Abstract
During mitosis, microtubules (MTs) are massively rearranged into three sets of highly dynamic MTs that are nucleated from the centrosomes to form the mitotic spindle. Tight regulation of spindle positioning in the dividing cell and chromosome alignment at the center of the metaphase spindle are required to ensure perfect chromosome segregation and to position the cytokinetic furrow that will specify the two daughter cells. Spindle positioning requires regulation of MT dynamics, involving depolymerase activities together with cortical and kinetochore-mediated pushing and pulling forces acting on astral MTs and kinetochore fibres. These forces rely on MT motor activities. Cortical pulling forces exerted on astral MTs depend upon dynein/dynactin complexes and are essential in both symmetric and asymmetric cell division. A well-established spindle positioning pathway regulating the cortical targeting of dynein/dynactin involves the conserved LGN (Leu-Gly-Asn repeat-enriched-protein) and NuMA (microtubule binding nuclear mitotic apparatus protein) complex.1 Spindle orientation is also regulated by integrin-mediated cell adhesion2 and actin retraction fibres that respond to mechanical stress and are influenced by the microenvironment of the dividing cell.3 Altering the capture of astral MTs or modulating pulling forces affects spindle position, which can impair cell division, differentiation and embryogenesis.
In this general scheme, the activity of mitotic kinases such as Auroras and Plk1 (Polo-like kinase 1) is crucial.4 Recently, the p21-activated kinases (PAKs) emerged as novel important players in mitotic progression. In our recent article, we demonstrated that PAK4 regulates spindle positioning in symmetric cell division.5 In this commentary, and in light of recent published studies, we discuss how PAK4 could participate in the regulation of mechanisms involved in spindle positioning and orientation.
At the onset of mitosis, the cellular cytoskeleton is significantly rearranged to allow subsequent assembly of a bipolar spindle, accurate segregation of chromosomes and completion of cytokinesis. The mitotic spindle is assembled from the duplicated centrosomes and requires MT nucleation and their dynamics. In metaphase the bipolar spindle with congressed chromosomes aligned to the metaphase plate is anchored to the cell cortex through astral MTs. In this configuration the spindle is submitted to tension with pulling and pushing forces emanating from different subcellular structures (the cellular cortex, spindle poles and kinetochores). The tension reaches a threshold when sister kinetochores become properly bioriented to spindle poles, allowing the onset of anaphase and chromatid segregation. A surveillance mechanism, the spindle assembly checkpoint, prevents the metaphase-anaphase transition until this exquisite tension is reached. Anchorage of the bipolar spindle to the cell cortex also defines the cell division axis and location. Disturbing spindle positioning, by altering astral MT capture or affecting pulling forces, induces spindle rotation that can compromise proper cell division and cell fate. In mammalian cells, the dynein complex is the major force generator at the cortex, where its recruitment depends on a conserved protein module that includes Gαi, LGN and NuMA-associated membrane proteins.Citation1 Essential functions of Auroras and Plk1 kinases in mitotic spindle formation and activity occur through the targeting of molecular motors and factors regulating MT dynamics during mitosis. Increasing evidence suggests that p21-activated kinases (PAKs) are also involved in the regulation of mitotic progression.Citation5-Citation10
