Abstract
Defective apoptosis comprises the main reason for tumor aggressiveness and chemotherapy tolerance in solid neoplasias. Among the BCL-2 family members, whose mRNA or protein expression varies considerably in different human malignancies, BCL2L12 is the one for which we have recently shown its propitious prognostic value in gastric cancer. The purpose of the current work was to investigate the expression behavior of BCL2L12, BAX, and BCL-2 in human stomach adenocarcinoma cells following their exposure to anti-tumor substances. The 3-(4,5-dimethyl thiazol-2-yl)-2,5-diphenyl tetrazolium bromide and trypan blue methods assessed the impact of doxorubicin, oxaliplatin and methotrexate on AGS cells’ viability and growth. Following isolation from cells, total RNA was reverse-transcribed to cDNA. Quantification of target genes’ expression was performed with real-time PCR using SYBR Green detection system. The relative changes in their mRNA levels between drug-exposed and untreated cells were calculated with the comparative Ct method (2-ddCt). All three drugs, as a result of their administration to AGS cancer cells for particular time intervals, provoked substantial fluctuations in the transcriptional levels of the apoptosis-related genes studied. While BAX was principally upregulated, striking similar were the notable changes regarding BCL-2 and BCL2L12 expression in our cellular system. Our findings indicate the growth suppressive effects of doxorubicin, oxaliplatin and methotrexate treatment on stomach carcinoma cells and the implication of BCL2L12, BAX, and BCL-2 expression profiles in the molecular signaling pathways triggered by chemotherapy.
Introduction
Gastric cancer, a malignancy of the digestive system, represents the fourth most common human neoplasia and it currently holds the second place in cancer-related deaths, worldwide.Citation1 Despite sophisticated surgical techniques and novel, efficacious chemotherapy schemes,Citation2,Citation3 gastric cancer continues to be a prevailing source of mortality, with the survival rates of the afflicted patients to be among the lowest in comparison with other types of cancer.Citation4 The dismal prognosis of stomach cancer is mostly attributed to its “silent” nature, an immediate result of which is the establishment of a clear diagnosis at advanced and often untreatable disease stages.Citation5 In such cases of unresectable and metastatic carcinomas, the outcome of cancer patients remains extremely poor and even total gastrectomy, the only curative approach providing the utmost survival advantage may prove to be inadequate.Citation6-Citation8
Even though gastric cancer has, for a very long time, been considered as a chemo-sensitive tumor, at present there has not been a definitive standard of treatment in the advanced and adjuvant settings, since management patterns vary worldwide and the survival gain from current multimodality regimens appears to have reached a plateau.Citation9,Citation10 Clinically useful chemotherapeutic compounds exert their broad spectrum anti-tumoral activity either directly by provoking DNA lesions and impeding processes vital for tumor cells’ growth or replication, such as blocking DNA, RNA, or protein synthesis or indirectly as a response to the stress caused by their interference with an intracellular metabolic pathway.Citation11,Citation12 The dual role of anti-cancer therapies lies in the potential of drugs to eradicate cancer cells and to overcome their tolerance by restoring any defects in the apoptotic signaling pathways.Citation12,Citation13 Therefore, the common denominator of all existing and novel treatment paradigms is apoptosis.
