Abstract
In addition to regulating the activation of nuclear factor (NF)-κB and c-Jun N-terminal kinase (JNK), TGF-β activated kinase 1 (TAK1) also upregulates the activation of AMP-activated protein kinase (AMPK) and autophagy. In the liver, TAK1-mediated autophagy plays a role in preventing excessive lipid accumulation induced by starvation and fat overload. TAK1-mediated autophagy and inhibition of mechanistic target of rapamycin complex 1 (mTORC1) prevent liver fibrosis and tumor development.
Abbreviations
| AMPK | = | AMP-activated protein kinase |
| FAO | = | fatty acid oxidation |
| IKK | = | IκB kinase |
| JNK | = | c-Jun N-terminal kinase (JNK) |
| LC3B | = | microtubule-associated protein 1 light chain 3 b |
| LKB1 | = | liver kinase B1 |
| NF-κB | = | nuclear factor κB |
| mTORC1 | = | mechanistic target of rapamycin complex 1 |
| HFD | = | high-fat diet |
| PPAR | = | peroxisome proliferator-activated receptor |
| TAK1 | = | TGF-β activated kinase 1 |
| Tak1ΔHEP mice | = | hepatocyte-specific Tak1-deficient mice |
| TLR | = | Toll-like receptor |
| ULK1 | = | unc-51 like autophagy activating kinase |
| WT | = | wild-type |
Main Text
TGF-β activated kinase 1 (TAK1), also known as MAP3K7, is a serine/threonine kinase that belongs to the MAP3K family. In the signaling pathways of IL-1β, toll-like receptors (TLRs), TNFα, and TGF-β, TAK1 regulates the activation of nuclear factor (NF)-κB and c-Jun N-terminal kinase (JNK).Citation1 To examine the physiological role of TAK1 in the liver, we generated hepatocyte-specific Tak1-deficient (Tak1ΔHEP) mice. Surprisingly, these mice develop spontaneous liver damage that leads to chronic liver inflammation, fibrosis, and carcinogenesis.Citation2 The ablation of Tak1 in hepatocytes promotes hepatocyte apoptosis and compensatory hepatocyte proliferation due to failure to activate the survival function of NF-κB. This aberrant hepatocyte regeneration, in combination with chronic liver inflammation, ultimately results in the development of hepatocellular carcinoma in TAK1ΔHEP mice. However, another group reported that additional deletion of Ikbkg (also known as Nemo), an essential modulator of IκB kinase (IKK) and NF-κB activation, reduces the spontaneous liver damage, inflammation, fibrosis, and cancer in Tak1ΔHEP mice.Citation3 This suggests that mechanisms regulated by TAK1 other than the NF-κB pathway are responsible for the regulation of the liver pathology that develops in Tak1ΔHEP mice.
Several studies have reported that TAK1 also activates AMP-activated protein kinase (AMPK) and autophagy.Citation4 Our recent work demonstrates the pathophysiological role of TAK1-mediated autophagy in the liver. Fasting is a physiological inducer of autophagy in the liver. Fasted wild-type (WT) mice show induction of hepatic autophagy accompanied by decreased expression of p62 (also known as sequestosome or SQSTM1Citation1) and activity of mechanistic target of rapamycin complex 1 (mTORC1). To our surprise, hepatic Tak1 deficiency suppressed autophagy induction and rescued the decrease in p62 expression and mTORC1 activity upon fasting.Citation5 Nutrient deprivation or sensing an increased AMP/ATP ratio activates AMPK through liver kinase B 1 (LKB1), which directly and indirectly phosphorylates unc-51 like autophagy activating kinase (ULK1) and subsequently induces autophagy. We found that starvation-induced phosphorylation of LKB1, AMPK, and ULK1 and autophagy are TAK1-dependent in hepatocytes. Using microtubule-associated protein 1 light chain 3 β (LC3B)-GFP transgenic mice, we found that basal levels of conversion of LC3BI to LC3BII protein, free GFP generation from the LC3B-GFP fusion protein, and mRNA levels of autophagy-related genes (e.g., Atg3, Atg5, and Ulk1) are downregulated in Tak1-deficient livers.Citation5 This indicates that TAK1 regulates not only autophagy induced through AMPK activation, but also basal autophagy through transcriptional control of the expression of autophagy-related genes. A recent report demonstrated that TAK1 also mediates autophagy through S6K1 independently of AMPK, which may be another mechanism for the regulation of basal autophagy through TAK1.Citation6