PAKs are serine/threonine kinases initially characterized as effectors of the Rho GTPases Rac and Cdc42.Citation11 We previously demonstrated that the Xenopus ortholog of PAK4 regulates MT dynamics in interphase epithelial cells and in mitotic egg extract.Citation7,Citation12 In this latter study, we showed that PAK4 acts through the regulation of the small GTPase Ran. Ran controls nucleocytoplasmic transport, mitosis and nuclear envelope formation. These functions are regulated by the binding of Ran to different partners and by the formation of a Ran-GTP gradient emanating from chromatin.Citation13 We showed that Ran is phosphorylated by PAK4 on serine 135, which is increased during mitosis. Endogenous phosphorylated Ran and active PAK4 associate with centrosomes from prophase to anaphase and with chromosomes from prophase to metaphase. Ran phosphorylation on serine 135 impedes its binding to two of its regulators, RCC1 and RanGAP1 which respectively promote GTP loading and GTP hydrolysis. Thus, Ran phosphorylation can either prevent Ran activation or sustain Ran activity. Finally, we showed that PAK4 depletion inhibits Ran phosphorylation and delays mitosis entry and potentially mitotic progression.Citation7 This study led us to hypothesize that Ran phosphorylation regulates the assembly of Ran-dependent complexes on the mitotic spindle. We have now investigated the role of PAK4 during mitosis and showed that PAK4 depletion inhibits mitotic progression by strongly delaying the metaphase-anaphase transition, earlier mitotic phases progressing normally.Citation5 This delay is associated with the dynamic rotation of the metaphase plate on the x, y and z axes, demonstrating an altered positioning and orientation of the spindle upon PAK4 depletion. PAK4-depleted cells present numerous mitotic defects ranging from centrosome splitting, multipolar spindles and chromosome lagging, but we assume that all these defects derive from a primary defect. Indeed, extensive metaphase delay results in sister chromatid cohesion fatigue, which leads to asynchronous chromatid segregation, loss of spindle tension and the splitting of spindle poles.Citation14,Citation15 Chromosome congression occurred normally in PAK4-depleted cells but was followed by an extensive spindle rotation, resulting in a long metaphase-like delay. We did not identify what causes this delay, but we made several observations. In PAK4-depleted cells astral MTs were defective and off-centered spindles often appeared to adhere to the cortex. In addition, dynein and dynactin were mislocalized within these cells. In light of recently published articles, we would like to hypothesize how PAK4 may participate in spindle positioning and orientation.
In PAK4-depleted cells, the defective astral MT array prevents the cortical anchoring of the mitotic spindle.Citation5 This could reflect a PAK4-dependent regulation of astral MT nucleation and/or dynamics and may be mediated through regulation of Ran activity at centrosomes and/or the regulation of catastrophe factors such as stathminCitation7 (). Another possibility would be that PAK4 regulates the cortical capture of astral MTs. Indeed, defects in astral MT capture does lead to their depolymerisation. MT plus-end tracking proteins (+TIPs) are known to facilitate interaction between growing MTs and their intracellular target, including the cell cortex.Citation16 In PAK4-depleted cells, the +TIP EB1 is properly localized to the few remaining astral MTs. However, we do not know following the loss of PAK4, the status of Kif18B, an EB1 binding protein that regulates the number and length of mitotic astral MTs.Citation17 Another +TIPs, CLASP1 is also involved in astral MTs capture at the cortex and its loss was shown to impair spindle positioning.Citation18 A more in depth study of the dynamic of astral MTs and their recruitment of +TIPs tracking proteins in PAK4 depleted cells is currently ongoing to pinpoint the primary event leading to spindle rotation induced by the loss of PAK4 ().
Figure 1. Hypothetical model for PAK4 regulation of mitotic spindle positioning and orientation. Centrosomal bound active PAK4 could locally regulate Plk1 activity and participate in cortical localization of dynein. Alternatively the kinase could modulate astral MT dynamics via Ran-GTP and/or MT binding proteins. Metaphase plate bound active PAK4 could control the Ran-GTP gradient emanating from chromatin that is required for the spatial cortical regulation of LGN-NuMA complex.
