Abstract
Mechanical stress plays a pivotal role in developing and maintaining tissues functionalities. Cells are constantly subjected to strain and compressive forces that are sensed by specialized membrane mechanosensors and converted in biochemical signals able to differently influence cellular behavior in terms of surviving, differentiation and extracellular matrix remodeling. This review focuses on the effects of mechanical strain on soft and hard tissues.
Unexpectedly, different cells share almost the same membrane mechanosensors and the relative intracellular pathways, but to ultimately obtain very different biological effects. The events occurring in cardiovascular and bone tissues are treated in details, showing that integrins, cadherins, growth factor receptors and ions channels specifically expressed in the different tissues are the major actors of the sight. However, MAPkinases and RhoGTPases are mainly involved in the biochemical intracellular signaling directed to nuclear modifications.
Bone and Mechanical Stress: Role of Strain on Cell Behavior
Bone is a very complex tissue useful for a variety of functions. In fact, skeleton offers structural support for the rest of the body, permits movement and locomotion by providing levers for the muscles, protects internal organs and structures, provides maintenance of mineral homeostasis and acid-base balance, serves as a reservoir of growth factors and cytokines and provides the environment for hematopoiesis within the marrow spaces. Each bone is constantly subjected to modeling during life to adapt its architecture and function to biomechanical forces, as well as remodeling to replace old and damaged bone with new to maintain bone strength.Citation1 Moreover, bone remodeling provides Ca2+ in case of need, as the mineralized extracellular matrix furnishes as reservoir, and this process is finely modulated by the endocrine system.Citation2 The adaption of bone to mechanical stress is controlled at the cellular level through the coordinated actions of osteoblasts, osteocytes and osteoclasts and these loading forces lead to an increase in bone mass through a positive shift in the balance between bone formation and bone resorption. It is known that bone is formed in areas where loads are elevated and bone is necessary, and resorbed where strains and stresses are absent and bone is excessive.Citation3-Citation5
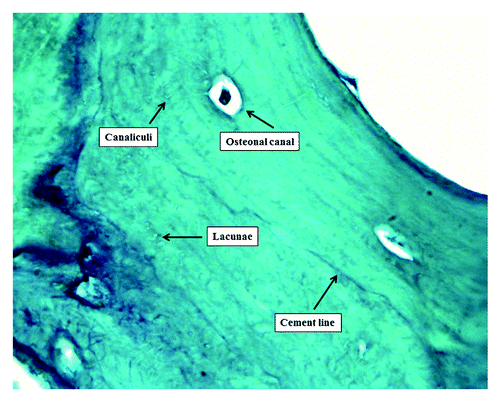
Many experimental data in vitro and in vivo indicate the osteocytes as the main sensor detecting mechanical forces in the bone tissue.Citation6-Citation9 Osteocytes comprise 90–95% of all bone cells in the adult skeleton, and they are located in the mineralized bone matrix within lacunae. Indeed, they have many cytoplasmic processes that stretch out within bone matrix channels called canaliculi. Through their complex communication network, osteocytes are able to mediate the effects of bone loading and respond by sending signals to the bone-forming osteoblasts and the bone-absorbing osteoclasts, thereby guiding the bone remodeling process. ()
The other candidates for the role of the mechanosensory cell in bone tissue are the osteoblasts, the bone lining cells and the osteoclasts, but because of their number, lifetime and function, the only other real candidate is represented by the osteoblast. However, because of their surface location they must have a great sensitivity to sense a small strain characteristic of the bone. The application of force to the skeletal system produces several potential stimuli for osteocyte function, including hydrostatic pressure, fluid-flow-induced shear stress and bone tissue strain.Citation7 The response of bone to mechanical solicitations will depend on magnitude, strain rate, strain distribution, number of loading cycles and frequency.
It has been shown that fluid flow and direct mechanical stimulation have different effects on bone cells. In this review we focused our attention on mechanical strain that is represented by the geometric deformation within the material and is expressed as the ratio between the length change and original length. Different kinds of strain are present in the bone, but we only considered the axial strain. By definition, axial strain comprises both compressive and tensile strain in the same direction as the applied load.Citation10 It is commonly believed that compressive and tensile strains are the most important mechanical stress because they are the main component for most kinds of activities. Osteocytes transduce stress signals from bending or stretching of bone into biologic activity. It has been shown that osteocytes are more sensitive to mechanical stress than osteoblasts.Citation11 Furthermore, osteocytes are more responsive to fluid flow shear stress than to other forms of mechanical strain, such as substrate stretching.Citation12-Citation14 As demonstrated, substrate stretching results in an increase of collagen or bone matrix synthesis. Indeed, bone mass and formation is increased with dynamic loading, while bone resorption is decreased. Although both tissue strain and fluid shear stress cause cell deformation, these stimuli might excite different signaling pathways related to bone growth and remodeling.
Molecular pathway involved in bone responses to mechanical strain
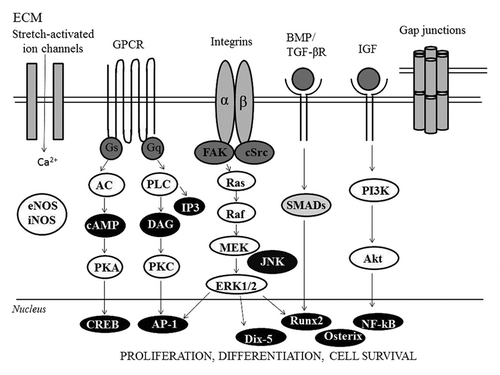
Mechanical strain has been reported to induce bone remodeling activity resulting in structural changes. This type of stimulation can promote the proliferation and anabolism of osteoblasts in order to facilitate bone tissue reconstruction, contributing to the homeostasis of bone tissue. In bones, mechanical stimuli are transmitted through the extracellular matrix (ECM) to osteoblasts, osteocytes, periosteal cells and osteoclasts. Osteoblasts are important mechanical receptors that can transform mechanical stimuli into biochemical signals and secrete bone matrix to promote bone matrix mineralization. Transduction of strain stress occurs at more regions of the membrane, such as intercellular junction (adherens junction) that involved cadherins (protein of adherens junctions), cell-matrix contacts called focal adhesions that involve integrins and stretch-activated channel that could respond directly to membrane perturbation.Citation15 Furthermore, expression of cadherins is increased by mechanical strain.Citation16
Integrins, involved in focal contacts, are membrane spanning proteins that couple the cell to the extracellular environment. Functional integrins are heterogeneous dimers made of α and β subunits. In osteoblast the β1 subunit has the predominant function role, dimerized with a subunits including α1 through α5. The ligands of integrins are varied, including collagen I and III and fibronectin.
