Abstract
STAT3 and Akt signaling have been validated as potential molecular targets for treatment of cancers including melanoma. These small molecule inhibitors of STAT3 or Akt signaling are promising for developing anti-melanoma therapeutic agents. MLS-2438, a novel 7-bromoindirubin, a derivative of the natural product indirubin, was synthesized with a bromo-group at the 7-position on one indole ring and a hydrophilic group at the 3′-position on the other indole ring. We tested the anticancer activity of MLS-2438 and investigated its mechanism of action in human melanoma cell lines. Here, we show that MLS-2438 inhibits viability and induces apoptosis of human melanoma cells associated with inhibition of STAT3 and Akt signaling. Several pro-apoptotic Bcl-2 family proteins are involved in the MLS-2438 mediated apoptosis. MLS-2438 inhibits Src kinase activity in vitro and phosphorylation of JAK2, Src, STAT3 and Akt in cultured cancer cells. In contrast to the decreased phosphorylation levels of JAK2, Src, STAT3 and Akt, phosphorylation levels of the MAPK (Erk1/2) signaling protein were not reduced in cells treated with MLS-2438. These results demonstrate that MLS-2438, a novel natural product derivative, is a Src inhibitor and potentially regulates kinase activity of JAK2 and Akt in cancer cells. Importantly, MLS-2438 suppressed tumor growth with low toxicity in a mouse xenograft model of human melanoma. Our findings support further development of MLS-2438 as a potential small-molecule therapeutic agent that targets both STAT3 and Akt signaling in human melanoma cells.
Introduction
Melanoma is the sixth most common cancer in the United States and it is the most malignant type of skin cancer. Although early stage primary melanoma is curable through surgery, late stage metastatic melanoma is very difficult to treat. Most standard chemotherapy cancer drugs have not passed large-scale clinical trials for this tumor. Treatment options for late stage or metastatic melanoma are limited.Citation1,Citation2 Using small-molecule inhibitors to target multiple intracellular signaling pathways is an emerging strategy in melanoma therapeutics.Citation3-Citation5 Searching for effective drugs to treat metastatic melanoma is a challenging task due to strong drug resistance of this disease. Vemurafenib (Zelboraf, PLX4032) has been approved by the US. Food and Drug Administration (FDA) recently for the treatment of patients with metastatic melanoma with the BRAFV600E mutation. However, acquired resistance develops partially due to activation or alterations of alternative signaling pathways including Src and Akt, which promote tumor progression.Citation6-Citation9
STAT3 and Akt are the central signaling proteins that promote growth and progression of tumors including melanoma.Citation10-Citation12 STAT3 is persistently activated in cancer cells due to aberrant activation of JAK, Src and/or other tyrosine kinases.Citation13-Citation19 Persistent activation of STAT3 signaling contributes to the malignancy of tumors by promoting tumor cell proliferation and survival, angiogenesis and immune evasion.Citation10,Citation20-Citation23 Akt or protein kinase B (PKB) is a potentially important mediator of the phosphatidylinositol-3-kinase (PI3K) signaling. The PI3K/Akt signaling has a key role in regulation of cell survival and apoptosis.Citation24-Citation26 and is constitutively activated in a wide range of cancers including melanoma.Citation11,Citation12 Thus, STAT3 and Akt signaling are promising molecular targets for cancer therapy.
Indirubin, a bis-indole alkaloid, is the active ingredient of Danggui Longhui Wan, a traditional Chinese herbal medicine for treatment of chronic myelocytic leukemia (CML).Citation27 Indirubin and its analogs can be found in certain terrestrial plants and sea shells. Natural bromoindirubins are restricted to marine sources.Citation28,Citation29 Comparing with indirubin, several indirubin derivatives, including some novel synthetic bromoindirubins, have shown enhanced anticancer activity in cancer cells.Citation30-Citation32 Synthetic 7-bromoindirubins are novel indirubin derivatives with potent anticancer activity, but the mechanism of action remains unclear.Citation33
In this study, we investigated a novel 7-bromoindirubin derivative, MLS-2438 in terms of anticancer activity and mechanisms of action particularly in human melanoma cells. We have found that MLS-2438 demonstrates potent anticancer activity and induces apoptosis of human melanoma cells. Furthermore, the bromoindirubin-mediated apoptosis is associated with inhibition of STAT3 and Akt signaling. Several pro-apoptotic Bcl-2 family proteins such as Bax, Bak, Bad and Bim are involved in the MLS-2438 mediated apoptosis in human melanoma cells. Our previous studies showed that a 6-bromoindirubin, 6-bromo-3′-oxime (6BIO), inhibits JAK/STAT3 signaling as a JAK inhibitor.Citation30 Interestingly, in this study MLS-2438 is identified as a Src inhibitor and inhibits phosphorylation of STAT3, JAK2, Src and Akt in cancer cells. Our findings indicate that Src may regulate kinase activity of JAK2 and/or Akt in human melanoma cells. We investigated the effects of MLS-2438 particularly on human melanoma cells, due to a need for more effective therapeutics for this tumor site. MLS-2438 as a Src inhibitor suppresses multiple signaling proteins and represents a promising lead compound for development of new anticancer therapeutics, especially for melanoma.