One of the most thoroughly described and well-recognized hallmarks of cancer is the evasion of apoptosis, a form of physiological and highly coordinated cell death with unique biochemical and morphological features that contributes to normal tissue homeostasis by eliminating malfunctioning cells.Citation14,Citation15 According to several reports, the deregulation of apoptotic mechanisms is directly associated with the development and progression of human malignancies from which gastric tumorigenesis is by no means an exception.Citation16,Citation17 Crucial mediators of the apoptotic response are the members of the BCL2 family of apoptosis-related genes, whose categorization as either pro-survival or pro-apoptotic, depends on their regulatory function, molecular structure and sub-cellular localization.Citation18
BCL2, a classical example of an anti-apoptotic gene that was first discovered as a proto-oncogene in non-Hodgkin B-cell lymphoma,Citation19 maps on the chromosomal locus 18q21.3. It encodes a transmembrane protein, mainly detected in the mitochondrial outer membrane, which inhibits apoptosis by hindering the mitochondrial disruption and the subsequent release of cytochrome c, an apoptogenic molecule indispensable for the activation of the caspase cascade through the formation of the apoptosome.Citation20-Citation22 On the other hand, BAX belongs to the BCL2 family subgroup of pro-apoptotic genes and is located at the genomic region 19q13.3–q13.4.Citation23 The activity of BAX cytosolic protein relies on its ability to oligomerize with other pro-apoptotic members of the family and integrate into the mitochondrial membrane upon apoptotic stimuli, facilitating in this way cytochrome c entrance to the cytoplasm. Therefore, BAX acts as a tumor suppressor, since its inactivation in numerous types of cancer is related to tumor progression by escaping apoptotic death.Citation24-Citation26 Alterations in the mRNA and protein expression patterns of BCL2 and BAX, usually reflecting different prognostic profiles for cancer patients, have been documented in human malignancies.Citation27-Citation29
A relative new addition to the BCL2 gene family is BCL2L12 that has been cloned from members of our group and resides at the chromosome 19q13.3–13.4.Citation30 BCL2L12 protein displays both nuclear and cytosolic localizationCitation31 and is highly expressed in a wide range of human tissues.Citation30 As far as its role in the apoptotic pathway is concerned, there are contradictory reports regarding its anti- or pro-apoptotic activity.Citation31-Citation34 In glioblastoma for example, it was observed that BCL2L12 not only blocks the post-mitochondrial apoptosis signaling by inhibiting the activation of two effector caspases, namely caspase-3 and -7,Citation32 but also it can interact with p53 in the nucleus and suppress p53-dependent transcription.Citation35 BCL2L12 has also been revealed to exert a pro-apoptotic function by sensitizing mouse embryonic fibroblasts to apoptotic stimuli induced by UV irradiationCitation34 or by promoting apoptosis in cisplatin-treated MDA-MB-231 breast carcinoma cells.Citation33 Furthermore, BCL2L12 was found to be differentially expressed at the mRNA level in a series of human malignancies with potential prognostic implications.Citation36-Citation38
In the present research work, the expression behavior of the apoptosis-associated genes BCL2L12, BAX, and BCL-2 was investigated and analyzed in the in vitro model system of stomach adenocarcinoma,Citation39 the AGS cells, after being subjected to the influence of the anti-neoplastic substances doxorubicin, oxaliplatin, or methotrexate. Our ultimate intent was the possible utilization of these functionally perplexing or distinct apoptotic gene members of the BCL-2 family as molecular indicators of prediction to chemotherapy in gastric cancer.
Results
Anti-tumor effect of doxorubicin, oxaliplatin, or methotrexate on the viability of human gastric adenocarcinoma cells
With the purpose of examining the response of the AGS stomach cancer cells to the cell death signals induced by anti-neoplastic agents with different mechanisms of action, these cells were exposed to a series of eight increasing concentrations of doxorubicin, oxaliplatin or methotrexate for up to three days during their exponential growth. In particular, the exposure time of the AGS cells to either doxorubicin (0–0.2 μΜ) or oxaliplatin (0–6.5 μΜ) lasted for 24, 36, 48, and 72 h, while in the case of methotrexate (0–100 μΜ), its relatively high cytotoxicity, allowed for incubation periods of 24, 36, and 48 h. By exploiting the use of the colorimetric MTT assay, the mean absorbance values measured, which were indicative of cells’ chemosensitivity to the employed anti-cancer substances, were directly proportional to the calculated viability rates of the treated-AGS population vs. the untreated control ().
Figure 1. Determination of stomach cancer cells’ viability (%) by conducting MTT tests up to 3 d following no treatment or in the presence of increasing concentrations of doxorubicin (A) or oxaliplatin (B) for 24, 36, 48, and 72 h or methotrexate (C) for 24, 36, and 48 h. Formazan release from untreated (control) cells is represented as viability set to 100%. The IC50 values for the drug-exposed AGS cells are shown in bold characters. Every point corresponds to the mean value of experiments performed in triplicates and repeated 3 times.
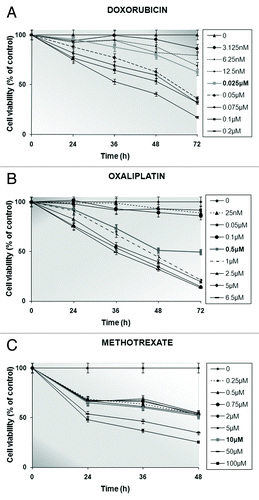
The principle of the semi-automated quantitative MTT test lies in the ability solely of the living cells to convert the tetrazolium salt to a blue formazan product, through their active mitochondrial dehydrogenases.Citation40 Based on that fact, as the number of living cells increases, the amount of formazan derivative per cell is also elevated proportionally. Consequently, the diminution in cells’ viability could be attributed to the anti-tumor effects of the individually administered chemotherapeutic drugs on the AGS cell culture for specifically chosen time intervals. As depicted in , the concentration and time dependence of the cytostatic action of doxorubicin () or oxaliplatin () was more apparent than that in the case of methotrexate treatment (). According to the calculated percentages of viable cells, the value of the cytotoxic index, which is capable of bringing about at least 50% inhibition of AGS cells’ survival (IC50), was 0.025 μM, 0.5 μM, and 10 μM for doxorubicin, oxaliplatin and methotrexate, respectively ().