Gap junctions are formed from membrane-spanning hexamers known as connexons (each subunits is called connexin) and mediate the interactions between cells. Gap junctions can pass small signaling molecules (< 1 kDa) such as calcium, inositol phosphates and cAMP.Citation7 Mechanical stimulation has been shown to increase expression of connexins in vitro and in vivo, suggesting that cells become better connected with their neighbors in presence of a dynamically stimulated enviroment.Citation17
Moreover, alteration in ion channel activity in osteoblasts have been associated with bone cell activation trough stretch/strain stress.Citation18 Cyclical strain has been shown to modulate the activity of certain channels—chronically strained osteoblasts had significantly larger increases in whole cell conductance when subjected to additional mechanical strain than unstrained controls.Citation19 In addition to direct activation of intracellular signaling cascades, influx of a charged species such as calcium can also alter membrane potential and activate voltage sensitive channels that are not directly mechanosensitive.Citation20 Thus, known intracellular signal transduction pathways, such as the intracellular Ca2+, Ins(1,4,5)P3 or cAMP dependent pathways, shown to play a role in other mechanosensitive cells, are involved.
More in details, prostaglandins (PGs) and nitric oxide (NO) involved in responses of bone tissue to stress, seem to be interesting candidates for intercellular communication within the three dimensional network of bone cells as they are rapidly released by mechanical stressed bone cells.Citation21
In osteocytes the mechanically induced synthesis and release of PGE2, are transmitted via the cytoskeleton which is physically linked to ions channels, as well as protein kinase C (PKC) and phospholipase A2. PGE2 mediates the gap junction intercellular communication in response to mechanical strain by increasing the number of functional gap junctions and the amount of connexin43 protein (major component of gap-junction).Citation22 Thus, the effect is mediated through EP2 receptors activation of cAMP dependent protein kinase A (PKA). PGE2 release due to mechanical stimulation has been reported to require a competent cytoskeleton and is increased with formation of focal adhesion and subsequent ERK and PKA signaling pathway in osteoblasts. PGE2 has been shown to have a regulatory effect on RANKL expression and therefore on osteoclastogenesis. Physiological levels of mechanical stress decrease RANKL expression and increase eNOS expression, which is mediated through ERK1/2.Citation21 Although the strain also activates c-jun kinase (JNK), JNK inhibitor does not prevent strain effect on either RANKL or eNOS. Reduced display of RANKL by cells present in bone downregulates the local osteoclastogenic potential. As a result of increased eNOS expression, nitric oxide (NO) is enhanced.Citation20
Finally, nitric oxide is a highly reactive, easily dissolved gas released by osteocytes and osteoblasts. Nitric oxide is produced by one of three isoforms of nitric oxide synthase (NOs): nNOS, eNOS and iNOS; all three isoforms are found in bone cells, but iNOS expression has also been shown in osteoblasts but not osteoclasts. Several studies have shown that NO production rapidly increases in response to mechanical stress in bone cells. In vitro, the release of NO from bone cells in response to mechanical stimulation has been involved in MAPK signaling, cytoskeletal adaptation and PGE2 signaling.Citation23
Anyway, PGs and NO are not the only targets involved by a mechanical strain. Mechanical stimulation leads also to the upregulation of growth factors, like IGF1 (insulin-like growth factor) and IGF2, VEGF (vascular endothelial growth factor), TGFβ, BMP2 and BMP4, which act via autocrine and paracrine mechanisms through their tyrosine and serine/threonine kinase receptors. These growth factors activate PI3K/AKT, MAPK and SMAD signal transduction pathways. Phospholipase C is also activated by GPCRs, resulting in synthesis of IP3 and DAG, the latter may stimulate PKC.
In particular, the BMP2-induced signaling pathway leads to the expression of three osteogenic master transcription factors: Osterix, Runx2 and Dix5.
In particular, Runx2, a Runt domain transcription factor family member, is a master regulator of osteoblast differentiation that binds to osteoblast-specific cis-acting element 2 (OSE2) sites, found in the promoter regions of all major osteoblast-specific genes, including osteocalcin (OCN), osteopontin, collagen type 1, bone sialoproteins and alkaline phosphatase.Citation24 Runx2 forms a heterodimeric complex with its partner cbf-b and binds to DNA directly, while cbf-b modulates the affinity of the complex for DNA.Citation25
The proteins and mRNAs levels, as well as the DNA-binding activity of Runx2, are increased after a low level mechanical stretching of cultured human osteoblastic cells. Runx2 activation may occur via several routes: through the interaction between ECM and cell surface integrins, through BMP, via MAPK, FGF2 and PTH signaling pathway and as a result of ATP modulation of intracellular Ca2+ mobilization by multiple p2 receptor. Specifically, the common target of MAPK induced by mechanical stress during osteoblast differentiation and function is believed to be the ERK pathway, which is mediated by ATP- dependent Ca2+ influx at calcium channel via PKC, Src family kinase, PKA, FAK and PYK2 (proline-rich tyrosine kinase 2).
Osterix (OSX) has been identified as a key transcription factor in osteoblast differentiation, which appears to be activated via Runx2-independent pathway. Both of these transcription factor regulators, Runx2 and Osterix seem to be induced early in the cellular response in mechanical strained osteoblastic cells.
Osteocytes are indeed able to respond to strain in vivo as shown by increased G6PD activity.Citation26 Kanno et al. shown that uniaxial sinusoidal stretching initiated the differentiation of preosteoblastic cells into osteogenic cells by inducing Runx2 expression in vitroCitation24 ().
Morpho-Functional Characteristics of Blood Vessels
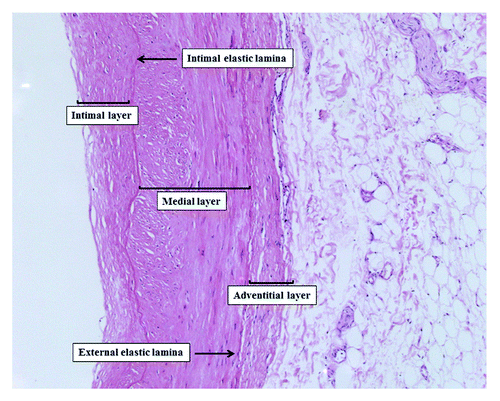
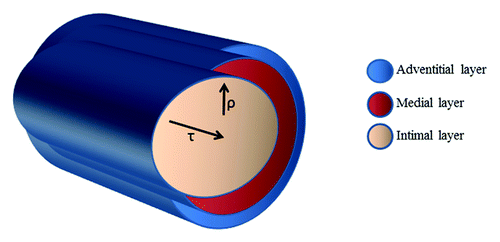
Blood vessels are constituted by a three-layered structure: an intimal lining constituted by an endothelial cell monolayer, surrounded by a medial layer containing extracellular matrix (ECM) and smooth muscle cells and an outer adventitial layer consisting of connective tissue with fibroblasts. The endothelial cells (ECs) monolayer not only provides a selective barrier for macromolecular permeability between the blood and the vessel wall but also serves a number of important homeostatic functions. Indeed, ECs modulate different biological processes like vascular remodeling (growth factors), hemostasis and thrombosis (prothrombotic and antithrombotic factors), inflammatory responses (adhesion of leukocytes) and contraction of vascular smooth muscle cells (VSMCs) through the release of vasoactive substances (dilators and vasoconstrictors).Citation27 Instead, VSMC are highly specialized cells whose principal function is the regulation of blood vessel tone, blood pressure and blood flow distributionCitation28-Citation30 ().