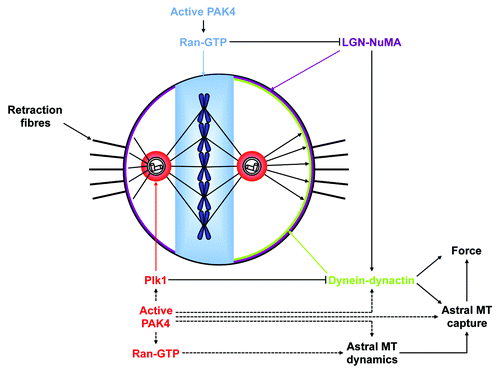
Nonetheless, defective spindle positioning and orientation in PAK4 depleted cells could also result of an impaired cortical localization and/or activity of dynein. Dynein is involved in multiple steps of mitotic progression. Cortical dynein is involved in regulating astral MT dynamic and tethering their anchor to the cortex.Citation19 Most importantly, cortical dynein regulates spindle anchoring and oscillations by pulling astral MTs and thus exerting forces toward centrosomes.Citation20 The recruitment of dynein to the cortex depends upon the spindle-positioning pathway that consists of Gαi, LGN and NuMA.Citation21,Citation22 Inhibition of this pathway leads to spindle rotation and mitotic delay.Citation23 LGN acts as a molecular switch that once bound to NuMA can interact with Gαi. Thus, the cortical localization of LGN and of NuMA depend on each other.Citation24 Interestingly, ABL1 kinase-mediated phosphorylation of NuMA maintains the cortical localization of NuMA during metaphase.Citation25 The LGN-NuMA complex is formed at the beginning of mitosis when NuMA is released from the nucleoplasm,Citation26 and reaches the cortex through interaction with membrane-associated Gαi. The cortical localization of dynein-dynactin has recently been shown to be dynamic and uneven.Citation22 As the metaphase spindle oscillates in the x- and y-axes, dynein-dynactin but not LGN dynamically accumulates, asymmetrically and synchronously with the oscillation, to the cortex facing the most distant spindle pole. Furthermore, cortical LGN-NuMA, and consequently dynein-dynactin, are excluded from the cortex near the spindle midzone.Citation22 Both of these dynamic localizations are controlled by two different mechanisms.
The mitotic kinase Plk1 is essential for spindle positioning.Citation27 Recently, a novel Plk1-dependent signaling pathway that regulates spindle oscillations required for positioning the metaphase plate at the center of the cell was described.Citation22 Indeed, when the spindle pole-to-cortex distance is below 2 µm, the centrosome-bound active Plk1 induces phosphorylation of dynein-dynactin components, and their dissociation from cortical LGN-NuMA (). This local displacement of dynein-dynactin consequently releases tensions exerted on the spindle. A second LGN-NuMA complex is excluded from the cortex in the vicinity of chromosomes in a Ran-GTP gradient-dependent mannerCitation22 (). This result is in disagreement with another report using a Drosophila cell model, that demonstrated a positive role for the Ran-GTP gradient in the recruitment of Mud (NuMA) to Pins (LGN) and the activation of the spindle orientation pathway.Citation28 Nevertheless, both reports highlight the importance of the Ran-GTP gradient in spindle positioning. Dynein is mislocalized in PAK4-depleted cells, and it would be of interest to study in finer detail whether LGN-NuMA at the “equatorial” cortex, and spindle pole Plk1-dependent release of dynein-dynactin at the “polar” cortex could be regulated by PAK4. Indeed, a number of pieces of evidence indicate that the PAK4 and Plk1 pathways may be linked. First, the mitotic localization of active PAK4 at the centrosomes and at the spindle midzone resembles the subcellular localization of Plk1 in mitosis, although active PAK4 stains the metaphase plate while Plk1 is restricted to kinetochores.Citation7,Citation29 Both Aurora A and B are known to be Plk1-activating kinases respectively at centrosomes in G2 and at centromeres in prometaphase, where Plk1 regulates MT-kinetochore attachment.Citation30-Citation32 Interestingly, PAK1 was also shown to phosphorylate Plk1 on serine 49 and participate in Plk1 activation.Citation10 Furthermore, PAKs, mammalian Ste20-like kinase and Aurora A were also identified from mitotic extractsCitation33 as Plk1 activating kinases and PAK1 regulates activation of centrosomal Aurora A.Citation9 Considering these data and our findings that PAK4 regulates spindle positioning, it is tempting to speculate that active centrosomal PAK4 may regulate Plk1 activity at centrosomes (). Further studies are now ongoing to test this hypothesis.
