Abstract
A steady increase in life expectancy has resulted in an equivalent increase in elderly patients who are more susceptible to diseases than young patients. In a recent study, we found that in both in vitro and in vivo models of ischemia/reperfusion (I/R), a loss of ATG4B is causatively associated with the increased sensitivity of the liver to I/R injury with age. Our work suggests that a restoration or enhancement of autophagy is a novel therapeutic modality to ameliorate liver function after I/R to aged livers.
The liver is a highly aerobic organ and innately vulnerable to hypoxic and ischemic stress, especially in pericentral regions. Although prolonged ischemia eventually causes cell death, severe tissue damage paradoxically does not occur until a recovery of blood flow and normal physiological pH, an event called reperfusion injury. I/R injury causes morbidity and mortality in veno-occlusive diseases, hemorrhagic shock, trauma, and cardiac arrest. I/R injury is also an important issue to a liver surgeon. During hepatectomy, no-flow ischemia may result from temporary hepatic inflow occlusion that minimizes intraoperative blood loss. Likewise, a substantial period of ischemia is unavoidable between the time of donor liver procurement and vascular reconstructions for transplantation. Subsequent hepatocellular damage after reperfusion has well been recognized as a mechanism of postoperative liver failure.
Aged tissues are more vulnerable to pathological stress and disease. The steady increase in life expectancy is likely causing a drastic increase in the number of elderly patients with hepatic malignancies. Though elderly patients may receive surgical treatment, the aged liver has a significantly lower reparative capacity after I/R injury associated with surgical operations. Currently, no therapeutic strategy is available to suppress this age-dependent I/R injury.
The mechanisms inducing I/R injury are multifactorial, and mitochondrial dysfunction is a key contributing factor. The inner membranes of normal mitochondria are virtually impermeable to all solutes except those for which specific membrane-bound carriers exist, which is essential for the proton motive force and oxidative phosphorylation. Although a decline in mitochondrial function with advancing age is evident in muscle and the brain, we have observed that the number of polarized mitochondria, and the mitochondrial membrane potential (ΔΨm) are indistinguishable between 3-mo- and 26-mo-old mouse livers, implying that aging per se minimally affects the liver mitochondria. However, exposure of aged livers to I/R rapidly induces the loss of mitochondrial membrane permeability barrier, a pathological phenomenon known as the mitochondrial permeability transition (MPT), which does not occur in young livers. MPT onset has been recognized as a key mechanism of I/R injury and drug toxicity. Once the permeability pores open, the mitochondria become freely permeable to solutes with a molecular mass of up to 1,500 Da. As a consequence, oxidative phosphorylation uncouples and the mitochondria depolarize, leading to ATP depletion and ultimately cell death. Our results with a time-lapse imaging analysis revealed that the mitochondria in old livers rapidly undergo inner membrane permeabilization after I/R, whereas those in young livers tolerate I/R rather well ().
What makes old livers susceptible to mitochondria-dependent I/R injury? In the liver most cellular proteins are long-lived. Macroautophagy (referred to as autophagy hereafter) not only degrades and recycles long-lived cytoplasmic proteins, but also targets and removes damaged or abnormal mitochondria by lysosome-dependent machinery. Hence, autophagy is of paramount importance for the quality control of intracellular organelles, such as the mitochondria. Numerous studies have demonstrated the protective role of autophagy in mitochondria-related diseases, including I/R injury, alcohol and drug toxicity, neurodegenerative diseases and aging. Our earlier study demonstrated that efficient autophagy is critical to mitochondrial function and hepatocyte survival after prolonged I/R. Enhancing autophagy promotes recovery of ΔΨm and blocks cell death after reperfusion, indicating a causative role of impaired or inefficient autophagy in I/R-induced mitochondrial dysfunction. In a recent work using immunoblotting, genetic and imaging approaches, we observed in both isolated hepatocytes and in vivo livers that I/R depletes aged livers of ATG4B in a calpain-dependent manner, which in turn impairs autophagic responsiveness to reperfusion and accumulates dysfunctional mitochondria, resulting in the MPT and ultimately cell death. Notably, the baseline levels of autophagy-related proteins and the basal autophagic flux are indiscernible between age groups. Furthermore, hepatocytes from both young and old mice display a robust autophagic flux under mildly stressed conditions such as normoxia and starvation, suggesting a minor impact of aging per se on hepatocellular basal autophagy. Although reduced blood flow and volume are often observed in aged livers, alterations in hepatic parenchymal structure and function have been known to be minimal with aging. Our results support this longstanding observation and further reveal that a striking reduction in autophagy occurs only when aged livers are subjected to I/R ().