Blood vessels are constantly subjected to various types of hemodynamic forces, including hydrostatic pressure, cyclic stretch, and fluid shear stress. Shear stress (τ) is parallel to the vessel wall and represents the frictional force that blood flow exerts mainly on the endothelial surface of the vessel wall. Instead, cyclic stretch (ρ) is the stress perpendicular to the vessel wall and represents the circumferential deformation of the blood vessel wall during distension and relaxation of the recurring cardiac cycle ().
Figure 4. Mechanical and hemodynamic forces associated with blood flow: Shear stress (τ) is parallel to the vessel wall and represents the frictional force that blood flow exerts mainly on the endothelial surface of the vessel wall. Instead, cyclic stretch (ρ) is the stress perpendicular to the vessel wall and represents the circumferential deformation of the blood vessel wall during distension and relaxation of the recurring cardiac cycle.
Mechanical or hemodynamic forces associated with blood flow play a central role in the homeostasis of the circulatory system. These forces act on the vascular wall, contributing to the regulation of myogenic tone, responses to vasoactive molecules, gene regulation, vascular permeability and remodeling. Disruption of normal hemodynamic loading can be responsible of the development of vascular diseases, including hypertension, thrombosis, aneurysms and atherosclerosis. While physiological cyclic stretch induces cell cycle arrest in VSMC, chronically increased blood pressure and vascular transmural stress activate vascular cell proliferation (eventually leading to luminal stenosis), collagen and fibronectin synthesis which results in thickening of the vascular wall as a feature of hypertension-induced vascular remodeling,Citation31,Citation32 finally altering arteries compliance. Although vascular endothelial cells and vascular smooth muscle cells are exposed to both types of mechanical forces, the shear stress resulting from blood flow is sensed mainly by ECs, whereas SMCs are primarily subjected to cyclic stretch resulting from pulsatile pressure. Vascular cells are equipped with numerous receptors that allow them to sense stimuli such as strain, pressure and fluid shear stress and transduce these mechanical signals into a biological response through a process named mechanotransduction. The cytoskeleton and other structural components have an established role in mechanotransduction, being able to transmit and modulate tension within the cell via focal adhesion sites, integrins, cellular junctions and the extracellular matrix. Measurements of circumferential cyclic strain in vivo average 2% in the human thoracic aorta at a frequency close to 1 Hz with increases upwards of 30% strain on arterial walls in hypertension.Citation33 The effects of cyclic strain on cell mechanobiology depend on rate, duration and species. Cyclic strain mediates the ability of ECs to remodel their ECM, proliferate, change shape, and signal to SMCs. Cyclic strain induce also ECs proliferation, morphological alignment and change in gene expression with consequences for cell phenotype and vessel wall homeostasis. In the second part of this section, more details of the molecular mechanisms involved will be discussed.
Molecular mechanisms involved in vascular responses to mechanical strain
Although blood flow imposes shear stress on the endothelial cells, cardiac pulsation generates circumferential stretch and impose mechanical stimulation on both endothelial and SMC. Mechanical stimuli need to be converted in biochemical signal in order to activate transcription factors and finally modulate gene expression. In fact, mechanical stretch on vascular cells has significant effects on the expression of genes related to vascular remodeling and cell functions such as cell proliferation, apoptosis, migration and control of cell phenotype. For instance, phenotypic responses of vascular cell exposed to cyclic stretch in vitro include increased expression of contractile and cytoskeletal proteins (myosin light chain kinase, smooth muscle myosin heavy chains and desmin).Citation34 A number of bioactive proteins regulated by cyclic strain have been also identified in endothelial cells and they include IL-8, TGFβ, FGF2 and VEGF.Citation35,Citation36
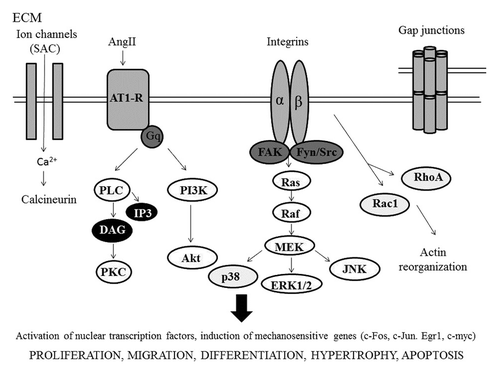
The mechanochemical transduction involves the activation at the cell membrane surface of specific mechanosensors and the related intracellular signaling pathways.Citation37 Thus, an intact cytoskeleton is important for the stretch-induced adjustments in vascular cells.Citation38 SMCs express many different receptors and ion channels, which can be activated by mechanical stress leading to the activation of multiple classic signaling pathways, such as G protein, kinases, calcium, cAMP, nitric oxide (NO), eNOS, MAPK and MKP-1. Moreover, mechanical stresses appeared simultaneously to initiate multiple signaling pathways.
In particular, integrins form a signaling interface between the extracellular matrix (ECM) and the cell. These proteins participate not only to cell attachment to the substrate but also to intracellular transmission of mechanical signal. Mechanical stresses stimulate conformational activation of cell integrins and increase cell binding to extracellular matrix. The dynamic formation of new ligand-integrin connections is required for stretch induced mechanotransduction. During the stimulation of vascular cells by mechanical factors such stretch, several signaling events are associated with the formation of focal adhesion, which comprise integrin cluster and cytoskeletal protein. The proteins present at focal adhesion become phosphorylated on tyrosine when the cells are stimulated, a FAK activation is an indicator in focal adhesion formation.Citation39,Citation40 Up cell attachment to ECM proteins, integrins became activated and form cluster at the cell surface thus initiating the formation of adhesion complexes which will enhance FAK’s catalytic activity and increase FAK tyrosine phosphorylation. FAK phosphorylation at tyrosine 397 (Y397) leads to the recruitment of Src and Src-family kinases as well as to an increased phosphorylation of other proteins present in the adhesion complex such as paxilin and p130Cos.Citation41,Citation42 Subsequent phosphorylation of specific tyrosine residues leads to the recruitment of additional SH2-domain-containing signaling proteins such as PI3K and Grb2.Citation43 Among all integrin receptors, integrin α5β3 has been identified as having particularly interesting expression pattern among the vascular cells during angiogenesis and vascular remodeling.Citation44,Citation45 In particular, integrin α5β3 binds numerous ECM ligands with exposed RGD peptide as fibronectin, vitronectin, fibrinogen and is involved in responses to mechanical strain. In the ‘90s Wilson and colleagues established a link between the mechanical stimuli and the action of growth factors. Their studies demonstrated that cyclic stretch increased VSMC proliferation when they are plated on collagen, fibronectin or vitronectin, but not on laminin or elastin; further, these effects required the production of PDGF suggesting a tight interaction between integrins and growth factors downstream signaling pathways related to mechanical stimuli.Citation46 Generally, growth factors receptors belong to two classes of membrane proteins: tyrosine kinase receptors and G-coupled receptors and G proteins.