In addition, active PAK4 also accumulates on the metaphase plate, and we showed that PAK4 regulates Ran activity.Citation7 As already mentioned, the phosphorylation of Ran on serine 135 by PAK4 prevents this GTPase from binding to its regulators RCC1 and RanGAP1. In addition, the level of Ran phosphorylated on serine 135 is significantly increased during mitosis, and phosphorylated Ran species localize to discrete substructures on the chromosomes of the metaphase plate.Citation7 Such a PAK4-mediated spatiotemporal phosphorylation of Ran on the metaphase plate could regulate its GTPase activity on the spindle, and participate in the controlled cortical targeting of the LGN-NuMA complex ().
A prerequisite to the validation of these potential pathways will indeed be a thorough investigation of the dynamic cortical localization of dynein and LGN-NUMA complexes in metaphase cells following loss of PAK4.
Finally, another interesting possibility to consider in spindle positioning is the role of extrinsic cues. At the onset of mitosis, cells round up but remain attached to the substrate through actin-rich retraction fibers. Retraction fibers exert forces by mechano-tension on the cell body and control spindle positioning.Citation3 This involves the polarization of a dynamic subcortical actin structure, which somehow stabilizes astral MTs at the level of the retraction fibers. Since PAKs are well known for regulating the actin cytoskeleton, it will also be interesting to study the function of retraction fibers in spindle positioning upon PAK4 depletion.
Perspectives
In summary, our recent data show that PAK4 plays important roles in spindle positioning and orientation. Considering the studies recently published in the literature, here we propose several pathways in which PAK4 may be involved in the regulation of spindle orientation. Further studies are ongoing in the lab to test these hypotheses.
Acknowledgments
We want to especially thank Dr. James Hutchins (IGH, CNRS UPR142, Montpellier, France) for critical reading and editing of the manuscript. This work was supported by a grant MEGAPAK to N.M. from the ANR (Agence Nationale pour la Recherche) GENOPAT.
References
- Morin X, Bellaïche Y. Mitotic spindle orientation in asymmetric and symmetric cell divisions during animal development. Dev Cell 2011; 21:102 - 19; http://dx.doi.org/10.1016/j.devcel.2011.06.012; PMID: 21763612
- Toyoshima F, Nishida E. Spindle orientation in animal cell mitosis: roles of integrin in the control of spindle axis. J Cell Physiol 2007; 213:407 - 11; http://dx.doi.org/10.1002/jcp.21227; PMID: 17654475
- Fink J, Carpi N, Betz T, Bétard A, Chebah M, Azioune A, et al. External forces control mitotic spindle positioning. Nat Cell Biol 2011; 13:771 - 8; http://dx.doi.org/10.1038/ncb2269; PMID: 21666685
- Lens SM, Voest EE, Medema RH. Shared and separate functions of polo-like kinases and aurora kinases in cancer. Nat Rev Cancer 2010; 10:825 - 41; http://dx.doi.org/10.1038/nrc2964; PMID: 21102634
- Bompard G, Rabeharivelo G, Cau J, Abrieu A, Delsert C, Morin N. P21-activated kinase 4 (PAK4) is required for metaphase spindle positioning and anchoring. Oncogene 2012; http://dx.doi.org/10.1038/onc.2012.98; PMID: 22450748