Growth inhibitory effects of diverse anti-neoplastic compounds on AGS cells
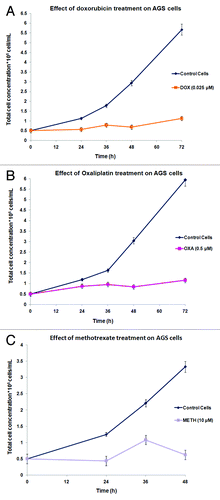
The anti-proliferative effects of the selected concentrations of doxorubicin, oxaliplatin, and methotrexate, on the AGS cancer cells, were determined with the construction of growth curves (). It is clearly illustrated that compared with the untreated (control) cells there is a drug- and also time-dependent growth suppression of the treated-AGS population. Consistent with this, the pattern of uninterrupted and continuous division of the control cells is severely disturbed once a sub-population of them was exposed to either of the pre-mentioned chemotherapeutic agents. Precisely, exposure to doxorubicin () resulted to a 5.04-fold drop in the multiplication rate of the treated cells in comparison to the untreated ones after about 72 h of treatment. Similar and more persistent was the growth inhibition of the oxaliplatin-treated AGS cells (), in which their propagation in culture was about 5.13 times lower than that of the control cells at 72 h. A steeper decrease in AGS proliferative capacity was observed particularly following methotrexate treatment (), since it appears that cell death is more probable on the treated cells from 36 h of incubation and afterwards. With the use of trypan blue dye method, the percentage of non-viable cells was found to be less than 15% (<15%) at about 72 h of AGS cells’ treatment with doxorubicin or oxaliplatin. These findings are suggestive of the limited toxicity of the dugs examined, possibly corresponding to low extent necrotic phenomena.
Figure 2. Anti-tumor effects of doxorubicin (A(, oxaliplatin (B), or methotrexate (C) on the proliferative capability of AGS cells. Results are expressed as total cell concentration of the drug-treated or untreated populations against incubation time periods. Each point represents the mean value of triplicates from 3 independent experiments.
The expression levels of BCL2L12, BAX, and BCL-2 genes are transcriptionally modulated by chemotherapy treatment in stomach cancer cells
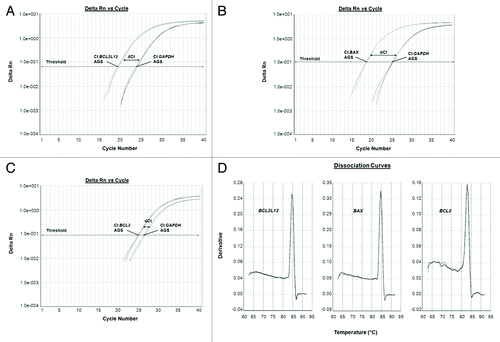
The mRNA levels of the apoptosis-related genes BCL2L12, BAX and BCL-2 were quantified through the ultra-sensitive and of great precision qPCR by using the SYBR® Green I chemical detection system. Following the completion of the amplification process, the values of the threshold cycles (Ct) for the endogenous control (GAPDH) and the target (BCL2L12, BAX, and BCL-2) genes were recorded. After that, the expression levels of each target gene were normalized to those of the reference gene, thus giving rise to dCt values for every drug-exposed or untreated AGS cells’ sample of all time periods studied. Representative examples of the amplification curves for BCL2L12, BAX, and BCL-2, as well as GAPDH PCR products are indicated in , where the dCt values are also labeled (). Verification of the formation of gene-specific PCR products and the complete absence of any primer-dimmers came from dissociation curve analyses, at the end of which a single peak is denoted per respective PCR products of interest ().
Figure 3. Representative examples of quantitative PCR reaction plots of the target genes BCL2L12 (A), BAX (B), and BCL-2 (C) for chemotherapy subjected AGS cells, accompanied by the dissociation curves of the corresponding PCR products (D). The reference gene (GAPDH) was amplified in parallel to the gene of interest, each time.