There is still another membrane structure which is involved in mechanosensing: ion channels. VSMCs stimulated by mechanical stress result in a transient increase in intracellular calcium and divalent cations and depolarization which maintain smooth muscle tension. Opening these channels causes Ca2+ and Na+ influx and membrane depolarization, which contributes to the myogenic response to mechanical stretch.Citation47 The altered stretch-activated channels in arterial SMCs may contribute to the enhanced myogenetic responses as well as the generation of hypertrophy and remodeling of arterial tissue in hypertension.
All the cited membrane mechanosensors have a common intracellular pathway often converging to the MAP kinase signaling. The MAP kinase comprises a ubiquitous family of threonine/tyrosine kinases, and includes extracellular signal-regulated kinases (ERK), stress activated protein kinases (SAPK) or c-Jun NH2-terminal kinases (JNK) and p38/ERK kinases.Citation48-Citation50 This cascade is an important pathway whereby signal originating from mechanical forces can lead to gene expression and protein synthesis. More in details, this pathway implicates the sequential phosphorylation and activation of the cytoplasmic protein kinases MEKK, MEK and finally MAP kinase. The MAP kinase cascade comprises three different pathways. Phosphorylation of a MAP kinase, which lies downstream of the MEKK Raf and is present in two isoforms termed ERK 1 and 2, leads to the activation of regulatory proteins both in the cytoplasm and the nucleus. A second branch of the MAP kinase family, termed stress-activated protein kinases (SAPK) because they are activated by UV light, heat shock, hypoxia or high osmolarity, includes kinases that phosphorylate the N-terminal of transcription factor c-jun (JNK). Finally, a third branch comprises p38, also activated by osmotic stress.Citation51
Related to mechanical strain, a cyclic stimulus activates both ERK1/2 and JNK in VSMC.Citation52 Moreover, it induces on vascular smooth muscle cells (VSMC) alignment and differentiation.Citation53,Citation54 The signaling pathway involved in cells alignment includes p38, MAPK (mitogen-activated protein kinase), NO (nitric oxide) and ROS (reactive oxygen species).Citation54-Citation56 and the mechano-sensor mainly involved is integrin β1.Citation55 Moreover, cyclic strain increases both smooth muscle α-actin protein expression and promoter activity. The induction of smooth muscle α-actin is mediate by activation of JNK and p38 MAPK pathway.Citation57 Thus mechanical strain leads to migration, proliferation and contraction. The primary genes encoding SMC contractile proteins are regulated by stretch-induced RhoA pathway and associated transcription factors.Citation58 Moreover, mechanical stress regulates VSMC migration trough ERK1/2 and MLCK (myosin light-chain kinase).Citation59 Also p38, MAPK and TGFβ1 are involved in migration of VSMC after mechanical stress. In fact, inhibition of p38 MAPK and TGFβ1 activity decreases migration activity.Citation60
The effects of mechanical strain on cell survival and proliferation are mainly related to α5β3 integrin expression, stabilization of PINCH1, a survival protein that is linked with integrine and the cytoskeleton.Citation61 siRNA (small interfering RNA) against intregrin β3, abolished the anti-apoptotic effect of mechanical stress. Also downregulation of Rho inhibits the proliferation of VSMC induced by stretch.Citation58 While, the proliferation of VSMCs induced by mechanical stretch is mediate by the activation of IGF-1 (insulin growth factors 1) and IGFR-1 (receptor). Anyway, mechanical stress induces apoptosis in differentiated VSMC, but not in proliferating VSMCs and the stretch-induced apoptosis in VSMC is associated with BAD expression. VEGF and overexpression of the anti-apoptotic protein bcl2 decreases the expression of BAD and apoptosis induced in response to stretch.Citation38
Finally stress strain also mediates vascular remodeling. The signaling involved in response to mechanical stretch includes ROS, NO, NFκB, EGFR, MAPK and PKC. Mechanical force can be transduced via ROS-dependent autocrine and paracrine EGFR activation, and may regulate VSMC proliferation and synthetic activity through the NFκB pathway.Citation62
Anyway, using cells from different species and modifications in type (cyclic or steady), intensity and duration of stretch may cause the different biological effects in vitro. For instance, cyclic but not steady mechanical strain activates vascular FAK, and Src and integrins are involved in steady pressure-induced FAK activation in the vessels.Citation42
Moreover, low (5%) or high (18%) magnitude cyclic stretch selectively involve small GTP-ases (Rac and Rho respectively) with direct consequence on actin polymerization and stress fibers formationCitation63 ().
The Heart
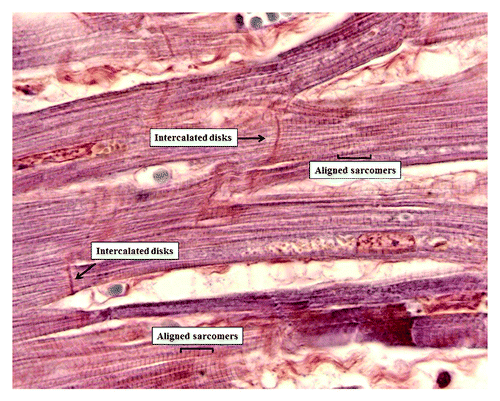
Cardiac tissue is exposed to dynamic mechanical stresses from the early development for all lifetime. Mechanical forces are transduced into biochemical and electrical responses through the mechanotransduction process. In the heart, this process is mediated by the cardiomyocytes, originating from cardiac progenitors, which represent the fundamental mechanical work unit of the heart. In normal myocardium, cardiac myocytes are cylindrical structures elongated and aligned along a longitudinal cellular axis, with the aim to facilitate a fast propagation of the electrical impulses and the uniaxial alignment of sarcomeres, both of which contribute to a contraction at regular intervals. Heart chamber filling and wall distension within diastole account for rapid changes in pressure and volume that are released by the wave of contraction that pumps blood through the body. At the cellular level, these pulsatile stimuli are experienced as cyclic strains (relative deformation) and stresses (force per unit area). In the heart, myocytes are subjected to strain in diastole before contraction (preload) and actively contract against imposed load in systole (afterload). Cardiac myocytes are primarily subjected to cyclic stretch due to pressure and volume overload.Citation38 It has been shown that cardiomyocytes respond to mechanical forces promoting cardiac hypertrophy and development, adaptative remodeling and changes in gene expression.Citation64,Citation65 In fact, stretch of cardiomyocytes cause transcriptional activation of gene like atrial natriuretic factor (ANF), skeletal α-actin and β-myosin heavy chain (MHC).65Mechanical stimuli can also affected cytoskeletal and sarcomeric organization to regulate cell shape and alignment changes in contractile performances.Citation66,Citation67 Dysregulation of mechanical signaling can cause cardiac remodeling, characterized by cardiomyocytes loss, interstitial fibrosis and collagen deposition, leading to heart failure, myocardial infarction and cardiomyopathies.Citation68 In both normal and pathological conditions, the cardiomyocytes cytoskeleton play key role in sensing mechanical stress and mediating structural remodeling and functional responses within the myocytes. Mechanical stimuli are sensed by protein complexes associated with the sarcomeres in order that actin filaments slide along the adjacent myosin filaments promoting the cardiac contractile forces. Let’s go further in the molecular mechanisms involved in cardiac tissue mechanostransduction ().
