Abstract
Adult skeletal muscles adapt their fiber size to workload. We show that serum response factor (Srf) is required for satellite cell-mediated hypertrophic muscle growth. Deletion of Srf from myofibers, and not satellite cells, blunts overload-induced hypertrophy, and impairs satellite cell proliferation and recruitment to pre-existing fibers. We reveal a gene network in which Srf within myofibers modulates interleukin-6 and cyclooxygenase-2/interleukin-4 expressions and therefore exerts a paracrine control of satellite cell functions. In Srf-deleted muscles, in vivo overexpression of interleukin-6 is sufficient to restore satellite cell proliferation, but not satellite cell fusion and overall growth. In contrast, cyclooxygenase-2/interleukin-4 overexpression rescues satellite cell recruitment and muscle growth without affecting satellite cell proliferation, identifying altered fusion as the limiting cellular event. These findings unravel a role for Srf in the translation of mechanical cues applied to myofibers into paracrine signals, which in turn will modulate satellite cell functions and support muscle growth.
Adult skeletal muscle is a highly plastic tissue, the mass of which changes in response to environmental cues and/or physiological stimuli. The basic cellular building blocks of adult muscle are the multinucleated myofibers, which undergo remodeling during post-natal growth, during regeneration following injury, and in response to functional demand such as external loads and to nutrient availability.
In addition to the multinucleated post-mitotic myofibers, there are mononucleated stem cells located under the basal lamina—the satellite cells. Quiescent satellite cells become activated to meet myofiber adaptive requirements. Once activated, satellite cells follow an ordered set of events including proliferation, migration and fusion to growing adult myofibers.Citation1
Mature myofibers can grow by different ways: (1) the increase of their cytoplasmic volume by making more sarcomeric proteins and (2) the acquisition of new genetic material by accretion of new nuclei provided by the satellite cells.
As the accumulation of contractile proteins within the fiber, and the loss of such proteins, are associated with muscle hypertrophy and atrophy respectively, muscle protein synthesis and degradation are believed to be crucial in the regulation of muscle mass. Mechanical stimuli and anabolic reagent (such as IGF-1) lead to the activation of the translational machinery via PI3K/Akt/mTOR pathway. Conversely, chronic mechanical unloading and catabolic agents (such as glucocorticoids, TNFα) result in the activation of FOXO and NF-kB and the subsequent expression of genes implicated in protein catabolism such as the muscle E3 ubiquitin ligases (MuRF1 and MAFbx) and autophagy-related genes.Citation2
The mechanisms controlling satellite cell function (activation, proliferation, migration and fusion) contribute as well to muscle growth by regulating the addition of new myonuclei to the growing fibers. Satellite cell functions are regulated by extrinsic signals such as growth factors and cytokines.Citation3 Among the secreted factors, interleukin 6 (IL6), a myokine detected at high concentrations in contracting muscle fibers and after increased load, enhances satellite cell proliferation and migration during muscle hypertrophy.Citation4-Citation6 Muscle-secreted interleukin 4 (IL4) promotes muscle regeneration and post-natal growth by facilitating the fusion of myoblasts to nascent myotubes.Citation7 Prostaglandins produced by cyclooxygenase (Cox) enzymes, which catalyze the rate-limiting step in their synthesis, are bioactive lipid mediators that can also regulate satellite cell behavior.Citation8-Citation10
While significant progress has been made in understanding the signaling pathways that control muscle mass, the molecules that translate muscle load into signals that support muscle growth are unclear. Furthermore, very little is known about the transcription factors and the target genes that are involved in promoting adult muscle growth.