Taking into account that the Relative Quantification (RQ) units are deduced by applying the 2-ddCt numeral relation, the number of fold change in the BCL2L12, BAX and BCL-2 mRNA levels was calculated after comparison of the RQ units of the drug-exposed AGS cells (for every separate time point) to those of the untreated cells.Citation41 A thorough look at the expression profiles of the BCL-2 family members disclosed intriguing and contemporaneously remarkable alterations in the mRNA levels of BCL2L12, BAX, and BCL-2 after chemotherapy administration to gastric adenocarcinoma cells, in vitro ( and ) compared with the control cells.
Figure 4. Analysis of the transcriptional activity of BCL2L12, BAX, and BCL-2 genes in the AGS cells after being exposed to doxorubicin (A) or oxaliplatin (B) or methotrexate (C) for the indicated time intervals. Any modifications in the expression levels of BCL2L12, BAX, and BCL-2 transcripts in the treated cells compared with the untreated (control) cells are presented as fold change and have a columnar form.
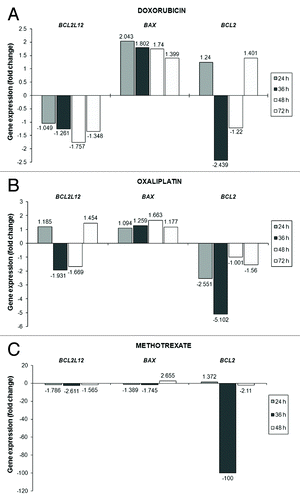
Table 1. Relative Quantification (RQ) units, 95% Confidence Intervals (95% CI) and fold change for each gene per different drug.
Exposure of AGS cells to doxorubicin (0.025 μM) did not result in notable changes in BCL2L12 transcript levels during the first 36 h of treatment. However, its levels descended by 1.76-fold at 48 h and maintained their falling pattern, though at a lower rate, until the end of the treatment (72 h). Entirely different was the behavior of the BAX gene following doxorubicin addition onto the AGS cells. In particular, the maximum peak (a 2.043-fold increase) of the upregulated BAX levels was observed at 24h and thereafter the increase followed a downward and time-dependent trend. The modifications in the case of BCL-2 started from a substantial drop in its levels at 36 h after treatment (by 2.439 times), then a minor decline was noticed, which was replaced by a slight (1.4-fold) increase in the next time point (72h) ( and ).
Cells’ incubation with 0.5 μM of oxaliplatin had as a consequence remarkable modulations in BCL2L12 profiles, which included an about 2-fold decrement in the gene’s levels at 36 h, followed by a lesser-degree drop (1.67-times) at 48 h of treatment. Interestingly, on the third day after the initial addition of oxaliplatin in cells’ growth media, a 1.45-fold increase in BCL2L12 expression was remarked. As regards BAX mRNA levels, the gradual increase in its levels from 24 h of treatment and onwards reached its peak (1.66-fold) on 48 h. Afterwards, in the final time interval examined (72 h), this gene’s levels were presented similar to those of the untreated (control) cells. The pattern of changes in BCL-2 expression of the oxaliplatin-treated gastric cancer cells in comparison to that of the control cells did not appear to be an unexpected finding. Specifically, the 2.55-fold notable reduction in BCL-2 levels from the first 24 h of incubation became even more enhanced (5.1-times decrease) in the next 12 h after treatment and eventually such a reduction was kept until the end of treatment, albeit at much lower extent ( and ).
The outcome of cells’ exposure to methotrexate (10 μM) resulted in striking fluctuations for all three genes of the BCL-2 family. Twenty-four hours soon after treatment, the initial important diminution in BCL2L12 transcript levels was greater in the following 12 h (2.6-fold decline), whereas by the end of treatment (48 h) it did not exceed the value of 1.57-fold decrease. Similar results, regarding BAX expression, indicated a slight (at 24h) and then a more apparent (at 36 h) change in its levels, although these were strongly augmented 2 d after treatment (2.66-times increase). In the first 24 h of methotrexate treatment, the BCL-2 gene was slightly upregulated, while the most dramatic negative modification of its mRNA levels was depicted at 36 h (100-fold decline) of cells’ incubation with this chemotherapeutic drug. By the end of the treatment procedure (48 h), the decrement in BCL-2 expression had become less severe (2.1-fold) in the drug-exposed cell population ( and ).
