Abstract
Autophagy is a cell-autonomous mechanism of innate immunity that protects the cytosol against bacterial infection. Invasive bacteria, including Listeria monocytogenes, have thus evolved strategies to counteract a process that limits their intracellular growth. ActA is a surface protein produced by L. monocytogenes to polymerize actin and mediate intra- and intercellular movements, which plays a critical role in autophagy escape. We have recently investigated the role of another L. monocytogenes surface protein, the internalin InlK, in the infection process. We showed that in the cytosol of infected cells, InlK interacts with the Major Vault Protein (MVP), the main component of cytoplasmic ribonucleoprotein particles named vaults. Although MVP has been implicated in a variety of key cellular process, its role remains elusive. We demonstrated that L. monocytogenes is able, via InlK, to decorate its surface with MVP in order to escape autophagic recognition. Strikingly, this new strategy used by L. monocytogenes to avoid autophagy is independent of ActA, suggesting that InlK-MVP interactions and actin polymerization are two processes that favor in the same manner the infection process. Understanding the role of MVP may provide new insights into bacterial infection and autophagy.
L. monocytogenes is a facultative intracellular pathogen responsible for listeriosis. It is able to interact with and subvert host molecules to invade cells and spread from cell-to-cell. Listeria has emerged as a paradigm to study host-pathogen interactions and fundamental processes in cell biology, and the study of actin rearrangements upon entry and intracellular movements is a well-recognized example of how bacterial-induced processes can yield insight into basic cellular processes. Listeria ActA triggers the recruitment of the Arp2/3 complex to mediate actin polymerization and propel bacteria from one infected cell to another without exposure to the extracellular milieu. Interestingly, ActA, independent of its role in bacterial motility, also disguises Listeria from autophagic recognition within the cytosol of infected cells. Listeria lacking ActA becomes rapidly ubiquitinated and targeted by the selective autophagy receptor p62 and autophagic degradation, leading to decreased survival as compared with wild-type (WT) bacteria. Thus, it is thought that Listeria efficiently uses ActA to camouflage itself to escape recognition by autophagy. While the role of ActA in autophagy is now well established, the role of many other surface proteins during Listeria infection remains elusive.
We recently investigated the role of InlK, a member of the internalin family specific to L. monocytogenes. We first demonstrated, using deletion analysis and in vivo infection, that InlK is a bona fide virulence factor of L. monocytogenes. We then showed that InlK is anchored to the surface of Listeria through its LPXTG peptidoglycan anchoring signal and that it is only expressed in vivo, making this virulence factor difficult to analyze in vitro without overexpression. Using InlK as bait in a yeast two-hybrid screen we identified the major vault protein (MVP) as a prey with a high interaction score. We then confirmed the InlK-MVP interaction by pull-down and co-immunoprecipitation assays. MVP is the main component of cytoplasmic ribonuleoprotein particles named vaults. Vault particles have been implicated in several cellular processes, including cellular differentiation, innate immunity, virus infection, signaling cascades, and cell survival. However their role remains enigmatic. Using InlK overexpressing L. monocytogenes to infect tissue cultured cells, we demonstrated that approximately 20% of the intracytosolic bacteria efficiently recruit MVP on their surface. Interestingly, we did not observe co-recruitment of MVP and actin to those intracellular bacteria, and, using live cell imaging, we demonstrated that MVP recruitment occurs before actin polymerization. We then verified that MVP is recruited independently of actin polymerization by infecting cells with Listeria ΔactA expressing InlK. Strikingly, MVP recruitment to these bacteria was more efficient than to WT bacteria expressing InlK.