Figure 6. Heart microanatomy showing the intercalated disks (Phosphotungstic acid hematoxylin staining 1,200× oil).
Cardiac tissue mechanotransduction
Mechanical stresses can deform the ECM and alter integrin structure leading to activation of second messenger pathway. Several mechanoreceptors are involved in mechanical stress. In particular the interaction between cardiomyocytes and ECM involved mainly integrins. The cardiac ECM is a dynamic entity, it is composed of basement membrane adhesion proteins such as fibronectin, collagen type IV, laminin and proteoglycans.Citation69 Integrins can be a mechanosensor that transmits mechanical signal to the cytoskeleton. The cardiomyocytes express a variety of α subunits; however, they primarily express β1-integrins. Integrins connect to actin through linking proteins such as talin, vinculin and α-actinin, but also with the N-terminal domain of focal adhesion tyrosine kinase (FAK), are also associated with signaling molecules such as small GTP-binding proteins Rho, Rac and Cdc42.Citation70 Vinculin is a crucial downstream effectors of myosin VI in E-cadherin-mediated adhesions. Vinculin plays a crucial role in cardiomyocytes mechanotransduction and is found at costameres as well as in the intercalated disk.Citation71 However several proteins interact with FAK, such as Src, Fyn, p130cas, Graf, Grb2 and PI-3-kinase. These signaling molecules further activate various downstream protein kinase cascades, including p21ras, mitogen-activated protein (MAP) kinase, protein kinase C (PKC) and p70 s6k. Intercellular communication is via the intercalated disk. Intercalated disc is made largely of desmosomes, gap junctions and adherens junctions. Desmosomes are particularly abundant in tissues such myocardium. Desmosomal adhesion molecules, desmocollin and desmoglen are members of cadherins family of calcium-dependent adhesion molecules.Citation72 Gap junctions connect the cytoplasm of neighboring cells allowing the passage of small molecules (1,000 kDa) and electrical current, in fact connexins form diffusion pores. Each gap junction is formed by two connexons, each of which is made of six identical or different proteins called connexins. Myocardial gap junction are composed of three connexin isotopes: connexin 40 (Cx40), connexin 43 (Cx43) and connexin 45 (Cx45), the most abundant isoform (43 is molecular weight in kDa) is upregulated after mechanical stress in cultured rat neonatal cardiomyocytes.Citation73 This increase in Cx43 expression corresponded to an increase in conduction velocity, and appears to be mediated by vascular endothelial growth factor (VEGF). AngII, VEGF and TGF-β may mediate the stretch-induced Cx expression. Gap junctions via actin are connected to integrins. Adherens junctions (fascia adherens junction) are composed mainly of N-cadherins, homophilic Ca2+-dependent cell-cell adhesion molecules. Cadherins activate the pathway of α-catenins and β-catenins.Citation74,Citation75 These mechanotransductions play an important role in maintaining the structural and mechanical organization of cardiac tissue during and after development.Citation76 The sensing of mechanical stress is realized via stretch activated ion channels (SAC). Typically stress-activated channels (SAC) allow the passage of cation K+, Na2+ and Ca2+.Citation76 Another mechanical sensor is titin (called connectin). Titin, an intracellular protein necessary for sarcomeric structure, contributes significantly to the mechanical properties of the sarcomere. Titin runs from the Z-disk to the M-band where bounds myosin, is involved in response to stress.
Downstream to the mechanosensors activation, several studies show that MAP kinase constitute major stress-activated signal pathway in cardiomyocytes.Citation77 Mechanical stress of neonatal cardiac myocytes has been shown to activated ERK1 and ERK2 and their downstream protein kinase pp90 rsk, also activates the upstream regulators of ERK including MAP kinase kinase (MEK1) and MAP kinase (Raf1).Citation78 Also c-Jun N-terminal kinases (JNK) can be activated by mechanical stress.Citation79
Mechanical stress causes activation of phospholipases, for example activation of phospholipase C (PLC). Phospholipases generate lipid-derived second messagers such as inositol-1,4,5-trisphospate (IP3) and diacylglycerol (DAG). DAG, a soluble molecule, causes Ca2+ release that activates protein kinase C (PKC).
Ultimately, mechanical stress activates intra-nuclear factors such as the serum response factors (SRE)-p&2 TCF complex via SRE, causing induction of c-Fos. SRF is a transcription factor that regulates many adhesion-related genes, and is regulated by a number of independent pathways. The first group of genes activated by mechanical stretch is immediate early genes such as c-Fos, c-Jun, Egr1 and c-myc.Citation80 Pathological levels of mechanical stress activate nuclear transcription factors like NFκB mediated by VEGF.Citation81
Furthermore, mechanical stress induces autocrine or paracrine secretion of growth factors. Presence of growth factors is one of the important mechanisms in the pathogenesis of stretch-induced organ hypertrophy.Citation82 After mechanical stress, cardiac myocytes synthesize growth factor like angiotensin II, endothelin 1 and basic FGF (fibroblast growth factor). Mechanical stress at physiological level does not induce damage of cardiac myocyte, but levels of pathological mechanical stress can induce apoptosis. It’s what happens in certain diseases such as hypertrophic cardiomyopathy. Altered mechanical stress associated with myocardial infarction leads the constitutively production of cytokine such as tumor necrosis factor α (TNF α), interleukin 1 (IL1) and IL6 via the MAPK and JAK-STAT pathways. These pathways activate nuclear transcription factor like NFκB and AP-1 that encode for TNF α and IL6.Citation83 These levels of cytokines increase metalloprotease MMP activity such as MMP2 and MMP9 in local matrix. Moreover, pathological levels of mechanical stress induce ROS production stretch-amplitude-dependent.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- Clarke B. Normal bone anatomy and physiology. Clin J Am Soc Nephrol 2008; 3:Suppl 3 S131 - 9; http://dx.doi.org/10.2215/CJN.04151206; PMID: 18988698
