Abstract
Multiple myeloma (MM) comprises 1% of all malignancies and 10% of all hematological malignancies. MM is a malignancy of plasma cells in the bone marrow where complex and dynamic interactions with the bone marrow microenvironment lead to tumor progression, skeletal destruction and angiogenesis. Despite the discovery of several novel treatments against MM, including the proteasome inhibitor bortezomib, it is considered to be an incurable disease with an average 4–5 years overall survival.
It is now recognized that MM rely on the activation of multiple receptor and nonreceptor tyrosine kinases (RTKs) for proliferation and survival. Despite this fact, tyrosine kinase inhibitors are not part of the therapeutic protocols currently used in the clinic. Considering the profound importance of receptor and nonreceptor tyrosine kinases in the tumorigenesis and progression of MM, we hypothesized that the usage of a multi-RTK inhibitor, rather than a target-specific RTK inhibitor, would disrupt multiple oncogenic signaling cascades and confer lethal damages to MM cells (). We found that sorafenib (Sor) induced cell death in a panel of MM cell lines with variable efficacy at early time points and reached high levels of cell death after 72 h. Interestingly the mode of cell death varied considerably between cell lines, with some displaying characteristics typical of apoptosis whereas others of caspase-independent cell death.
Figure 1. Multitargeted therapies for multiple myeloma. Treatment of MM cells with Sor leads to the inhibition of several tyrosine kinase signaling cascades. Development of resistance to Sor may be mediated by the induction of autophagy or by the uptake of fibroblast-derived secreted factors. Co-treatment of MM cells with either the autophagy inhibitor chloroquine or the BCL-2 antagonist ABT737 may revert the resistance and improve the efficacy of Sor.
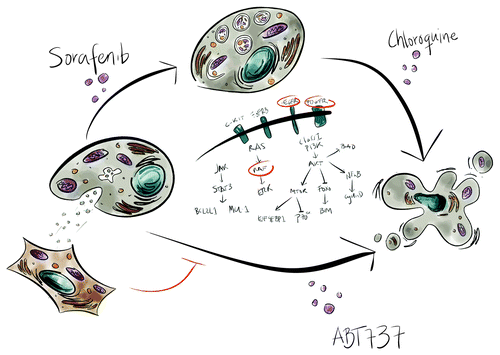
Apart from the cell death pathways we examined the role of autophagy in Sor-induced cell death. We found that Sor induced autophagy in LP1, RPMI 8226 cells, patient samples and in mice as shown by SQSTM1/p62 degradation, LC3 lipidation and appearance of GFP-LC3-positive foci. On the one hand, pre-treatment of MM cell lines with chemical inhibitors of early, 3-methyladenine (3MA), and late, chloroquine (CQ), autophagy lead to a potentiation of Sor-induced cell death in LP-1, OPM-2 and NCI cells (). On the other hand, co-treatment of MM cell lines with Sor and the autophagy inducer rapamycin (Rapa) partially protects LP-1 and OPM-2 cell lines from Sor-induced cell death. Together, these data indicate that Sor-induced autophagy is cytoprotective, and inhibition of this pathway can potentiate the efficacy of Sor. We also showed that Sor induced autophagy and cell death in CD138-enriched cells from myeloma patient samples.
The mechanism behind the induction of autophagy by tyrosine kinase inhibitors remains elusive. We found no evidence of direct cellular damage (e.g., mitochondrial damage, ER stress), which also promotes the activation of the autophagic program. Another potential mechanism is via the inhibition of the PI3K-AKT pathway, which leads to the inactivation of MTOR, a negative regulator of autophagy. The activity of MTOR is determined by the levels of phosphorylation of its downstream targets such as RPS6K/p70S6K or EIF4EBP1. We found that Sor inhibits the phosphorylation of EIF4EBP1 in several MM cell lines suggesting that inhibition of MTOR activity may be responsible for the induction of autophagy in these cells.
The role of the anti-apoptotic BCL2 family members in regulating the activation of autophagy is well established. Apart from BCL2L1 and Bcl-xL and their interaction with the BH3-only protein BECN1, it was recently found that MCL1 acts as a stress sensor and regulates activation of autophagy by interacting with BECN1. Interestingly, one of the most dramatic effects induced by Sor is the post-transcriptional downregulation of MCL1. It is tempting to speculate that this downregulation may serve two purposes, one being to lower the apoptotic threshold required for the induction of cell death and the other to allow for the induction of autophagy to counteract the cytotoxic effects induced by Sor.
One of the important findings in our study is that bone marrow stroma cells can protect some of the MM cell lines and the primary samples from sorafenib-induced cell death. It is becoming more and more evident that the tumor microenvironment is a strong “resistance to therapy” factor (). Interestingly, the combination of Sor with the BCL2 antagonist ABT737 reverts the resistance to Sor conferred by the presence of bone marrow fibroblasts in a trans-well setting. ABT737 also induces autophagy presumably by disrupting the interaction of BCL2 and/or BCL2L1 with BECN1. It would be interesting to examine whether one of the reasons of resistance to Sor is the induction of tumor microenvironment-promoting autophagy in the MM cells.
We examined in vivo Sor efficacy using the 5T33MM mouse model. C57BL/KaLwRijHsd mice inoculated with 5T33MMvv cells were either treated with Sor or placebo. Survival rate increased significantly (p < 0.001) in mice treated with Sorafenib compare with the placebo group. At day 63, tumor burden in the bone marrow in the nontreated group was ranging from 52–97%, whereas it was ranging from 16–34% in the Sor-treated group, indicating that Sor significantly reduces tumor development. We showed in vitro that Sor combined with rapamycin or chloroquine have stronger cytotoxic efficacy than Sor monotherapy. The in vivo efficacy of these two agents should also be examined.
In summary, sorafenib induced autophagy in several MM cell lines, in primary MM patient samples and in mice. The autophagic program induced by Sor in the MM cell lines attenuates cell death induced by this multi-tyrosine kinase inhibitor. The combination of Sor with either chemical inhibitors (e.g., chloroquine) of early and late autophagy or with autophagy inducers (e.g., rapalogs) potentiates the cytotoxic efficacy of Sor as a monotherapy. Thus, the combination of sorafenib with autophagy inhibitors may be a novel therapeutic strategy against MM, which needs to be evaluated in clinical trials.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.