To test if MVP recruitment could lead to autophagic escape, we analyzed the recruitment of selective autophagy markers (ubiquitin, p62 and GFP-LC3) around MVP-positive bacteria. Irrespective of their ability to polymerize actin (i.e., using either WT or Listeria ΔactA), we found that the vast majority of MVP-positive bacteria were completely devoid of autophagy marker labeling, and none of them were totally engulfed in an autophagosome. In some rare cases, MVP-positive bacteria exhibit a recruitment of p62 or GFP-LC3 at one pole. We examined these bacteria by live-cell-imaging, and showed that the membrane elongation leading to the autophagosome formation fails to occur. To confirm these findings, we analyzed the total level of lipidated LC3 (i.e., LC3-II) in cells infected with ΔactA or ΔactA expressing InlK bacteria, and found that InlK expression leads to a significant decrease of autophagy induction in response to infection.
As cells use autophagy to restrict the growth of intracellular microbes, we tested whether MVP-dependent autophagy escape could lead to an increase in bacterial survival. We first showed that, as expected, the intracellular survival of Listeria lacking ActA is significantly lower than WT bacteria. Strikingly, we observed that the overexpression of InlK by the ΔactA strain restores intracellular survival levels to that of WT bacteria, indicating that InlK could functionally replace ActA in its role of autophagy escape. To confirm the role of InlK-MVP interaction in bacterial survival, we showed that the InlK-overexpressing ΔactA strain did not replicate more than the ΔactA strain in MVP-depleted cells.
Together, our results show that L. monocytogenes is able to decorate its surface, via InlK, with MVP in order to escape autophagy (). It has not yet been determined whether MVP-dependent autophagy escape is strictly due to the disguising of intracytosolic bacteria (as previously described for ActA-dependent autophagy escape) or if the bacteria somehow exploits unknown anti-autophagic properties of MVP. To fully understand the role of MVP in autophagy, MVP will have to be analyzed using different autophagy scenarios. Indeed, MVP and/or vault particles have been mainly described as scaffolding proteins involved in diverse cellular processes that have links to autophagy, including apoptosis. A more comprehensive understanding of MVP biology should provide new insights into how bacteria can subvert autophagic processes.
Figures and Tables
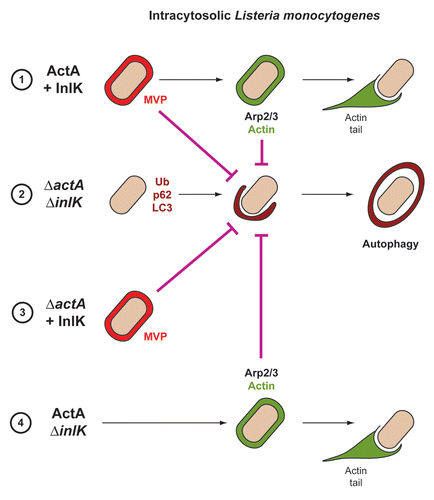
Figure 1 Strategies used by L. monocytogenes to avoid autophagic recognition. Listeria is able to avoid autophagic recognition using two independent virulence factors, ActA and InlK. Depending on ActA and InlK expression, four possibilities can be distinguished: (1) ActA and InlK are coexpressed by the bacterium: InlK recruits MVP (red) to the surface of the bacterium. ActA subsequently replaces InlK, and actin (green) replaces MVP to disguise the bacteria and prevent ubiquitinated protein (Ub) recruitment/formation, p62 recognition and LC3 recruitment; (2) neither ActA nor InlK is expressed: Listeria is surrounded by ubiquitinated proteins, p62 and LC3, leading to autophagy; (3) In the absence of ActA, InlK recruits MVP and efficiently protects bacteria from ubiquitinated protein recruitment/formation, p62 recognition and LC3 recruitment; (4) ActA is expressed, but not InlK: the recruitment of the Arp2/3 complex and actin is sufficient to prevent ubiquitinated protein recruitment/formation, p62 recognition and LC3 recruitment.
Punctum to: Dortet L, Mostowy S, Samba Louaka A, Gouin E, Nahori MA, Wiemer EAC, et al. Recruitment of the Major Vault Protein by InlK: a Listeria monocytogenes strategy to avoid autophagy. PLoS Pathog 2011; 7:e1002168; PMID: 21829365; http://dx.doi.org/10.1371/journal.ppat.1002168