In this context, we focused our attention on the transcription factor Srf (Serum Response Factor) that is highly expressed in skeletal muscles and that controls the expression of genes specifically expressed in skeletal muscle (dystrophin, muscle creatine kinase, myoD), including several genes encoding sarcomeric proteins (such as α skeletal actin, myosin light chain, tropomyosin).Citation11 Data obtained from mouse genetic models with skeletal muscle specific loss of Srf or Mrtfs functions emphasize their crucial role in post-natal muscle growth.Citation12,Citation13 In the adult, Srf activity could also be important for the control of skeletal muscle mass. Evidence of an increase in Srf expression during overload-induced hypertrophy and a decrease in Srf expression during disuse-induced muscle atrophy and aging reinforces this hypothesis.Citation14-Citation16
In order to decipher the role of Srf in the control of muscle mass in the adult, mice in which the deletion of the Srf gene was induced in myofibers (Srfflox/flox:HSA-Cre-ERT2 mice injected with tamoxifen) were subjected to overload-induced plantaris muscle hypertrophy achieved by the incapacitation of two synergic muscles, the soleus and the gastrocnemius. During the compensatory hypertrophy phase, growth was completely blunted in the Srf-deleted plantaris muscle, demonstrating that Srf is necessary for overload-induced myofiber hypertrophy.
Unexpectedly, we showed that the lack of Srf in myofibers affected satellite cells proliferation and fusion to the growing fibers. In our genetic mouse model, Cre recombinase is expressed only in myofibers and not in satellite cells. This suggested a paracrine control of satellite cell functions by the myofibers that we were able to corroborate using cultured muscle cells.
To identify the secreted molecules mediating these effects and whose expression is under the control of Srf, we used a global transcriptomic approach allowing the identification of genes activated by Srf. We focused our attention on genes encoding the secreted factors IL6, IL4 and on Cox2 (which encodes a key enzyme for prostaglandin synthesis).
The potential roles of these factors in the lack of hypertrophic growth of muscles lacking Srf was tested by in vivo AAV-driven overexpression of IL6, IL4 or Cox2 in plantaris muscles prior to overload. In Srf-deleted muscles, the overexpression of IL6 is sufficient to restore satellite cell proliferation, but not satellite cell fusion and overall growth. In contrast, Cox2/IL4 overexpression rescues satellite cell recruitment and muscle growth without affecting satellite cell proliferation, identifying altered fusion as the limiting cellular event precluding hypertrophic growth of Srf-deleted muscles. In addition, we demonstrated that expressions of Cox2 and of IL4 genes are linked and that Cox2 is a direct Srf target gene which in turn controls IL4 expression. Thus, IL4 could mediate at least some of the action of Cox2 on satellite cell recruitment during muscle overload hypertrophy.
The contribution of satellite cells to muscle hypertrophy has been a controversial issue.Citation17,Citation18 Our data support a role for satellite cells in activity-induced hypertrophy and are in line with an elegant study showing that addition of nuclei precedes increased fiber size during compensatory hypertrophy and that this constitutes the major cause of hypertrophy.Citation19 In addition, recent data from Larsson’s group suggested that hypertrophy must be accompanied by new myonuclear incorporation for the maintenance of muscle-specific force and that there is a critical cytoplasmic volume that individual myonuclei can support efficiently.Citation20 Interestingly, although satellite cells appear to be involved in muscle hypertrophy in normal circumstances, satellite cell-depleted muscles undergo effective fiber hypertrophy (McCarthy et al., 2011).Citation21 The compensatory mechanism allowing growth in satellite cell-depleted skeletal muscle may be impaired in our model because of the lack of Srf expression in myofibers.
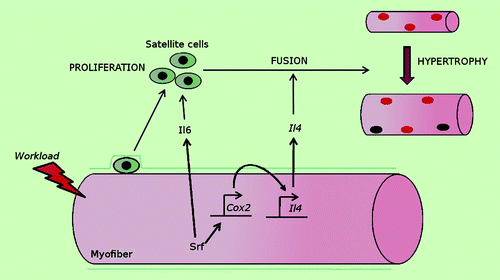
Together our findings unravel a role for Srf in the translation of mechanical cues applied to myofibers into paracrine signals, which in turn modulate satellite cell functions and support muscle growth ().Citation22 We provide evidence for a gene network operating in myofibers during overload-induced muscle growth in which Srf modulates IL6 and Cox2/IL4 expression levels, which control satellite cell proliferation and fusion, respectively. Interestingly, Srf is required for muscle growth in response to increased loading, but is dispensable for Myr-Akt induced muscle hypertrophy, which occurs in the absence of increased mechanical signals. Our future studies will focus on the identification of the mechanical signals and the underlying signaling pathways that can be interpreted by Srf.