Results
MLS-2438 demonstrates anticancer activity in human melanoma cells and in a mouse xenograft model
We screened a group of 7-bromoindirubins (Fig. S1), using MTS cell viability assays in various human cancer cells and MLS-2438 showed the best anticancer activity (Table S1). MLS-2438 () was selected for further study in A2058, A375, G361 and MeWo human melanoma cells. Cells were treated with MLS-2438 at various concentrations for 24 h. We compared the anticancer effects of MLS-2438 on the four human melanoma cell lines by analyzing cell viability. Cell viability was inhibited in all of the four melanoma cell lines. IC50 values are less than 2 μmol/L. A2058 and A375 cells are more sensitive to the treatment of MLS-2438 than G361 and MeWo cells (). At the treatment of 5 μmol/L of MLS-2438, cell viability was reduced dramatically.
Figure 1. MLS-2438 suppresses viability of human melanoma cells and tumor growth in a mouse xenograft model. (A) structure of MLS-2438. Indirubin molecule was derivatized with a bromo-group at the 7-position on one indole ring and a hydrophilic group at the 3′-position on the other indole ring. The synthesis of this 7-bromoindirubin derivative was reported previously.Citation31 (B) A2058, A375, G361 and MeWo human melanoma cells were treated with MLS-2438 at various concentrations for 24 h. Viable cells were counted by using a cell viability analyzer. The values of cell viability were calculated as percentages of viable cell numbers from bromoindirubin-treated cells to viable cell numbers from the DMSO-treated cells. (C) MLS-2438 suppressed tumor growth of MeWo human melanoma xenografts in NSG mice. (D) Body weight change. MLS-2438-or vehicle- treated mice were weighed on the same dates as tumors were measured. Body weight change is shown as percentage to the weight on the starting date. Points, mean (n = 8); bars, SE; *p < 0.01 vs. control.
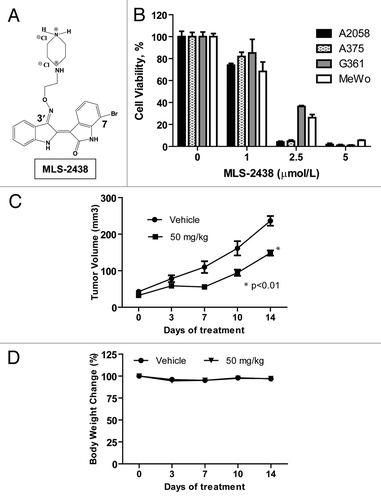
The anticancer activity of MLS-2438 in vivo was studied with a MeWo human melanoma xenograft mouse model. We first tested the toxicity of MLS-2438 in BALB/c normal mice. MLS-2438, at a dose of 100 mg/kg was found safe to the mice. Then we used a dose of 50 mg/kg for MLS-2438 therapy study in NSG mouse xenograft model by oral administration once daily for 2 weeks. As shown in , the tumor growth was significantly suppressed. No side effects were observed in the MLS-2438- treated mice. The body weight change is shown as a percentage of the treated mouse body weight to the untreated mouse body weight on the starting date (). These findings show the antitumor activity of MLS-2438 in vivo against human melanoma cells.
MLS-2438 induces apoptosis of human melanoma cells
To characterize the MLS-2438-induced cell death, we conducted apoptosis analysis by flow cytometry using Annexin V-FITC and DAPI double staining. MLS-2438 induced apoptosis of human melanoma cells in a dose-dependent manner. The concentrations to induce 50% apoptosis are less than 2 μmol/L (). The patterns for inhibition of cell viability () and induction of apoptosis () are consistent in the four melanoma cell lines. When cells were treated with MLS-2438, viability decreased, whereas apoptosis increased.
Figure 2. MLS-2438 induces apoptosis of human melanoma cells. A2058, A375, G361 and MeWo human melanoma cells were treated with MLS-2438 at various concentrations for 24 h. (A) apoptosis was analyzed by flow cytometry by using Annexin V-FITC and DAPI double staining. (B) cells were lysed for Western blot analysis using antibodies specific to PARP, Caspase-3 and β-Actin.
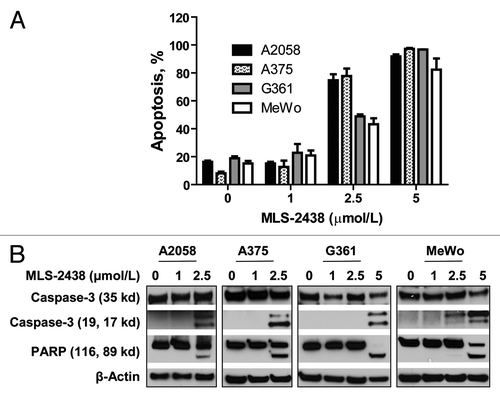
We also analyzed apoptosis by using Western blotting analysis to detect two apoptosis markers, cleaved Caspase-3 and PARP in human melanoma cells. As shown in , cleavage of Caspase-3 and PARP was detected at 2.5 μmol/L in A2058 and A375 cells and at 5 μmol/L in G361 and MeWo cells. This observation provides more pieces of evidence for the MLS-2438-mediated apoptosis in human melanoma cells.