- Faure S, Vigneron S, Dorée M, Morin N. A member of the Ste20/PAK family of protein kinases is involved in both arrest of Xenopus oocytes at G2/prophase of the first meiotic cell cycle and in prevention of apoptosis. EMBO J 1997; 16:5550 - 61; http://dx.doi.org/10.1093/emboj/16.18.5550; PMID: 9312014
- Bompard G, Rabeharivelo G, Frank M, Cau J, Delsert C, Morin N. Subgroup II PAK-mediated phosphorylation regulates Ran activity during mitosis. J Cell Biol 2010; 190:807 - 22; http://dx.doi.org/10.1083/jcb.200912056; PMID: 20805321
- Li F, Adam L, Vadlamudi RK, Zhou H, Sen S, Chernoff J, et al. p21-activated kinase 1 interacts with and phosphorylates histone H3 in breast cancer cells. EMBO Rep 2002; 3:767 - 73; http://dx.doi.org/10.1093/embo-reports/kvf157; PMID: 12151336
- Zhao ZS, Lim JP, Ng YW, Lim L, Manser E. The GIT-associated kinase PAK targets to the centrosome and regulates Aurora-A. Mol Cell 2005; 20:237 - 49; http://dx.doi.org/10.1016/j.molcel.2005.08.035; PMID: 16246726
- Maroto B, Ye MB, von Lohneysen K, Schnelzer A, Knaus UG. P21-activated kinase is required for mitotic progression and regulates Plk1. Oncogene 2008; 27:4900 - 8; http://dx.doi.org/10.1038/onc.2008.131; PMID: 18427546
- Manser E, Leung T, Salihuddin H, Zhao ZS, Lim L. A brain serine/threonine protein kinase activated by Cdc42 and Rac1. Nature 1994; 367:40 - 6; http://dx.doi.org/10.1038/367040a0; PMID: 8107774
- Cau J, Faure S, Comps M, Delsert C, Morin N. A novel p21-activated kinase binds the actin and microtubule networks and induces microtubule stabilization. J Cell Biol 2001; 155:1029 - 42; http://dx.doi.org/10.1083/jcb.200104123; PMID: 11733543
- Clarke PR, Zhang C. Spatial and temporal coordination of mitosis by Ran GTPase. Nature reviews 2008; 9:464-77.
- Daum JR, Potapova TA, Sivakumar S, Daniel JJ, Flynn JN, Rankin S, et al. Cohesion fatigue induces chromatid separation in cells delayed at metaphase. Curr Biol 2011; 21:1018 - 24; http://dx.doi.org/10.1016/j.cub.2011.05.032; PMID: 21658943
- Stevens D, Gassmann R, Oegema K, Desai A. Uncoordinated loss of chromatid cohesion is a common outcome of extended metaphase arrest. PLoS One 2011; 6:e22969; http://dx.doi.org/10.1371/journal.pone.0022969; PMID: 21829677
- Pearson CG, Bloom K. Dynamic microtubules lead the way for spindle positioning. Nat Rev Mol Cell Biol 2004; 5:481 - 92; http://dx.doi.org/10.1038/nrm1402; PMID: 15173827
- Stout JR, Yount AL, Powers JA, Leblanc C, Ems-McClung SC, Walczak CE. Kif18B interacts with EB1 and controls astral microtubule length during mitosis. Mol Biol Cell 2011; 22:3070 - 80; http://dx.doi.org/10.1091/mbc.E11-04-0363; PMID: 21737685
- Samora CP, Mogessie B, Conway L, Ross JL, Straube A, McAinsh AD. MAP4 and CLASP1 operate as a safety mechanism to maintain a stable spindle position in mitosis. Nat Cell Biol 2011; 13:1040 - 50; http://dx.doi.org/10.1038/ncb2297; PMID: 21822276
- Laan L, Pavin N, Husson J, Romet-Lemonne G, van Duijn M, López MP, et al. Cortical dynein controls microtubule dynamics to generate pulling forces that position microtubule asters. Cell 2012; 148:502 - 14; http://dx.doi.org/10.1016/j.cell.2012.01.007; PMID: 22304918