Discussion
The relative sensitivity of stomach carcinoma to chemotherapy has intensely spurred the research interest to the integration of anti-neoplastic drugs as essential components of comprehensive treatment for this malignancy.Citation3,Citation9 In spite of the efficacy of diverse chemical compounds (such as doxorubicin, oxaliplatin, and methotrexate) in treating gastric cancer,Citation42-Citation44 treatment responses to conventional chemotherapeutics exhibit high variability even within the same pathological stage and patients’ prognosis with advanced disease remains feeble.Citation3 These findings reveal that the choice of any chemotherapy regimen is essentially dependent upon each patient’s profile.Citation45 Consequently, any difficulties related to currently available therapies could be surmounted via the necessary exploration of gastric cancer-specific molecular indicators. Their future incorporation in clinical practice will be expected to assist in better forecasting response to chemotherapy and general monitoring of disease outcome.
In view of the fact that most anti-neoplastic drugs exert their anti-tumor action through the induction of apoptotic cell death programs, refractoriness of stomach carcinoma that may be attributed to its non-susceptibility to such mechanisms remains a hindrance toward its complete eradication. It has been quite understandable that any alterations in the apoptotic machinery probably influence drug sensitivity, thus compromising their competence to treat cancer. Therefore, reduced apoptosis or its tolerance, which favors cell proliferation and survival, plays a pivotal role in the initiation and progression of carcinogenesis.Citation13,Citation14 This is owed to the disturbed balance between cell division and death, a property characteristic of cancer cells whose expansion is enhanced once these become insensitive to the received death stimuli.Citation13 On the basis of our results, the differential chemosensitivity of AGS gastric adenocarcinoma cells to the employed anti-neoplastic substances doxorubicin, oxaliplatin and methotrexate is mirrored by the dose- and time-dependent decrease in their viability (). These findings lie in conformity with the considerably extenuated rates of cell proliferation as evidenced in . Consequently, the growth suppressive effects of the anti-tumor compounds combined with their limited (<15% at about 72 h of incubation) cytotoxicity, most likely entail the triggering of apoptosis signaling pathways on the treated population of the AGS cells.
There is no dispute, nowadays, that the BCL-2 family members of pro-survival and pro-apoptotic factors possess a prominent position as common targets for deregulation in several types of human cancer. The intricate interplay among the various members, through upregulation of the anti-apoptotic and mutations or defects in the pro-apoptotic genes, is thought to contribute essentially to tumor development, maintenance and resistance to therapy, by reducing the capability of cancer cells to enter apoptosis.Citation13,Citation46
It is of exceptional significance to note that the expression of the innumerable members of the BCL-2 family of apoptosis-associated genes is quite variable in different human cancers in which a general pattern of overexpression or downregulation of these genes cannot be stated explicitly. The founder of this family, the BCL-2 pro-survival gene, functions so as to block or delay apoptotic cell death.Citation20 Despite the so far meagerly determined mechanism responsible for BCL-2 upregulation in solid tumors, its prognostic value is debatable, since there is some evidence indicating that the elevated expression of BCL-2 mRNA or protein may be correlated with better overall survival,Citation29 poor prognosisCitation47,Citation48 or may have no effect.Citation49 The death repressor function of BCL-2 is counteracted by another BCL-2-related protein, BAX, which possesses a tumor suppressor activity by stimulating apoptosis.Citation26 Notwithstanding the variable expression of BAX in gastric carcinomas,Citation28 its status may be suggestive of its role being played in the development and evolution of this type of malignant tumors, since BAX-negativity has been related to shorter survival periods of patients surgically treated for their disease.Citation50 As regards the relatively novel member of the BCL-2 family, the BCL2L12 gene,Citation30 its peculiar attributes as either a pro-survivalCitation31,Citation32,Citation51 or pro-apoptoticCitation33,Citation34,Citation36-Citation38,Citation52 factor have been a pole of attraction for constant research endeavors. In fact, a series of data has demonstrated the usefulness of BCL2L12 expression in human solid tumors, including stomach adenocarcinomas, as a molecular indicator of favorableCitation36-Citation38,Citation52 or adverseCitation51 prognosis.