BECN1 plays a central role in autophagy initiation and cell survival by interacting with numerous cofactors. We have also shown that specific overexpression of BECN1 promotes autophagic flux and mitophagy and prevents cell death and MPT onset after I/R. The physiological relevance of such a relationship was confirmed and extended in I/R experiments conducted in vivo. Interestingly, BECN1 overexpression markedly suppresses reperfusion-induced ATG4B loss. Though the precise mechanisms are yet to be elucidated, a physical interaction between BECN1 and ATG3 appears to be important. A critical contribution of ATG3 to BECN1-mediated protection was verified in wild-type (WT) and knockout mouse embryonic fibroblasts: (1) ATG3-null cells are highly prone to the MPT and cell death after I/R, and (2) BECN1 overexpression confers cytoprotection only in ATG3-WT cells, but not in ATG3-deficient cells. Taken together, these findings suggest that a BECN1-ATG3 interaction, at least in part, contributes to the cytoprotective effects in old hepatocytes. Intriguingly, BECN1 may be a multifunctional protein that interacts with a variety of autophagic proteins and unknown proteins that are seemingly unrelated to autophagic pathways.
In conclusion, our study shows in both in vitro and in vivo models that impaired autophagic responsiveness to I/R results in the increased sensitivity of aged livers to reperfusion injury. Enhancing autophagy could be a novel strategy to ameliorate liver function after liver resection and transplantation surgery in elderly patients.
Figures and Tables
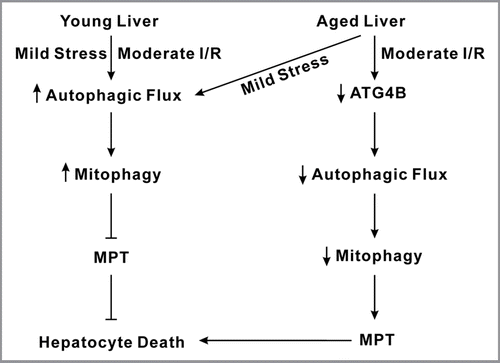
Figure 1 Scheme of hepatocyte death after I/R in aged livers. Young livers have a robust autophagic responsiveness to mild stress, such as normoxia and starvation, as well as moderate ischemia/reperfusion (I/R). As a consequence of increased mitophagy that can eliminate abnormal or dysfunctional mitochondria in a timely manner, MPT onset and hepatocyte death do not occur in young livers. Despite a strong tolerance against mild stress, aged livers poorly tolerate I/R injury due to impaired autophagy, which in turn promotes the onset of the MPT and cell death.
Acknowledgments
This work was supported in part by the National Institutes of Health National Institute of Diabetes and Digestive and Kidney Diseases grant DK079879 (J.-S. K.) and National Institute on Aging AG 17994 (C.L.), AG 21042 (C.L.) and the University of Florida Institute on Aging, Claude D. Pepper Older Americans Independence Center (1 P30 AG028740).
Punctum to: Wang JH, Ahn IS, Fischer TD, Byeon JI, Dunn WA Jr, Behrns KE, et al. Autophagy suppresses age-dependent ischemia and reperfusion injury in livers of mice. Gastroenterology 2011; 141:2188 - 2199; e6 PMID: 21854730; http://dx.doi.org/10.1053/j.gastro.2011.08.005