- O’Connell CB, Wang YL. Mammalian spindle orientation and position respond to changes in cell shape in a dynein-dependent fashion. Mol Biol Cell 2000; 11:1765 - 74; PMID: 10793150
- Peyre E, Jaouen F, Saadaoui M, Haren L, Merdes A, Durbec P, et al. A lateral belt of cortical LGN and NuMA guides mitotic spindle movements and planar division in neuroepithelial cells. J Cell Biol 2011; 193:141 - 54; http://dx.doi.org/10.1083/jcb.201101039; PMID: 21444683
- Kiyomitsu T, Cheeseman IM. Chromosome- and spindle-pole-derived signals generate an intrinsic code for spindle position and orientation. Nat Cell Biol 2012; 14:311 - 7; http://dx.doi.org/10.1038/ncb2440; PMID: 22327364
- Woodard GE, Huang NN, Cho H, Miki T, Tall GG, Kehrl JH. Ric-8A and Gi alpha recruit LGN, NuMA, and dynein to the cell cortex to help orient the mitotic spindle. Mol Cell Biol 2010; 30:3519 - 30; http://dx.doi.org/10.1128/MCB.00394-10; PMID: 20479129
- Du Q, Macara IG. Mammalian Pins is a conformational switch that links NuMA to heterotrimeric G proteins. Cell 2004; 119:503 - 16; http://dx.doi.org/10.1016/j.cell.2004.10.028; PMID: 15537540
- Matsumura S, Hamasaki M, Yamamoto T, Ebisuya M, Sato M, Nishida E, et al. ABL1 regulates spindle orientation in adherent cells and mammalian skin. Nat Commun 2012; 3:626; http://dx.doi.org/10.1038/ncomms1634; PMID: 22252550
- Merdes A, Ramyar K, Vechio JD, Cleveland DW. A complex of NuMA and cytoplasmic dynein is essential for mitotic spindle assembly. Cell 1996; 87:447 - 58; http://dx.doi.org/10.1016/S0092-8674(00)81365-3; PMID: 8898198
- Sakai D, Dixon J, Dixon MJ, Trainor PA. Mammalian neurogenesis requires Treacle-Plk1 for precise control of spindle orientation, mitotic progression, and maintenance of neural progenitor cells. PLoS Genet 2012; 8:e1002566; http://dx.doi.org/10.1371/journal.pgen.1002566; PMID: 22479190
- Wee B, Johnston CA, Prehoda KE, Doe CQ. Canoe binds RanGTP to promote Pins(TPR)/Mud-mediated spindle orientation. J Cell Biol 2011; 195:369 - 76; http://dx.doi.org/10.1083/jcb.201102130; PMID: 22024168
- Petronczki M, Léńrt P, Peters JM. Polo on the Rise-from Mitotic Entry to Cytokinesis with Plk1. Dev Cell 2008; 14:646 - 59; http://dx.doi.org/10.1016/j.devcel.2008.04.014; PMID: 18477449
- Seki A, Coppinger JA, Jang CY, Yates JR, Fang G. Bora and the kinase Aurora a cooperatively activate the kinase Plk1 and control mitotic entry. Science 2008; 320:1655 - 8; http://dx.doi.org/10.1126/science.1157425; PMID: 18566290
- Macůrek L, Lindqvist A, Lim D, Lampson MA, Klompmaker R, Freire R, et al. Polo-like kinase-1 is activated by aurora A to promote checkpoint recovery. Nature 2008; 455:119 - 23; http://dx.doi.org/10.1038/nature07185; PMID: 18615013
- Carmena M, Pinson X, Platani M, Salloum Z, Xu Z, Clark A, et al. The chromosomal passenger complex activates Polo kinase at centromeres. PLoS Biol 2012; 10:e1001250; http://dx.doi.org/10.1371/journal.pbio.1001250; PMID: 22291575
- Ji JH, Hwang HI, Lee HJ, Hyun SY, Kang HJ, Jang YJ. Purification and proteomic identification of putative upstream regulators of polo-like kinase-1 from mitotic cell extracts. FEBS Lett 2010; 584:4299 - 305; http://dx.doi.org/10.1016/j.febslet.2010.09.025; PMID: 20869364