By exploiting the power of real-time PCR, we proceeded toward the examination and extensive analysis of BCL2L12, BAX and BCL-2 mRNA profiles following exposure of AGS cancer cells to broadly applied anti-cancer agents ( and ). Collectively, noticeable alterations were caused by the action of doxorubicin and methotrexate in the expression levels of BCL2L12. These were found to be similar to both drugs and to follow an overall declining pattern, but the differences among them were more intense in the case of methotrexate until 36 h of treatment than in that of doxorubicin. The results of cells’ exposure to oxaliplatin concerned important downregulation of BCL2L12 from 36 h until 48 h, which was replaced by the interesting finding of a slight increase in its levels at the end of the treatment procedure ( and ). In an earlier study from our lab, the usage of cisplatin, a second generation platinum analog, led to exactly the opposite fluctuations in BCL2L12 transcript levels at two time points (24h and 48h).Citation53 Moreover, the response of MCF-7Citation33,Citation54 and MDA-MB-231Citation33 to cisplatin generated comparable results with ours with respect to the expression of this gene. The time-dependent (up to 24 h) enhanced transcriptional activity of BCL2L12 upon incubation of MDA-MB-231 with cisplatin in conjunction with the overexpression of the corresponding protein promoted apoptosis induced by this particular drug.Citation33 On the contrary, in another mammary carcinoma cell line, BT-20, this gene’s levels did not present any detectable modulations under the influence of either doxorubicin or platinum compounds. When the same drugs were used in ovarian cancer cells, BCL2L12 expressional behavior was mostly reduced.Citation55 With the exception of the negatively variable BAX levels for up to 36h of methotrexate administration onto AGS cells, it is deduced that for doxorubicin and oxaliplatin treatments this gene’s profiles were substantially augmented. Subtle differences were observed between the findings of our study with oxaliplatin and those of another with cisplatin.Citation53 Increased cell death probably justifies the decrement in growth of different gastric cancer cell lines after the induction of BAX overexpression.Citation56 BAX profiles were unaffected, as a result of OVCAR-3 and BT-20 cancer cells’ response to carboplatin, cisplatin or doxorubicin addition, or they became under-expressed in doxorubicin-treated MCF-7 breast carcinoma cells.Citation54,Citation55 Such discrepancies could be explained on the context of concrete features of the cellular systems examined as well as by the discrete pathways that are induced as a consequence of the cytotoxic action of every different chemotherapeutic agent utilized. An overview of the BCL-2 alterations that is in general line with recent literature,Citation53-Citation55 included drastic (in methotrexate treatment) or great (for doxorubicin or oxaliplatin-treated AGS cells) reductions in its mRNA levels. However, some minor upregulations in BCL-2 transcriptional activity at particular time intervals of either doxorubicin or methotrexate exposure are not an unusual phenomenon, since even BCL-2 itself could ensure that cell death is executed in certain situations.Citation57,Citation58 The striking similarities in the expression levels of BCL2L12 and BCL-2 genes in our cellular system and under the precise conditions pursued, might attribute to BCL2L12 a potential anti-apoptotic roleCitation31,Citation32 without excluding, though, the possibility of apoptosis promotion as it can be valid for BCL-2.Citation57,Citation58 Although the present findings seem opposing to earlier data where the elevated expression of BCL2L12 in stomach cancer tumors of early TMN stages was associated with favorable patients’ prognosis, thereby suggesting for it a possible pro-apoptotic function, it is not a paradox for a gene to exhibit variable expression patterns between a cancer tissue and its corresponding cell type. This bimodal activity of BCL2L12, with its upregulation in well-differentiated mammary tumorsCitation38 and its under-expression in MCF-7 cancer cells after being exposed to doxorubicin or cisplatin treatment,Citation54 has also been indicated in the case of breast cancer. The heterogeneity of gastric tumors, the different endogenous need of stomach carcinoma cells for this particular gene, as well as the distinguished pathways of cellular demise that are signalized by each drug utilized might be explanations for the distinctively modified BCL2L12 levels in this type of cancer. Nonetheless, it is important to note that the differences in the mRNA expression of the studied genes do not necessarily reflect changes in their protein levels and therefore we cannot reliably describe their function in the apoptotic machinery.
As far as we know, this is the first study pointing out that doxorubicin, oxaliplatin or methotrexate drugs exert their anti-tumor effects on human gastric carcinoma cells by inhibiting their growth and the treated cells respond to the received cytotoxic signals by discretely modifying the transcriptional behavior of BCL2L12, BAX, and BCL-2 apoptosis-related genes. Our results support the concept of the possible exploitation of the differential profiles of these genes as emerging predictors of chemotherapy response in gastric cancer. In order to obtain a clear view of how the genes BCL2L12, BAX, and BCL-2 are involved in the apoptotic mechanisms that are activated subsequent to anti-cancer agent exposure of gastric cancer cells, it would be necessary to study their respective products with well-established indicators of apoptosis. Delineation of the biological role and the delicate and complex interactions of several BCL-2 family members in stomach carcinogenesis are grounds for optimism for more accurate and tailored therapeutic interventions.