Levels of pro-apoptotic and pro-survival proteins changed in human melanoma cells
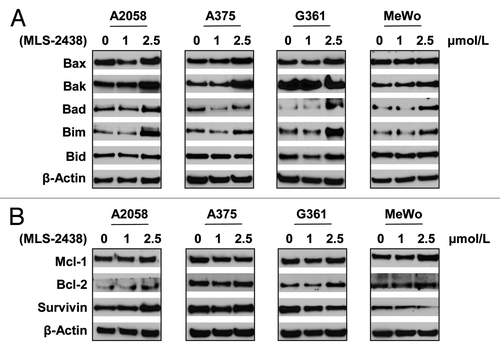
We further investigated level changes of apoptosis-related proteins such as pro-apoptotic Bcl-2 family proteins, Bax, Bak, Bad, Bim and Bid and pro-survival proteins such as Mcl-1, Bcl-2 and Survivin. These proteins are regulated by Akt and/or STAT3 signaling.Citation10,Citation24,Citation25,Citation34,Citation35 We analyzed expression levels of these apoptosis-related proteins in four human melanoma cell lines by Western blotting analysis (). As shown in , expression levels of Bax, Bak, Bad and Bim increased in A2058 human melanoma cells at the treatment of 2.5 μmol/L, whereas levels of Bax, Bak and Bim were elevated in A375 cells. In G361 cells, levels of Bax, Bad and Bim increased at the treatment of 2.5 μmol/L, whereas levels of Bak, Bad and Bim were higher in MeWo cells treated with 2.5 μmol/L of MLS-2438. Bid levels were not changed in the four cell lines treated with MLS-2438. The increased levels of these pro-apoptotic Bcl-2 family proteins are consistent with the induction of apoptosis starting at 2.5 μmol/L. Among the three pro-survival proteins Mcl-1, Bcl-2 and Survivin, only Survivin levels were decreased in response to the treatment of MLS-2438 in G361 and MeWo cells (). Mcl-1 levels were not changed in the four cell lines. Bcl-2 levels were increased slightly in the four cell lines and Survivin levels were also increased in A2058 and A375 cells. These findings indicate that pro-apoptotic Bcl-2 family proteins such as Bax, Bak, Bad and Bim are involved in the MLS-2438 mediated apoptosis in human melanoma cells and changes of these proteins are specific to cell types.
Figure 3. Expression levels of pro- apoptotic and pro-survival proteins in human melanoma cells treated with MLS-2438. A2058, A375, G361 and MeWo human melanoma cells were treated with MLS-2438 at various concentrations for 24 h. Cells were lysed for Western blot analysis using antibodies specific to pro-apoptotic Bcl-2 family proteins such as Bax, Bak, Bad, Bim and Bid and pro-survival proteins such as Mcl-1, Bcl-2 and Survivin. β-Actin was used as a loading control.
MLS-2438 inhibits STAT3 and Akt signaling in human melanoma cells
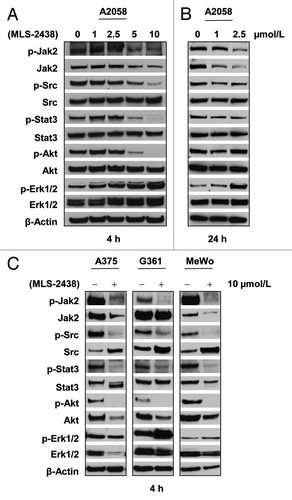
To elucidate which signaling pathway was involved in MLS-2438-mediated apoptosis in human melanoma cells, we investigated three major signaling pathways, STAT3, Akt and MAPK in four human melanoma cell lines. We treated A2058 cells with MLS-2438 at various concentrations for 4 h () and 24 h (). A375, G361 and MeWo cells were treated with10 μmol/L of MLS-2438 for 4 h (). As shown in , phosphorylation of JAK2, Src and STAT3 was inhibited in A2058 cells treated with MLS-2438 for 24 h at concentrations of 1 and 2.5 μmol/L, but phosphorylation of Akt was not inhibited in A2058 cells at the 24-h treatment of MLS-2438. At the 4-h treatment of MLS-2438, phosphorylation levels of JAK2, Src, STAT3 and Akt were reduced in a dose-dependent manner in A2058 cells () and dramatically decreased at a treatment of 10 μmol/L in the entire four cell lines ( and ). In contrast to the reduction in phosphorylation of JAK2, Src, STAT3 and Akt, increased phosphorylation of Erk1/2 was detected in A2058 cells in a dose-dependent manner at both 4- and 24- hour treatments ( and ) and in G361 and MeWo cells at the 4-h treatment with 10 μmol/L of MLS-2438 (). Phosphorylation of Erk1/2 was only slightly inhibited in A375 cells at the treatment with 10 μmol/L of MLS-2438 for 4 h (). These findings suggest that MLS-2438 induces apoptosis associated with inhibition of STAT3 and Akt signaling in human melanoma cells.