- Confavreux CB. Bone: from a reservoir of minerals to a regulator of energy metabolism. Kidney Int Suppl 2011; 121:S14 - 9; http://dx.doi.org/10.1038/ki.2011.25; PMID: 21346725
- Henriksen K, Neutzsky-Wulff AV, Bonewald LF, Karsdal MA. Local communication on and within bone controls bone remodeling. Bone 2009; 44:1026 - 33; http://dx.doi.org/10.1016/j.bone.2009.03.671; PMID: 19345750
- Skerry TM. The response of bone to mechanical loading and disuse: fundamental principles and influences on osteoblast/osteocyte homeostasis. Arch Biochem Biophys 2008; 473:117 - 23; http://dx.doi.org/10.1016/j.abb.2008.02.028; PMID: 18334226
- Turner CH, Warden SJ, Bellido T, Plotkin LI, Kumar N, Jasiuk I, et al. Mechanobiology of the skeleton. Sci Signal 2009; 2:pt3; http://dx.doi.org/10.1126/scisignal.268pt3; PMID: 19401590
- Aarden EM, Nijweide PJ, van der Plas A, Alblas MJ, Mackie EJ, Horton MA, et al. Adhesive properties of isolated chick osteocytes in vitro. Bone 1996; 18:305 - 13; http://dx.doi.org/10.1016/8756-3282(96)00010-5; PMID: 8726386
- Bonewald LF. Mechanosensation and transduction in osteocytes. Bonekey Osteovision 2006; 3:7 - 15; http://dx.doi.org/10.1138/20060233; PMID: 17415409
- Burger EH, Klein-Nulend J, van der Plas A, Nijweide PJ. Function of osteocytes in bone--their role in mechanotransduction. J Nutr 1995; 125:Suppl 2020S - 3S; PMID: 7602386
- Burger EH, Klein-Nulend J. Mechanotransduction in bone--role of the lacuno-canalicular network. FASEB J 1999; 13:Suppl S101 - 12; PMID: 10352151
- Yang PF, Brüggemann GP, Rittweger J. What do we currently know from in vivo bone strain measurements in humans?. J Musculoskelet Neuronal Interact 2011; 11:8 - 20; PMID: 21364270
- Mikuni-Takagaki Y, Suzuki Y, Kawase T, Saito S. Distinct responses of different populations of bone cells to mechanical stress. Endocrinology 1996; 137:2028 - 35; http://dx.doi.org/10.1210/en.137.5.2028; PMID: 8612544
- Cowin SC. Mechanosensation and fluid transport in living bone. J Musculoskelet Neuronal Interact 2002; 2:256 - 60; PMID: 15758447
- Klein-Nulend J, van der Plas A, Semeins CM, Ajubi NE, Frangos JA, Nijweide PJ, et al. Sensitivity of osteocytes to biomechanical stress in vitro. FASEB J 1995; 9:441 - 5; PMID: 7896017
- Westbroek I, Ajubi NE, Alblas MJ, Semeins CM, Klein-Nulend J, Burger EH, et al. Differential stimulation of prostaglandin G/H synthase-2 in osteocytes and other osteogenic cells by pulsating fluid flow. Biochem Biophys Res Commun 2000; 268:414 - 9; http://dx.doi.org/10.1006/bbrc.2000.2154; PMID: 10679219
- Guharay F, Sachs F. Stretch-activated single ion channel currents in tissue-cultured embryonic chick skeletal muscle. J Physiol 1984; 352:685 - 701; PMID: 6086918
- Thompson WR, Rubin CT, Rubin J. Mechanical regulation of signaling pathways in bone. Gene 2012; 503:179 - 93; http://dx.doi.org/10.1016/j.gene.2012.04.076; PMID: 22575727
- Alford AI, Jacobs CR, Donahue HJ. Oscillating fluid flow regulates gap junction communication in osteocytic MLO-Y4 cells by an ERK1/2 MAP kinase-dependent mechanism small star, filled. Bone 2003; 33:64 - 70; http://dx.doi.org/10.1016/S8756-3282(03)00167-4; PMID: 12919700
- Duncan RL, Hruska KA, Misler S. Parathyroid hormone activation of stretch-activated cation channels in osteosarcoma cells (UMR-106.01). FEBS Lett 1992; 307:219 - 23; http://dx.doi.org/10.1016/0014-5793(92)80771-8; PMID: 1379539
- Duncan RL, Hruska KA. Chronic, intermittent loading alters mechanosensitive channel characteristics in osteoblast-like cells. Am J Physiol 1994; 267:F909 - 16; PMID: 7528987
- Rubin J, Rubin C, Jacobs CR. Molecular pathways mediating mechanical signaling in bone. Gene 2006; 367:1 - 16; http://dx.doi.org/10.1016/j.gene.2005.10.028; PMID: 16361069
- Liedert A, Kaspar D, Blakytny R, Claes L, Ignatius A. Signal transduction pathways involved in mechanotransduction in bone cells. Biochem Biophys Res Commun 2006; 349:1 - 5; http://dx.doi.org/10.1016/j.bbrc.2006.07.214; PMID: 16930556
- Jiang JX, Gu S. Gap junction- and hemichannel-independent actions of connexins. Biochim Biophys Acta 2005; 1711:208 - 14; http://dx.doi.org/10.1016/j.bbamem.2004.10.001; PMID: 15955305
- Castillo AB, Jacobs CR. Mesenchymal stem cell mechanobiology. Curr Osteoporos Rep 2010; 8:98 - 104; http://dx.doi.org/10.1007/s11914-010-0015-2; PMID: 20425617
- Kanno T, Takahashi T, Tsujisawa T, Ariyoshi W, Nishihara T. Mechanical stress-mediated Runx2 activation is dependent on Ras/ERK1/2 MAPK signaling in osteoblasts. J Cell Biochem 2007; 101:1266 - 77; http://dx.doi.org/10.1002/jcb.21249; PMID: 17265428
- Lian JB, Javed A, Zaidi SK, Lengner C, Montecino M, van Wijnen AJ, et al. Regulatory controls for osteoblast growth and differentiation: role of Runx/Cbfa/AML factors. Crit Rev Eukaryot Gene Expr 2004; 14:1 - 41; http://dx.doi.org/10.1615/CritRevEukaryotGeneExpr.v14.i12.10; PMID: 15104525
- Skerry TM, Bitensky L, Chayen J, Lanyon LE. Early strain-related changes in enzyme activity in osteocytes following bone loading in vivo. J Bone Miner Res 1989; 4:783 - 8; http://dx.doi.org/10.1002/jbmr.5650040519; PMID: 2816520
- Rubanyi GM. The role of endothelium in cardiovascular homeostasis and diseases. J Cardiovasc Pharmacol 1993; 22:Suppl 4 S1 - 14; http://dx.doi.org/10.1097/00005344-199322004-00002; PMID: 7523767
- Albinsson S, Hellstrand P. Integration of signal pathways for stretch-dependent growth and differentiation in vascular smooth muscle. Am J Physiol Cell Physiol 2007; 293:C772 - 82; http://dx.doi.org/10.1152/ajpcell.00622.2006; PMID: 17507430
- Hayashi K, Takahashi M, Nishida W, Yoshida K, Ohkawa Y, Kitabatake A, et al. Phenotypic modulation of vascular smooth muscle cells induced by unsaturated lysophosphatidic acids. Circ Res 2001; 89:251 - 8; http://dx.doi.org/10.1161/hh1501.094265; PMID: 11485975