Materials and Methods
Gastric cancer cells’ culture and treatment conditions
The propagation of the stomach carcinoma, AGS, cells was ensured in complete RPMI 1640 (PAA, R15–802) inside a 37 °C humidified incubator with balanced air and 5% (v/v) CO2. The culture medium was enriched with the constituents l-glutamine (0.3 g/L), NaHCO3 (0.85 g/L), fetal bovine serum (10% v/v), as well as the antibiotics streptomycin (0.1 g/L) and penicillin (100 KU/L). Grown in the form of an adherent monolayer for up to 3 d, in which complete confluence was not reached, the AGS cells either remained intact to proliferate continuously or were treated with increasing concentrations of chemotherapeutic agents for different time periods during their exponential phase. All dose-response cell suspensions, obtained by trypsinization with 0.05% (v/v) trypsin in 0.53 mM EDTA (Cambrex Bio Science, 091689149), were gathered in order to be further analyzed for the expression of the genes of interest.
Cell viability as measured with the MTT assay
The colorimetric 3-(4,5-dimethyl thiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) (Sigma Chemical Co., M2128-1G) assayCitation40 was employed so as to assess AGS cells’ chemosensitivity by determining their viability after anti-cancer drug administration. One hundred microliters of cancer cells’ suspension in complete culture medium (corresponding to the optimal seeding density of 0.5 × 105 cells/mL) was placed, in triplicates, in 96-well microtiter plates. The plates were returned to the incubator, in order for cell adherence to take place for 6 h at 37 °C. Several increasing concentrations of the anti-cancer substances doxorubicin (Ebewe Pharma), oxaliplatin (Sanofi-Aventis) and methotrexate (Lederle Pharmaceuticals) were added in the AGS cells, whose incubation lasted for 24, 36, 48 h and up to 72 h, respectively. After each time of exposure to either of these agents, 10 μL of the MTT stock solution in PBS (1×) (at a final concentration of 5 mg/mL) was added per well, in the presence of which the cells were further incubated at 37 °C for an additional 4 h. Afterwards, the culture supernatant was discarded and the formazan crystals, produced by the mitochondrial dehydrogenases of the living cells, were solubilized with 100 μL of 12.5% (w/v) sodium dodecyl sulfate (SDS) (Sigma Chemical Co., L4390–1KG) containing 45% (v/v) formamide (Merck KGaA, 344206-1L) in the dark at 37 °C overnight.
An ELISA multiwell reader was used to measure all absorbances at 545 nm with a reference wavelength of 690 nm. The controls of the experiments included wells containing media and cells (constituting the untreated cell population) and wells set up only with media (to check for nonspecific dye reduction). Repetition of the experiments took place three times, independently. Cell viability (percent) that was expressed as the ratio of the mean absorbance of drug-treated cells vs. that of the control (untreated) ones was calculated as follows:
Cell viability (%) = [mean Abs (drug-treated cells) / mean Abs (control-untreated cells)] × 100, where Abs: absorbance values
All subsequent cell work was based on the IC50 value for each drug tested, which is equivalent to at least 50% reduction in the absorbance of the control cells at the end of every time point of the treatment procedure.
Multiplication capability of the AGS cells and trypan blue staining
In the absence or presence of the chosen, with the MTT method, IC50-specific doses of the anti-tumoral compounds examined, the number of dividing gastric cancer cells mirrored their capability for proliferation. More specifically, a quantity (0.5 × 105 cells/mL) of log-phase growing cells was plated inside 75 mm2 tissue culture flasks (total volume of 20 mL in each), in triplicates. Soon after the 6 h incubation period for cell attachment to be completed, each drug, to which the cells were uninterruptedly exposed, was supplied for the pre-mentioned time intervals. 50 μL of the trypsinized and re-suspended in culture medium cell samples was diluted ten times in PBS (1×). From the diluted cell suspension, 18 μL was removed and thoroughly mixed with 2 μL of 0.4% (w/v) trypan blue dye (Sigma Chemical Co., T8154-100ML). When the mixture was allowed to stand for 5 min at room temperature, the reaction was developed and the non-viable cells, which absorb this stain, were visualized under an inverted optical microscope as distinctively colored blue.
With the aid of a hemocytometer, the total cell number and the number of trypan blue positively-stained cells were counted. The percentage of non-viable cells, in every sample, was calculated by applying the following arithmetic formula:
Trypan blue stained cells (%) = [total number of non-viable cells per mL / total number of cells per mL] × 100.
In the case of the proliferative potential of the treated or untreated AGS cells, the results were expressed as a function of total cell concentration plotted against different time periods.