Figure 4. MLS-2438 inhibits phosphorylation of JAK2, Src, STAT3 and Akt in human melanoma cells. A2058 human melanoma cells were treated with MLS-2438 at various concentrations for 4 h (A) and 24 h (B). C, A375, G361 and MeWo human melanoma cells were treated with 10 μmol/L of MLS-2438 for 4 h. Cells were lysed for Western blot analysis using antibodies specific to p-JAK2, JAK2, p-Src, Src, p-STAT3, STAT3, p-Akt, Akt, p-Erk1/2, Erk1/2 and β-Actin.
MLS-2438 is a Src inhibitor and inhibits phosphorylation of Src, Akt and JAK2 in cells
Our data have demonstrated that MLS-2438 inhibits phosphorylation of kinases such as JAK2, Src and Akt in human melanoma cells (). To further identify that MLS-2438 is a kinase inhibitor, we had MLS-2438 tested by in vitro kinase assays by using purified recombinant Src, Akt and JAK2 proteins with catalytic domains and relevant substrates. As shown in , MLS-2438 is a strong Src inhibitor with an IC50 value of 0.2 μmol/L and a mild inhibitor of Akt, but not an inhibitor of JAK2 in vitro. We actually tested Akt kinase family (Akt1, Akt2 and Akt3) and JAK family (JAK1, JAK2, JAK3 and TYK2) in vitro (data not shown). The IC50 value of Akt shown in is an average value of the IC50 values of Akt1, Akt2 and Akt3. Comparing with Akt and JAK family kinases, Src kinase activity was most potently inhibited in vitro by MLS-2438.
Figure 5. MLS-2438 inhibits Src kinase activity in vitro and phosphorylation of Src, Akt and JAK2 in A2058 cells. (A) In vitro kinase assays of Src, Akt and JAK2 were conducted using recombinant proteins, relevant substrates and 33P-labeled ATP in the presence of MLS-2438 at various concentrations. Radioactivity was measured for determination of kinase activity. (B) A2058 human melanoma cells were treated with 10 μmol/L of MLS-2438 in a short time course from 5 to 30 min. Cells were lysed for Western blot analysis using antibodies specific to p-Src, Src, p-Akt, Akt, p-JAK2, JAK2 and β-Actin.
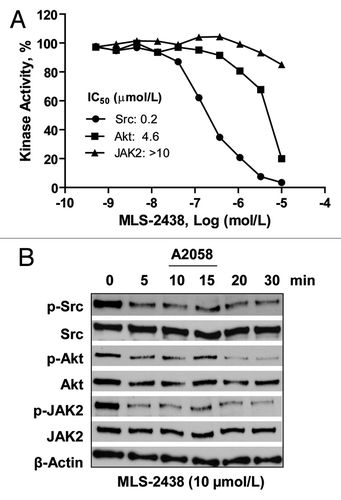
Data in show that MLS-2438 inhibits phosphorylation of Src, Akt and JAK2 in human melanoma cells at the 4-h treatment with 5 and 10 μmol/L of MLS-2438. To investigate the inhibitory effect at very early time points such as in minutes, A2058 cells were treated with 10 μmol/L of MLS-2438 from 5 to 30 min. We analyzed phosphorylated protein levels of Src, Akt and JAK2 on the treated A2058 cells in the short time course. As shown in , phosphorylated protein levels of Src, Akt and JAK2 were reduced in a similar time-dependent manner and started at 5 min. It is interesting that MLS-2438 exhibited differential inhibition of Src, Akt and JAK2 kinase activity in vitro (), whereas it inhibited phosphorylation of Src, Akt and JAK2 in similar patterns in human melanoma cells ( and ). These findings suggest that a possible interaction among Src, Akt and JAK2 may regulate their kinase activity in human melanoma cells.
Discussion
In a prior study, we showed that 6BIO, a 6-bromoindirubin derivative, targets JAK/STAT3 signaling as a pan-JAK inhibitor,Citation30 as well as targeting CDKs and GSK-3.Citation36 Both MLS-2438 and 6BIO have a hydrophilic group at the 3′-position, which may improve their water solubility. The major difference between the two compounds is the positions of the bromo-substitutions. 6BIO has a bromo-group at the 6-position, whereas MLS-2438 has a bromo-group at the 7-position. In this study, we have found the MLS-2438 is a Src inhibitor and inhibits phosphorylation of Src, JAK2 and Akt. These findings suggest that the bromo-substitution at the 7- position of indirubin molecule may modify the molecular binding affinity to different targets.
Previous studies have reported that Src could regulate the activity of Akt and JAK in cells.Citation37,Citation38 In this study, MLS-2438 inhibited phosphorylation of Src, Akt and JAK2 in cells ( and ). It is interesting that MLS-2438 displays differential inhibition among Src, Akt and JAK2 kinase activity in an in vitro system using recombinant kinases and relevant substrates (). MLS-2438 is a Src inhibitor with an IC50 value of 0.2 μmol/L, but not a JAK inhibitor in vitro. The IC50 value of Akt inhibition is relatively high in an in vitro system. These findings suggest a possible interaction between Src and Akt, Src and JAK2 in cells and this interaction may potentially regulate their kinase activity in cells. The proposed mechanism of action is shown in . MLS-2438 induces apoptosis of human melanoma cells at least in part associated with inhibition of STAT3 and Akt signaling pathways.