AMPK and mTORC1 are suggested to be downstream of TAK1. Indeed, overexpression of a constitutively active form of AMPK and treatment with rapamycin, an mTORC1 inhibitor, induces autophagy in Tak1-deficient hepatocytes.Citation5 Rapamycin treatment of TAK1-deficient hepatocytes significantly induces autophagy, but to a lower level than that in WT cells. This suggests that the regulation of autophagy by TAK1 is modulated not only by AMPK and mTORC1, but also by other pathways such as IKK signaling, as reported previously.Citation7 A physiological function of TAK1-mediated autophagy is to prevent excessive lipid accumulation through lipophagy, a type of autophagy that degrades intracellular lipid droplets. Fasting increases hepatic uptake of circulating fat released from peripheral fat tissues, but hepatic fat accumulation is tightly regulated by lipophagy to prevent excessive fat accumulation. In contrast, upon fasting the livers of Tak1ΔHEP mice dramatically changed to a white color, reflecting excessive accumulation of fat in hepatocytes due to the lack of lipophagy.Citation5 We observed autophagic vesicles attached to lipid droplets in WT livers using electron microscopy, but autophagic vesicles were rarely seen in Tak1ΔHEP livers.
Although IkbkbΔHEP mice have reduced autophagy induction,Citation7 fasting does not cause a fatty liver appearance in these mice (Seki, unpublished observation). This suggests the existence of another mechanism in addition to autophagy that promotes hepatic lipid degradation. mTORC1 is known to negatively regulate peroxisome proliferator-activated receptor (PPAR) α-mediated functions.Citation8 Given the pronounced upregulation of mTORC1 activity, hepatic PPARα activity is suppressed in Tak1ΔHEP livers. Expression of PPARα-inducible genes (e.g., Acox1, Cpt1, and Hmgcs2) and PPARα-mediated β-oxidation were markedly suppressed by the loss of Tak1.Citation5 Suppression of lipophagy and PPARα-mediated β-oxidation promotes lipid accumulation in the liver. Accordingly, a high-fat diet (HFD) challenge augmented hepatic steatosis and inflammation in Tak1ΔHEP mice as a result of the strong suppression of autophagy and β-oxidation by Tak1-deficiency.Citation5 Intriguingly, insulin resistance is comparable between WT and Tak1ΔHEP mice with high fat diet (HFD) feeding, which suggests a dissociation between hepatosteatosis/ steatohepatitis and insulin resistance.
Alternatively, the role of TAK1-mediated autophagy can be examined through forced induction of autophagy by administration of rapamycin to Tak1ΔHEP mice. In contrast to the severe fatty liver appearance in fasted Tak1ΔHEP mice, rapamycin treatment suppressed hepatic fat accumulation in these mice.Citation5 We confirmed that the effect of rapamycin on the reduction of fat accumulation is largely dependent on autophagy using Atg5-silenced hepatocytes.Citation5 We also found decreased hepatic expression of TAK1 in advanced fatty liver disease, suggesting a mechanism whereby a decrease in TAK1 in advanced fatty liver suppresses autophagy and increases mTORC1 activity, which further augments hepatosteatosis and liver inflammation and fibrosis, ultimately causing carcinogenesis .
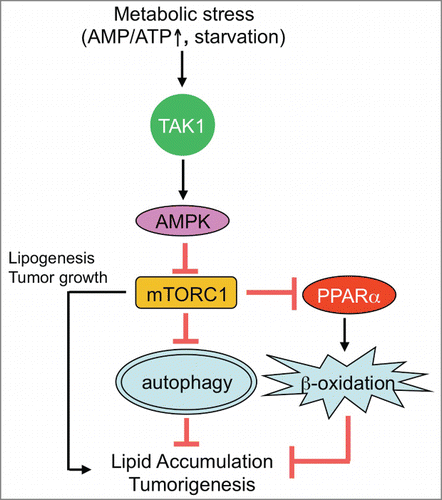
Figure 1. TAK1-mediated autophagy prevents hepatic steatosis and tumorigenesis. Upon nutrition deprivation, TGF-β activated kinase 1 (TAK1) mediates autophagy through activation of AMP-activated protein kinase (AMPK) and inhibition of mechanistic target of rapamycin complex 1 (mTORC1). TAK1 is also associated with induction of peroxisome proliferator-activated receptor (PPAR) α-mediated fatty acid oxidation (FAO) through suppression of mTORC1. Both autophagy and FAO contribute to lipid breakdown to prevent excessive lipid accumulation in hepatocytes. Autophagy prevents, and mTORC1 enhances, spontaneous tumorigenesis in the liver.