Figure 1. Schematic model, in response to increased workload, Srf within myofibers modulates IL6 and Cox2/IL4 expression and, therefore, exerts a paracrine control of satellite cell proliferation and fusion, respectively, which in turn support skeletal muscle hypertrophy.
Hypertrophy induced by overload is greatly attenuated in older animals and we previously reported a decreased expression of Srf in aged human and mouse muscles.Citation16,Citation23,Citation24 Accordingly, loss of Srf within myofibers of young adult mice induced premature skeletal muscle aging.Citation16 Therefore, during aging, there is a further link between Srf activity and muscle hypertrophic capacities. Thus, the identification of Srf as a master controller of physiological hypertrophy carries potential significance for the search for muscle atrophy therapies and treatments alleviating muscular atrophy during muscle aging and disease.
References
- Le Grand F, Rudnicki MA. Skeletal muscle satellite cells and adult myogenesis. Curr Opin Cell Biol 2007; 19:628 - 33; http://dx.doi.org/10.1016/j.ceb.2007.09.012; PMID: 17996437
- Rüegg MA, Glass DJ. Molecular mechanisms and treatment options for muscle wasting diseases. Annu Rev Pharmacol Toxicol 2011; 51:373 - 95; http://dx.doi.org/10.1146/annurev-pharmtox-010510-100537; PMID: 20936944
- Kuang S, Gillespie MA, Rudnicki MA. Niche regulation of muscle satellite cell self-renewal and differentiation. Cell Stem Cell 2008; 2:22 - 31; http://dx.doi.org/10.1016/j.stem.2007.12.012; PMID: 18371418
- Carson JA, Nettleton D, Reecy JM. Differential gene expression in the rat soleus muscle during early work overload-induced hypertrophy. FASEB J 2002; 16:207 - 9; PMID: 11744623
- Penkowa M, Keller C, Keller P, Jauffred S, Pedersen BK. Immunohistochemical detection of interleukin-6 in human skeletal muscle fibers following exercise. FASEB J 2003; 17:2166 - 8; PMID: 12958150
- Serrano AL, Baeza-Raja B, Perdiguero E, Jardí M, Muñoz-Cánoves P. Interleukin-6 is an essential regulator of satellite cell-mediated skeletal muscle hypertrophy. Cell Metab 2008; 7:33 - 44; http://dx.doi.org/10.1016/j.cmet.2007.11.011; PMID: 18177723
- Horsley V, Jansen KM, Mills ST, Pavlath GK. IL-4 acts as a myoblast recruitment factor during mammalian muscle growth. Cell 2003; 113:483 - 94; http://dx.doi.org/10.1016/S0092-8674(03)00319-2; PMID: 12757709
- Bondesen BA, Mills ST, Pavlath GK. The COX-2 pathway regulates growth of atrophied muscle via multiple mechanisms. Am J Physiol Cell Physiol 2006; 290:C1651 - 9; http://dx.doi.org/10.1152/ajpcell.00518.2005; PMID: 16467402
- Otis JS, Burkholder TJ, Pavlath GK. Stretch-induced myoblast proliferation is dependent on the COX2 pathway. Exp Cell Res 2005; 310:417 - 25; http://dx.doi.org/10.1016/j.yexcr.2005.08.009; PMID: 16168411
- Shen W, Prisk V, Li Y, Foster W, Huard J. Inhibited skeletal muscle healing in cyclooxygenase-2 gene-deficient mice: the role of PGE2 and PGF2alpha. J Appl Physiol 2006; 101:1215 - 21; http://dx.doi.org/10.1152/japplphysiol.01331.2005; PMID: 16778000
- Pipes GC, Creemers EE, Olson EN. The myocardin family of transcriptional coactivators: versatile regulators of cell growth, migration, and myogenesis. Genes Dev 2006; 20:1545 - 56; http://dx.doi.org/10.1101/gad.1428006; PMID: 16778073