Figure 6. Schematic representation of MLS-2438-mediated apoptosis in human melanoma cells. MLS-2438, as a Src inhibitor, inhibits phosphorylation of Src, JAK2, STAT3 and Akt and induces apoptosis in human melanoma cells. The MLS-2438-mediated apoptosis at least in part is associated with inhibition of STAT3 and Akt signaling.
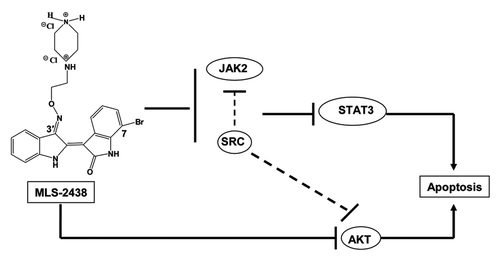
Among STAT3, Akt and MAPK signaling pathways, STAT3 and Akt signaling pathways were inhibited although MAPK signaling was not inhibited by MLS-2438. This finding suggests that MLS-2438 might be applied either as a single agent or in combination with inhibitors of MAPK signaling. Currently there are limited effective drugs to treat metastatic melanoma. Several combination agents are available for treatment of melanoma.Citation7-Citation9,Citation39,Citation40 MLS-2438 inhibits STAT3 and Akt signaling in melanoma, suggesting it may be a candidate for combination with the inhibitor of MAPK signaling, PLX 4032 or other inhibitors to treat metastatic melanoma more effectively by overcoming drug resistance.
MLS-2438 displays anticancer activity in various cancer cells. We used human melanoma as an example to investigate the mechanism of action for MLS-2438 and examined the three major signaling pathways, STAT3, Akt and MAPK, in human melanoma cells. Our results show that MLS-2438 selectively inhibits STAT3 and Akt signaling among these three major signaling pathways. However, we cannot exclude other signaling pathways being involved in melanoma, or other cancers. In summary, MLS-2438 is promising for development as a novel therapeutic agent targeting STAT3 and Akt signaling in melanoma and potentially other cancers. Furthermore, it may be more effective when combined with MAPK signaling inhibitors. Future studies will be directed toward in vivo pre-clinical evaluation of MLS-2438 using a metastatic tumor mouse model of human melanoma and other tumors.
In summary, we have found that MLS-2438 demonstrates potent anticancer activity and induces apoptosis of human melanoma cells associated with inhibition of STAT3 and Akt signaling. MAPK signaling is not involved in this induction of apoptosis. MLS-2438 is an inhibitor of Src and inhibits phosphorylation of STAT3, JAK2, Src and Akt in cancer cells. Our findings suggest that Src may regulate kinase activity of JAK2 and/or Akt in human melanoma cells. Importantly, MLS-2438 suppresses tumor growth in a MeWo human melanoma xenograft mouse model. Thus, MLS-2438 represents a promising lead compound for development of new anticancer therapeutics, especially targeting STAT3 and Akt in melanoma.
Materials and Methods
Reagents
The preparation of 7-bromoindirubins including MLS-2438 has been described previously.Citation31 The compound MLS-2438 was dissolved in dimethyl sulfoxide (DMSO) at a concentration of 20 mmol/L and stocked at -20°C before use for in vitro experiments and treatments in cells. For in vivo experiments, MLS-2438 was freshly prepared in 30% Solutol (Basf) at a concentration of 10 mg/mL. Anti-Survivin was from Novus. Anti-β-Actin was from Sigma. Horeseradish peroxidase (HRP)-labeled anti-mouse and anti-rabbit secondary antibodies were from GE Healthcare. All other antibodies were from Cell Signaling.
Cell lines and culture
The A2058, A375, G361 and MeWo human melanoma cell lines were obtained from American Type Culture Collection. Cells were maintained in RPMI 1640 medium. The cell culture medium was supplemented with 10% heat-inactivated FBS and 1% penicillin/streptomycin (P/S).
Cell viability analysis
Human cancer cells were seeded at 12-well plates with 25,000 cells per well. After 24-h incubation, cells were treated with MLS-2438 or DMSO as the vehicle control for 24 h. Dead cells were removed by washing with PBS buffer solution. Then viable cells were collected by trypsinization. Viable cells were counted by Vi-CELLTM XR Cell Viability Analyzer. The values of cell viability were calculated as percentages of viable cell numbers from bromoindirubin-treated cells to viable cell numbers from the DMSO-treated cells.
Flow cytometric analysis of apoptosis
Cells were seeded on 6-well plates with 50,000 cells per well in 3 ml of RPMI 1640 medium supplemented with 10% FBS and 1% P/S. After 24-h incubation, cells were treated with MLS-2438 or DMSO for 24 h. After treatment, both living and dead cells were collected and apoptotic cells were detected by flow cytometry by the Annexin V-FITC Apoptosis Detection Kit (BD Biosciences) according to the manufacturer’s instructions.