Since our previous study showing that autophagy defects are associated with liver tumor development,Citation9 we have examined the role of autophagy defects in the development of liver fibrosis and tumorigenesis in our mouse model. Rapamycin-induced autophagy significantly suppressed spontaneous hepatocyte injury, liver fibrosis, and tumorigenesis in Tak1ΔHEP mice.Citation5 This suggests that TAK1-mediated autophagy plays a role in preventing spontaneous liver fibrosis and cancer. The clinical relevance of TAK1 in human non-alcoholic steatohepatitis (NASH) and hepatocarcinogenesis is currently unknown, but once its clinical relevance in human liver disease is understood, targeting TAK1 or TAK-1–mediated autophagy could be an important option for the prevention and treatment of human liver disease.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed
Funding
This work is supported by NIH grants R01AA02172, R01DK085252, and P42 ES010337.
References
- Roh YS, Song J, Seki E. TAK1 regulates hepatic cell survival and carcinogenesis. J Gastroenterol 2014; 49:185-194; PMID:24443058; http://dx.doi.org/10.1007/s00535-013-0931-x
- Inokuchi S, Aoyama T, Miura K, Osterreicher CH, Kodama Y, Miyai K, Akira S, Brenner DA, Seki E. Disruption of TAK1 in hepatocytes causes hepatic injury, inflammation, fibrosis, and carcinogenesis. Proc Natl Acad Sci U S A 2010; 107:844-849; PMID:20080763; http://dx.doi.org/10.1073/pnas.0909781107
- Bettermann K, Vucur M, Haybaeck J, Koppe C, Janssen J, Heymann F, Weber A, Weiskirchen R, Liedtke C, Gassler N, et al. TAK1 suppresses a NEMO-dependent but NF-kappaB-independent pathway to liver cancer. Cancer Cell 2010; 17:481-496; PMID:20478530; http://dx.doi.org/10.1016/j.ccr.2010.03.021
- Herrero-Martin G, Hoyer-Hansen M, Garcia-Garcia C, Fumarola C, Farkas T, Lopez-Rivas A, Jaattela M. TAK1 activates AMPK-dependent cytoprotective autophagy in TRAIL-treated epithelial cells. EMBO J 2009; 28:677-685; PMID:19197243; http://dx.doi.org/10.1038/emboj.2009.8
- Inokuchi-Shimizu S, Park EJ, Roh YS, Yang L, Zhang B, Song J, Liang S, Pimienta M, Taniguchi K, Wu X, et al. TAK1-mediated autophagy and fatty acid oxidation prevent hepatosteatosis and tumorigenesis. J Clin Invest 2014; 124:3566-3578; PMID:24983318; http://dx.doi.org/10.1172/JCI74068
- Shin JH, Min SH, Kim SJ, Kim YI, Park J, Lee HK, Yoo OJ. TAK1 regulates autophagic cell death by suppressing the phosphorylation of p70 S6 kinase 1. Sci Rep 2013; 3:1561.
- Criollo A, Senovilla L, Authier H, Maiuri MC, Morselli E, Vitale I, Kepp O, Tasdemir E, Galluzzi L, Shen S, et al. The IKK complex contributes to the induction of autophagy. EMBO J 2010;29:619-631; PMID:19959994; http://dx.doi.org/10.1038/emboj.2009.364
- Sengupta S, Peterson TR, Laplante M, Oh S, Sabatini DM. mTORC1 controls fasting-induced ketogenesis and its modulation by ageing. Nature 2010;468:1100-1104; PMID:21179166; http://dx.doi.org/10.1038/nature09584
- Ichimura Y, Waguri S, Sou YS, Kageyama S, Hasegawa J, Ishimura R, Saito T, Yang Y, Kouno T, Fukutomi T, et al. Phosphorylation of p62 activates the Keap1-Nrf2 pathway during selective autophagy. Mol Cell 2013;51:618-631; PMID:24011591; http://dx.doi.org/10.1016/j.molcel.2013.08.003