- Charvet C, Houbron C, Parlakian A, Giordani J, Lahoute C, Bertrand A, et al. New role for serum response factor in postnatal skeletal muscle growth and regeneration via the interleukin 4 and insulin-like growth factor 1 pathways. Mol Cell Biol 2006; 26:6664 - 74; http://dx.doi.org/10.1128/MCB.00138-06; PMID: 16914747
- Li S, Czubryt MP, McAnally J, Bassel-Duby R, Richardson JA, Wiebel FF, et al. Requirement for serum response factor for skeletal muscle growth and maturation revealed by tissue-specific gene deletion in mice. Proc Natl Acad Sci U S A 2005; 102:1082 - 7; http://dx.doi.org/10.1073/pnas.0409103102; PMID: 15647354
- Flück M, Carson JA, Schwartz RJ, Booth FW. SRF protein is upregulated during stretch-induced hypertrophy of rooster ALD muscle. J Appl Physiol 1999; 86:1793 - 9; PMID: 10368339
- Lamon S, Wallace MA, Léger B, Russell AP. Regulation of STARS and its downstream targets suggest a novel pathway involved in human skeletal muscle hypertrophy and atrophy. J Physiol 2009; 587:1795 - 803; http://dx.doi.org/10.1113/jphysiol.2009.168674; PMID: 19255118
- Lahoute C, Sotiropoulos A, Favier M, Guillet-Deniau I, Charvet C, Ferry A, et al. Premature aging in skeletal muscle lacking serum response factor. PLoS One 2008; 3:e3910; http://dx.doi.org/10.1371/journal.pone.0003910; PMID: 19079548
- McCarthy JJ, Esser KA. Counterpoint: Satellite cell addition is not obligatory for skeletal muscle hypertrophy. J Appl Physiol 2007; 103:1100 - 2, discussion 1102-3; http://dx.doi.org/10.1152/japplphysiol.00101.2007a; PMID: 17724306
- O’Connor RS, Pavlath GK. Point:Counterpoint: Satellite cell addition is/is not obligatory for skeletal muscle hypertrophy. J Appl Physiol 2007; 103:1099 - 100; http://dx.doi.org/10.1152/japplphysiol.00101.2007; PMID: 17289912
- Bruusgaard JC, Johansen IB, Egner IM, Rana ZA, Gundersen K. Myonuclei acquired by overload exercise precede hypertrophy and are not lost on detraining. Proc Natl Acad Sci U S A 2010; 107:15111 - 6; http://dx.doi.org/10.1073/pnas.0913935107; PMID: 20713720
- Qaisar R, Renaud G, Morine K, Barton ER, Sweeney HL, Larsson L. Is functional hypertrophy and specific force coupled with the addition of myonuclei at the single muscle fiber level?. FASEB J 2012; 26:1077 - 85; http://dx.doi.org/10.1096/fj.11-192195; PMID: 22125316
- McCarthy JJ, Mula J, Miyazaki M, Erfani R, Garrison K, Farooqui AB, et al. Effective fiber hypertrophy in satellite cell-depleted skeletal muscle. Development 2011; 138:3657 - 66; http://dx.doi.org/10.1242/dev.068858; PMID: 21828094
- Guerci A, Lahoute C, Hébrard S, Collard L, Graindorge D, Favier M, et al. Srf-dependent paracrine signals produced by myofibers control satellite cell-mediated skeletal muscle hypertrophy. Cell Metab 2012; 15:25 - 37; http://dx.doi.org/10.1016/j.cmet.2011.12.001; PMID: 22225874
- Alway SE, Degens H, Krishnamurthy G, Smith CA. Potential role for Id myogenic repressors in apoptosis and attenuation of hypertrophy in muscles of aged rats. Am J Physiol Cell Physiol 2002; 283:C66 - 76; PMID: 12055074
- Carson JA, Yamaguchi M, Alway SE. Hypertrophy and proliferation of skeletal muscle fibers from aged quail. J Appl Physiol 1995; 78:293 - 9; PMID: 7713826