Western blot analysis
Cells were treated with DMSO or MLS-2438. After treatment, cell lysates were prepared in radioimmunoprecipitation assay (RIPA) buffer supplemented with inhibitors of proteases (Roche Diagnostics GmbH) and sodium orthovanadate, an inhibitor of phosphotases (Aldrich). Protein concentrations were determined by BioMate Spectrometer (Thermo) and protein assay (Bio-Rad). A sample of 40 μg or 20 μg of each protein was resolved in 8% or between 8% and 16% gradient SDS-PAGE gels (Pierce). After gel electrophoresis, proteins were transferred to Hybond-C membranes (Amersham). The membranes were blocked in 5% nonfat milk in PBS containing 0.1% Tween 20 (Polysorbate 20; PBST) at room temperature for 1 to 3 h followed by an overnight incubation at 4°C with primary antibodies in PBST containing 5% nonfat milk. The membranes were then washed with PBST and incubated with HRP-conjugated secondary antibody in 5% nonfat milk/PBST solution for 1 to 3 h at room temperature, or overnight at 4°C. Specific proteins were detected by exposure to X-ray film by using Super Signal West Pico Chemiluminescent Substrate or Super Signal West Dura Extended Duration Substrate (Pierce).
In vitro kinase assay
Recombinant JAK2, Src and Akt proteins, substrates and 33P-labeled ATP were used for the in vitro kinase assays. The recombinant protein catalytic domains were tagged with glutathione S-transferase (GST) and purified from insect cells. The substrates are Crosstide (GRPRTSSFAEG) for Akt and pEY (mg/ml, Glu;Tyr = 4:1, Molecular weight = 5, 000–20, 000) for c-Src and JAK2. The substrate was prepared in freshly constituted Base Reaction Buffer [comprising 20 mmol/L HEPES, pH 7.5 ; 10 mmol/LMgCl2; 1 mmol/L EGTA; 0.02% BRIJ-35; 0.02 mg/ml BSA; 0.1 mmol/L Na3VO4; 2 mmol/L dithiothreitol (DTT); and1% DMSO]. Then, required cofactors and kinase were added into the substrate solution. MLS-2438 in DMSO was delivered into the kinase reaction mixture and then 33P-labeled ATP (specific activity: 0.01 μCi/μl final) was added into the reaction mixture to initiate the reaction. The kinase reaction mixture was incubated for 120 min at room temperature. Reactions were spotted onto P81 ion- exchange paper (Whatman) for measurement of radioactivity.
In vivo therapeutic efficacy
BALB/c mice (6–8 weeks old) were purchased from the National Cancer Institute for toxicity study. Immunodeficient NOD/SCID/IL2Rgamma null (NSG) mice (female; 6–8 weeks old) were purchased from The Jackson Laboratory for use as the xenograft model. The experimental protocol for animal experiments was approved by the Institutional Animal Care and Use Committee (IACUC) of the Beckman Research Institute at City of Hope Medical Center. MeWo human melanoma cells at a density of 2.5 × 106 cells in 0.1 mL serum-free medium were inoculated subcutaneously into the dorsal area of NSG mice to create the xenograft model. MLS-2438 was freshly prepared in vehicle, 30% Soluto (Basf). When tumors became palpable, MLS-2438 or vehicle control was administered via oral gavage once daily at a dose of 50 mg/kg body weight. Tumor growth was monitored every other day. Tumor volumes and mice’s body weights were measured every 3 or 4 d. Tumor volumes were calculated by the formula: 0.5 × (larger diameter) × (small diameter).Citation2
Statistical analysis
A 2-sided t test was used to evaluate statistical significance of differences between treated and control groups. P < 0.05 was considered statistically significant.
| Abbreviations: | ||
| STAT | = | signal transducer and activator of transcription |
| JAK | = | Janus activated kinase |
| MAPK | = | mitogen-activated protein kinase |
| PKB | = | protein kinase B |
| PI3K | = | phosphatidylinositol-3-kinase |
| CDK | = | cyclin-dependent kinase |
| GSK-3 | = | glycogen synthase kinase-3 |
| CML | = | chronic myelocytic leukemia |
| 6BIO | = | 6-bromoindirubin-3′-oxime |
| DAPI | = | 4,6-diamidino-2-phenylindole |
| PARP | = | poly (ADP-ribose) polymerase |
Additional material
Download Zip (267.9 KB)Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
This work was supported in part by R01-CA115674 from NIH (R.J.).
Supplemental Materials
Supplemental Materials may be found here:
http://www.landesbioscience.com/journals/cbt/article/21781/
References
- Serrone L, Zeuli M, Sega FM, Cognetti F. Dacarbazine-based chemotherapy for metastatic melanoma: thirty-year experience overview. J Exp Clin Cancer Res 2000; 19:21 - 34; PMID: 10840932
- Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin 2009; 59:225 - 49; http://dx.doi.org/10.3322/caac.20006; PMID: 19474385
- Bogenrieder T, Herlyn M. The molecular pathology of cutaneous melanoma. Cancer Biomark 2010; 9:267 - 86; PMID: 22112480
- Garber K. Cancer research. Melanoma drug vindicates targeted approach. Science (New York, NY 2009; 326:1619.