- Owens GK, Kumar MS, Wamhoff BR. Molecular regulation of vascular smooth muscle cell differentiation in development and disease. Physiol Rev 2004; 84:767 - 801; http://dx.doi.org/10.1152/physrev.00041.2003; PMID: 15269336
- Hipper A, Isenberg G. Cyclic mechanical strain decreases the DNA synthesis of vascular smooth muscle cells. Pflugers Arch 2000; 440:19 - 27; PMID: 10863993
- O’Callaghan CJ, Williams B. Mechanical strain-induced extracellular matrix production by human vascular smooth muscle cells: role of TGF-beta(1). Hypertension 2000; 36:319 - 24; http://dx.doi.org/10.1161/01.HYP.36.3.319; PMID: 10988258
- Lee T, Sumpio BE. Cell signalling in vascular cells exposed to cyclic strain: the emerging role of protein phosphatases. Biotechnol Appl Biochem 2004; 39:129 - 39; http://dx.doi.org/10.1042/BA20030104; PMID: 15032733
- Birukov KG, Shirinsky VP, Stepanova OV, Tkachuk VA, Hahn AW, Resink TJ, et al. Stretch affects phenotype and proliferation of vascular smooth muscle cells. Mol Cell Biochem 1995; 144:131 - 9; http://dx.doi.org/10.1007/BF00944392; PMID: 7623784
- Cheng GC, Briggs WH, Gerson DS, Libby P, Grodzinsky AJ, Gray ML, et al. Mechanical strain tightly controls fibroblast growth factor-2 release from cultured human vascular smooth muscle cells. Circ Res 1997; 80:28 - 36; http://dx.doi.org/10.1161/01.RES.80.1.28; PMID: 8978319
- Zheng W, Seftor EA, Meininger CJ, Hendrix MJ, Tomanek RJ. Mechanisms of coronary angiogenesis in response to stretch: role of VEGF and TGF-beta. Am J Physiol Heart Circ Physiol 2001; 280:H909 - 17; PMID: 11158993
- Chien S, Li S, Shyy YJ. Effects of mechanical forces on signal transduction and gene expression in endothelial cells. Hypertension 1998; 31:162 - 9; http://dx.doi.org/10.1161/01.HYP.31.1.162; PMID: 9453297
- Shyu KG. Cellular and molecular effects of mechanical stretch on vascular cells and cardiac myocytes. Clin Sci (Lond) 2009; 116:377 - 89; http://dx.doi.org/10.1042/CS20080163; PMID: 19175356
- Boccafoschi F, Mosca C, Bosetti M, Cannas M. The role of mechanical stretching in the activation and localization of adhesion proteins and related intracellular molecules. J Cell Biochem 2011; 112:1403 - 9; http://dx.doi.org/10.1002/jcb.23056; PMID: 21321993
- Lehoux S, Castier Y, Tedgui A. Molecular mechanisms of the vascular responses to haemodynamic forces. J Intern Med 2006; 259:381 - 92; http://dx.doi.org/10.1111/j.1365-2796.2006.01624.x; PMID: 16594906
- Arias-Salgado EG, Lizano S, Sarkar S, Brugge JS, Ginsberg MH, Shattil SJ. Src kinase activation by direct interaction with the integrin beta cytoplasmic domain. Proc Natl Acad Sci U S A 2003; 100:13298 - 302; http://dx.doi.org/10.1073/pnas.2336149100; PMID: 14593208
- Lehoux S, Esposito B, Merval R, Tedgui A. Differential regulation of vascular focal adhesion kinase by steady stretch and pulsatility. Circulation 2005; 111:643 - 9; http://dx.doi.org/10.1161/01.CIR.0000154548.16191.2F; PMID: 15668343
- Lehoux S, Tedgui A. Cellular mechanics and gene expression in blood vessels. J Biomech 2003; 36:631 - 43; http://dx.doi.org/10.1016/S0021-9290(02)00441-4; PMID: 12694993
- Aplin AE, Howe A, Alahari SK, Juliano RL. Signal transduction and signal modulation by cell adhesion receptors: the role of integrins, cadherins, immunoglobulin-cell adhesion molecules, and selectins. Pharmacol Rev 1998; 50:197 - 263; PMID: 9647866
- Eliceiri BP, Cheresh DA. The role of alphav integrins during angiogenesis: insights into potential mechanisms of action and clinical development. J Clin Invest 1999; 103:1227 - 30; http://dx.doi.org/10.1172/JCI6869; PMID: 10225964
- Wilson E, Sudhir K, Ives HE. Mechanical strain of rat vascular smooth muscle cells is sensed by specific extracellular matrix/integrin interactions. J Clin Invest 1995; 96:2364 - 72; http://dx.doi.org/10.1172/JCI118293; PMID: 7593624
- Marrero MB, Paxton WG, Schieffer B, Ling BN, Bernstein KE. Angiotensin II signalling events mediated by tyrosine phosphorylation. Cell Signal 1996; 8:21 - 6; http://dx.doi.org/10.1016/0898-6568(95)02016-0; PMID: 8777137
- Davis RJ. The mitogen-activated protein kinase signal transduction pathway. J Biol Chem 1993; 268:14553 - 6; PMID: 8325833
- Herlaar E, Brown Z. p38 MAPK signalling cascades in inflammatory disease. Mol Med Today 1999; 5:439 - 47; http://dx.doi.org/10.1016/S1357-4310(99)01544-0; PMID: 10498912
- Karin M. Mitogen-activated protein kinase cascades as regulators of stress responses. Ann N Y Acad Sci 1998; 851:139 - 46; http://dx.doi.org/10.1111/j.1749-6632.1998.tb08987.x; PMID: 9668616
- Seger R, Krebs EG. The MAPK signaling cascade. FASEB J 1995; 9:726 - 35; PMID: 7601337
- Reusch HP, Chan G, Ives HE, Nemenoff RA. Activation of JNK/SAPK and ERK by mechanical strain in vascular smooth muscle cells depends on extracellular matrix composition. Biochem Biophys Res Commun 1997; 237:239 - 44; http://dx.doi.org/10.1006/bbrc.1997.7121; PMID: 9268693
- Lehoux S, Esposito B, Merval R, Loufrani L, Tedgui A. Pulsatile stretch-induced extracellular signal-regulated kinase 1/2 activation in organ culture of rabbit aorta involves reactive oxygen species. Arterioscler Thromb Vasc Biol 2000; 20:2366 - 72; http://dx.doi.org/10.1161/01.ATV.20.11.2366; PMID: 11073839
- Standley PR, Cammarata A, Nolan BP, Purgason CT, Stanley MA. Cyclic stretch induces vascular smooth muscle cell alignment via NO signaling. Am J Physiol Heart Circ Physiol 2002; 283:H1907 - 14; PMID: 12384468
- Chen Q, Li W, Quan Z, Sumpio BE. Modulation of vascular smooth muscle cell alignment by cyclic strain is dependent on reactive oxygen species and P38 mitogen-activated protein kinase. J Vasc Surg 2003; 37:660 - 8; http://dx.doi.org/10.1067/mva.2003.95; PMID: 12618707
- Liu B, Qu MJ, Qin KR, Li H, Li ZK, Shen BR, et al. Role of cyclic strain frequency in regulating the alignment of vascular smooth muscle cells in vitro. Biophys J 2008; 94:1497 - 507; http://dx.doi.org/10.1529/biophysj.106.098574; PMID: 17993501
