respiratory complexes. Biochim Biophys Acta 2006; 1757:1357-1365; PMID:16979579; http://dx.doi.org/10.1016/j.bbabio.2006.07.007
- D’Eletto M, Farrace MG, Falasca L, Reali V, Oliverio S, Melino G, Griffin M, Fimia GM, Piacentini M. Transglutaminase 2 is involved in autophagosome maturation. Autophagy 2009; 5:1145-1154; http://dx.doi.org/10.4161/auto.5.8.10040
- Rossin F, D’Eletto M, Macdonald D, Farrace MG, Piacentini M. TG2 transamidating activity acts as a reostat controlling the interplay between apoptosis and autophagy. Amino Acids 2012; 42:1793-1802; PMID:21479826; http://dx.doi.org/10.1007/s00726-011-0899-x
- D’Eletto M, Farrace MG, Rossin F, Strappazzon F, Giacomo GD, Cecconi F, Melino G, Sepe S, Moreno S, Fimia GM, et al. Type 2 transglutaminase is involved in the autophagy-dependent clearance of ubiquitinated proteins. Cell Death Differ 2012; 19:1228-1238; http://dx.doi.org/10.1038/cdd.2012.2
- Lu H, Li G, Liu L, Feng L, Wang X, Jin H. Regulation and function of mitophagy in development and cancer. Autophagy 2013; 9:1720-1736; PMID:24091872; http://dx.doi.org/10.4161/auto.26550
- Chhabra A, Verma A, Mehta K. Tissue transglutaminase promotes or suppresses tumors depending on cell context. Anticancer Res 2009; 29:1909-1919; PMID:19528447