- Smalley KS, Herlyn M. Targeting intracellular signaling pathways as a novel strategy in melanoma therapeutics. Ann N Y Acad Sci 2005; 1059:16 - 25; http://dx.doi.org/10.1196/annals.1339.005; PMID: 16382039
- Villanueva J, Vultur A, Herlyn M. Resistance to BRAF inhibitors: unraveling mechanisms and future treatment options. Cancer Res 2011; 71:7137 - 40; http://dx.doi.org/10.1158/0008-5472.CAN-11-1243; PMID: 22131348
- Atefi M, von Euw E, Attar N, Ng C, Chu C, Guo D, et al. Reversing melanoma cross-resistance to BRAF and MEK inhibitors by co-targeting the AKT/mTOR pathway. PLoS One 2011; 6:e28973; http://dx.doi.org/10.1371/journal.pone.0028973; PMID: 22194965
- Deng W, Vashisht Gopal YN, Scott A, Chen G, Woodman SE, Davies MA. Role and therapeutic potential of PI3K-mTOR signaling in de novo resistance to BRAF inhibition. Pigment Cell Melanoma Res 2011.
- Vergani E, Vallacchi V, Frigerio S, Deho P, Mondellini P, Perego P, et al. Identification of MET and SRC activation in melanoma cell lines showing primary resistance to PLX4032. Neoplasia 2011; 13:1132 - 42; PMID: 22241959
- Yu H, Jove R. The STATs of cancer--new molecular targets come of age. Nat Rev Cancer 2004; 4:97 - 105; http://dx.doi.org/10.1038/nrc1275; PMID: 14964307
- Bunney TD, Katan M. Phosphoinositide signalling in cancer: beyond PI3K and PTEN. Nat Rev Cancer 2010; 10:342 - 52; http://dx.doi.org/10.1038/nrc2842; PMID: 20414202
- Carnero A. The PKB/AKT pathway in cancer. Curr Pharm Des 2010; 16:34 - 44; http://dx.doi.org/10.2174/138161210789941865; PMID: 20214616
- Frame MC. Newest findings on the oldest oncogene; how activated src does it. J Cell Sci 2004; 117:989 - 98; http://dx.doi.org/10.1242/jcs.01111; PMID: 14996930
- Niu G, Bowman T, Huang M, Shivers S, Reintgen D, Daud A, et al. Roles of activated Src and Stat3 signaling in melanoma tumor cell growth. Oncogene 2002; 21:7001 - 10; http://dx.doi.org/10.1038/sj.onc.1205859; PMID: 12370822
- Sinibaldi D, Wharton W, Turkson J, Bowman T, Pledger WJ, Jove R. Induction of p21WAF1/CIP1 and cyclin D1 expression by the Src oncoprotein in mouse fibroblasts: role of activated STAT3 signaling. Oncogene 2000; 19:5419 - 27; http://dx.doi.org/10.1038/sj.onc.1203947; PMID: 11114718
- Darnell JE Jr., Kerr IM, Stark GR. Jak-STAT pathways and transcriptional activation in response to IFNs and other extracellular signaling proteins. Science 1994; 264:1415 - 21; http://dx.doi.org/10.1126/science.8197455; PMID: 8197455
- Wojcik EJ, Sharifpoor S, Miller NA, Wright TG, Watering R, Tremblay EA, et al. A novel activating function of c-Src and Stat3 on HGF transcription in mammary carcinoma cells. Oncogene 2006; 25:2773 - 84; http://dx.doi.org/10.1038/sj.onc.1209306; PMID: 16407846
- Sam MR, Elliott BE, Mueller CR. A novel activating role of SRC and STAT3 on HGF transcription in human breast cancer cells. Mol Cancer 2007; 6:69; http://dx.doi.org/10.1186/1476-4598-6-69; PMID: 17967179
- Hbibi AT, Lagorce C, Wind P, Spano JP, Des Guetz G, Milano G, et al. Identification of a functional EGF-R/p60c-src/STAT3 pathway in colorectal carcinoma: analysis of its long-term prognostic value. Cancer Biomark 2008; 4:83 - 91; PMID: 18503159
- Gao SP, Bromberg JF. Touched and moved by STAT3. Sci STKE 2006; 2006:pe30; http://dx.doi.org/10.1126/stke.3432006pe30; PMID: 16835434
- Yu H, Pardoll D, Jove R. STATs in cancer inflammation and immunity: a leading role for STAT3. Nat Rev Cancer 2009; 9:798 - 809; http://dx.doi.org/10.1038/nrc2734; PMID: 19851315
- Ghoreschi K, Laurence A, O’Shea JJ. Janus kinases in immune cell signaling. Immunol Rev 2009; 228:273 - 87; http://dx.doi.org/10.1111/j.1600-065X.2008.00754.x; PMID: 19290934
- Kortylewski M, Jove R, Yu H. Targeting STAT3 affects melanoma on multiple fronts. Cancer Metastasis Rev 2005; 24:315 - 27; http://dx.doi.org/10.1007/s10555-005-1580-1; PMID: 15986140