- Qu MJ, Liu B, Wang HQ, Yan ZQ, Shen BR, Jiang ZL. Frequency-dependent phenotype modulation of vascular smooth muscle cells under cyclic mechanical strain. J Vasc Res 2007; 44:345 - 53; http://dx.doi.org/10.1159/000102278; PMID: 17713348
- Numaguchi K, Eguchi S, Yamakawa T, Motley ED, Inagami T. Mechanotransduction of rat aortic vascular smooth muscle cells requires RhoA and intact actin filaments. Circ Res 1999; 85:5 - 11; http://dx.doi.org/10.1161/01.RES.85.1.5; PMID: 10400905
- Goldman J, Zhong L, Liu SQ. Negative regulation of vascular smooth muscle cell migration by blood shear stress. Am J Physiol Heart Circ Physiol 2007; 292:H928 - 38; http://dx.doi.org/10.1152/ajpheart.00821.2006; PMID: 17012348
- Li C, Wernig F, Leitges M, Hu Y, Xu Q. Mechanical stress-activated PKCdelta regulates smooth muscle cell migration. FASEB J 2003; 17:2106 - 8; PMID: 12958154
- Xu Z, Fukuda T, Li Y, Zha X, Qin J, Wu C. Molecular dissection of PINCH-1 reveals a mechanism of coupling and uncoupling of cell shape modulation and survival. J Biol Chem 2005; 280:27631 - 7; http://dx.doi.org/10.1074/jbc.M504189200; PMID: 15941716
- Lemarié CA, Tharaux PL, Esposito B, Tedgui A, Lehoux S. Transforming growth factor-α mediates nuclear factor kappaB activation in strained arteries. Circ Res 2006; 99:434 - 41; http://dx.doi.org/10.1161/01.RES.0000237388.89261.47; PMID: 16857964
- Putman AJ, Cunningham JJ, Pillemer VB, Mooney DJ. External mechanical strain regulates membrane targeting of Rho GTPases by controlling microtubule assembly. Am J Physiol Cell Physiol 2002; 284:C627 - 39; PMID: 12409284
- Komuro I, Kaida T, Shibazaki Y, Kurabayashi M, Katoh Y, Hoh E, et al. Stretching cardiac myocytes stimulates protooncogene expression. J Biol Chem 1990; 265:3595 - 8; PMID: 2105950
- Sadoshima J, Jahn L, Takahashi T, Kulik TJ, Izumo S. Molecular characterization of the stretch-induced adaptation of cultured cardiac cells. An in vitro model of load-induced cardiac hypertrophy. J Biol Chem 1992; 267:10551 - 60; PMID: 1534087
- De R, Zemel A, Safran SA. Do cells sense stress or strain? Measurement of cellular orientation can provide a clue. Biophys J 2008; 94:L29 - 31; http://dx.doi.org/10.1529/biophysj.107.126060; PMID: 18192355
- Gopalan SM, Flaim C, Bhatia SN, Hoshijima M, Knoell R, Chien KR, et al. Anisotropic stretch-induced hypertrophy in neonatal ventricular myocytes micropatterned on deformable elastomers. Biotechnol Bioeng 2003; 81:578 - 87; http://dx.doi.org/10.1002/bit.10506; PMID: 12514807
- Paradis P, Dali-Youcef N, Paradis FW, Thibault G, Nemer M. Overexpression of angiotensin II type I receptor in cardiomyocytes induces cardiac hypertrophy and remodeling. Proc Natl Acad Sci U S A 2000; 97:931 - 6; http://dx.doi.org/10.1073/pnas.97.2.931; PMID: 10639182
- Fomovsky GM, Thomopoulos S, Holmes JW. Contribution of extracellular matrix to the mechanical properties of the heart. J Mol Cell Cardiol 2010; 48:490 - 6; http://dx.doi.org/10.1016/j.yjmcc.2009.08.003; PMID: 19686759
- Shewan AM, Maddugoda M, Kraemer A, Stehbens SJ, Verma S, Kovacs EM, et al. Myosin 2 is a key Rho kinase target necessary for the local concentration of E-cadherin at cell-cell contacts. Mol Biol Cell 2005; 16:4531 - 42; http://dx.doi.org/10.1091/mbc.E05-04-0330; PMID: 16030252
- Tokuyasu KT, Dutton AH, Geiger B, Singer SJ. Ultrastructure of chicken cardiac muscle as studied by double immunolabeling in electron microscopy. Proc Natl Acad Sci U S A 1981; 78:7619 - 23; http://dx.doi.org/10.1073/pnas.78.12.7619; PMID: 6801654
- Garrod D, Chidgey M. Desmosome structure, composition and function. Biochim Biophys Acta 2008; 1778:572 - 87; http://dx.doi.org/10.1016/j.bbamem.2007.07.014; PMID: 17854763
- Zhuang J, Yamada KA, Saffitz JE, Kléber AG. Pulsatile stretch remodels cell-to-cell communication in cultured myocytes. Circ Res 2000; 87:316 - 22; http://dx.doi.org/10.1161/01.RES.87.4.316; PMID: 10948066
- Aberle H, Butz S, Stappert J, Weissig H, Kemler R, Hoschuetzky H. Assembly of the cadherin-catenin complex in vitro with recombinant proteins. J Cell Sci 1994; 107:3655 - 63; PMID: 7706414
- Jou TS, Stewart DB, Stappert J, Nelson WJ, Marrs JA. Genetic and biochemical dissection of protein linkages in the cadherin-catenin complex. Proc Natl Acad Sci U S A 1995; 92:5067 - 71; http://dx.doi.org/10.1073/pnas.92.11.5067; PMID: 7761449
- Salameh A, Dhein S.. Effect of mechanical forces and stretch on intercellular gap junction coupling. Biochim Biophys Acta 2013; 1828:147 - 56; http://dx.doi.org/10.1016/j.bbamem.2011.12.030; PMID: 22245380
- Baines CP, Molkentin JD. STRESS signaling pathways that modulate cardiac myocyte apoptosis. J Mol Cell Cardiol 2005; 38:47 - 62; http://dx.doi.org/10.1016/j.yjmcc.2004.11.004; PMID: 15623421
- Yamazaki T, Komuro I, Kudoh S, Zou Y, Shiojima I, Mizuno T, et al. Mechanical stress activates protein kinase cascade of phosphorylation in neonatal rat cardiac myocytes. J Clin Invest 1995; 96:438 - 46; http://dx.doi.org/10.1172/JCI118054; PMID: 7615816
- Komuro I, Kudo S, Yamazaki T, Zou Y, Shiojima I, Yazaki Y. Mechanical stretch activates the stress-activated protein kinases in cardiac myocytes. FASEB J 1996; 10:631 - 6; PMID: 8621062
- Sadoshima J, Izumo S. The cellular and molecular response of cardiac myocytes to mechanical stress. Annu Rev Physiol 1997; 59:551 - 71; http://dx.doi.org/10.1146/annurev.physiol.59.1.551; PMID: 9074777
- Leychenko A, Konorev E, Jijiwa M, Matter ML. Stretch-induced hypertrophy activates NFkB-mediated VEGF secretion in adult cardiomyocytes. PLoS One 2011; 6:e29055; http://dx.doi.org/10.1371/journal.pone.0029055; PMID: 22174951
- Vandenburgh HH. Mechanical force and their second messengers in stimulating cell growth in vitro. Am J Physiol 1992; 262:350 - 5
- Nian M, Lee P, Khaper N, Liu P. Inflammatory cytokines and postmyocardial infarction remodeling. Circ Res 2004; 94:1543 - 53; http://dx.doi.org/10.1161/01.RES.0000130526.20854.fa; PMID: 15217919