- Franke TF, Kaplan DR, Cantley LC. PI3K: downstream AKTion blocks apoptosis. Cell 1997; 88:435 - 7; http://dx.doi.org/10.1016/S0092-8674(00)81883-8; PMID: 9038334
- Burgering BM, Coffer PJ. Protein kinase B (c-Akt) in phosphatidylinositol-3-OH kinase signal transduction. Nature 1995; 376:599 - 602; http://dx.doi.org/10.1038/376599a0; PMID: 7637810
- Franke TF, Yang SI, Chan TO, Datta K, Kazlauskas A, Morrison DK, et al. The protein kinase encoded by the Akt proto-oncogene is a target of the PDGF-activated phosphatidylinositol 3-kinase. Cell 1995; 81:727 - 36; http://dx.doi.org/10.1016/0092-8674(95)90534-0; PMID: 7774014
- Xiao Z, Hao Y, Liu B, Qian L. Indirubin and meisoindigo in the treatment of chronic myelogenous leukemia in China. Leuk Lymphoma 2002; 43:1763 - 8; http://dx.doi.org/10.1080/1042819021000006295; PMID: 12685829
- Cheng LH. The study on the extraction method of indirubin from Indigo Naturalis. Zhong Yao Cai 2007; 30:50 - 2; PMID: 17539304
- Meijer L. [Mediterranean purple indirubins: a source of GSK-3 inhibitors]. Med Sci (Paris) 2004; 20:516 - 8; http://dx.doi.org/10.1051/medsci/2004205516; PMID: 15190467
- Liu L, Nam S, Tian Y, Yang F, Wu J, Wang Y, et al. 6-Bromoindirubin-3′-oxime inhibits JAK/STAT3 signaling and induces apoptosis of human melanoma cells. Cancer Res 2011; 71:3972 - 9; http://dx.doi.org/10.1158/0008-5472.CAN-10-3852; PMID: 21610112
- Ferandin Y, Bettayeb K, Kritsanida M, Lozach O, Polychronopoulos P, Magiatis P, et al. 3′-Substituted 7-halogenoindirubins, a new class of cell death inducing agents. J Med Chem 2006; 49:4638 - 49; http://dx.doi.org/10.1021/jm060314i; PMID: 16854069
- Choi SJ, Lee JE, Jeong SY, Im I, Lee SD, Lee EJ, et al. 5,5′-substituted indirubin-3′-oxime derivatives as potent cyclin-dependent kinase inhibitors with anticancer activity. J Med Chem 2010; 53:3696 - 706; http://dx.doi.org/10.1021/jm100080z; PMID: 20361800
- Ribas J, Bettayeb K, Ferandin Y, Knockaert M, Garrofé-Ochoa X, Totzke F, et al. 7-Bromoindirubin-3′-oxime induces caspase-independent cell death. Oncogene 2006; 25:6304 - 18; http://dx.doi.org/10.1038/sj.onc.1209648; PMID: 16702956
- Pugazhenthi S, Nesterova A, Sable C, Heidenreich KA, Boxer LM, Heasley LE, et al. Akt/protein kinase B up-regulates Bcl-2 expression through cAMP-response element-binding protein. J Biol Chem 2000; 275:10761 - 6; http://dx.doi.org/10.1074/jbc.275.15.10761; PMID: 10753867
- Mills JR, Hippo Y, Robert F, Chen SM, Malina A, Lin CJ, et al. mTORC1 promotes survival through translational control of Mcl-1. Proc Natl Acad Sci U S A 2008; 105:10853 - 8; http://dx.doi.org/10.1073/pnas.0804821105; PMID: 18664580
- Polychronopoulos P, Magiatis P, Skaltsounis AL, Myrianthopoulos V, Mikros E, Tarricone A, et al. Structural basis for the synthesis of indirubins as potent and selective inhibitors of glycogen synthase kinase-3 and cyclin-dependent kinases. J Med Chem 2004; 47:935 - 46; http://dx.doi.org/10.1021/jm031016d; PMID: 14761195
- Thomas SM, Brugge JS. Cellular functions regulated by Src family kinases. Annu Rev Cell Dev Biol 1997; 13:513 - 609; http://dx.doi.org/10.1146/annurev.cellbio.13.1.513; PMID: 9442882
- Zhang Y, Turkson J, Carter-Su C, Smithgall T, Levitzki A, Kraker A, et al. Activation of Stat3 in v-Src-transformed fibroblasts requires cooperation of Jak1 kinase activity. J Biol Chem 2000; 275:24935 - 44; http://dx.doi.org/10.1074/jbc.M002383200; PMID: 10823829
- Lee JT, Herlyn M. MEK’ing the most of p53 reactivation therapy in melanoma. J Invest Dermatol 2012; 132:263 - 5; http://dx.doi.org/10.1038/jid.2011.362; PMID: 22241441
- Inamdar GS, Madhunapantula SV, Robertson GP. Targeting the MAPK pathway in melanoma: why some approaches succeed and other fail. Biochem Pharmacol 2010; 80:624 - 37; http://dx.doi.org/10.1016/j.bcp.2010.04.029; PMID: 20450891