Total RNA isolation and cDNA synthesis
Total RNA was extracted from both untreated and drug-treated AGS cells by dissolving the different time-point cell populations in TRI-Reagent (Ambion, AM9738) according to the manufacturer’s protocol. The isolated RNA pellet was subsequently solubilized in RNA storage solution (Ambion, AM7001) and stored at -80 °C. The determination of total RNA concentration was accomplished spectrophotometrically at 260 nm, while its purity was assessed by calculating the absorbance ratio at 260 and 280 nm.
Following the quantitative and qualitative control of the extracted RNA, the production of first-strand cDNA from 2 μg of total RNA was performed via reverse transcription using oligo-dT primers. The final volume of the reaction was 20 μL and the enzymes used for this procedure were the M-MuLV Reverse Transcriptase (Finnzymes, F-572S/L) and the Human Placental RNase Inhibitor (HT Biotechnology Ltd., RI01a).
BCL-2-specific polymerase chain reaction (PCR)
A pre-amplification PCR-based step was performed for BCL-2 in a thermal cycler (Labnet International Inc., PO Box 841, Woodbridge NJ 07095, USA). The BCL-2 specific primers used in the regular PCR reaction were one forward 5′-TTTGAGTTCG GTGGGGTCAT-3′ and one reverse primer 5′-TGACTTCACT TGTGGCCCAG-3′, giving rise to an amplicon of 275 bp. The reaction mixture contained 1 μL of cDNA, 1.5 mM MgCl2, 200 μΜ dNTPs, 1 μΜ of each primer, 1.25 U of Taq DNA polymerase and 1 × PCR buffer, pH 8.8 (HyTest Ltd., 7T1). The final volume of the mixture was adjusted to 50 μL by adding nuclease-free water. The thermal cycling profile applied to this reaction consisted of a 2 min preliminary denaturation step at 95 °C, followed by 20 cycles of denaturation at 95 °C for 30 sec, primer annealing at 60 °C for 30 sec and elongation by Taq polymerase at 72 °C for 1 min. The BCL-2 PCR product derived from this procedure was then subjected to a 10-fold dilution and used as a template in the subsequent real-time PCR experiments.
Quantitative real-time PCR (qPCR)
The quantitative mRNA expression analysis of BCL2L12, BAX, and BCL-2 apoptosis-related genes was made feasible by applying the real-time PCR methodology of excellent sensitivity and accuracy, developed with the use of SYBR® Green I fluorescent dye chemistry. A pair of gene-specific primers was designed and synthesized for every gene under study, as well as for GAPDH, taking into account their published mRNA reference sequences on the NCBI database (GenBank accession numbers NM_000633.2, ΝΜ_138761.3, ΝΜ_138639.1 and NM_002046.4 for BCL-2, BAX, BCL2L12, and GAPDH, respectively). GAPDH, the gene that codes for glyceraldehyde-3-phosphate dehydrogenase, played the central role as the reference gene in all qPCR experiments. The oligonucleotide sequences of the used primers for BCL-2 (forward 5′-TCGCCCTGTG GATGACTGA-3′ and reverse 5′-CAGAGACAGC CAGGAGAAAT CA-3′), BAX (forward 5′-TGGCAGCTGA CATGTTTTCT GAC-3′ and reverse 5′-TCACCCAACC ACCCTGGTCT T-3′), BCL2L12 (forward 5′-CCCTCGGCCT TGCTCTCT-3′ and reverse 5′-TCCGCAGTAT GGCTTCCTTC-3′) and GAPDH (forward 5′-CCTCCCGCTT CGCTCTCT-3′ and reverse 5′-CCGTTGACTC CGACCTTCAC-3′), gave rise to PCR products of 134, 195, 182, and 116 bp, respectively.
The qPCR reaction for each sample was performed on an ABI PrismTM 7500 Thermal Cycler (Applied Biosystems) in triplicates, so as to address any data reproducibility issues. The 10 μL reaction mixture consisted of 0.5 μL of cDNA (for BAX, BCL2L12 and GAPDH) or 10-fold diluted BCL-2 PCR product, 50 nM of gene-specific primers and 5 μL Power SYBR® Green I PCR Master Mix (Applied Biosystems, 4367659), containing AmpliTaq Gold DNA polymerase. The thermal conditions included an initial denaturation and activation of hot-start DNA polymerase step at 95 °C for 10 min, after which the reaction mixture was subjected to 40 cycles of denaturation at 95 °C for 15 sec and primer annealing and extension at 60 °C for 1 min. After the successful completion of the amplification process, a dissociation curve analysis was conducted in order to distinguish the amplified sequences of interest from any non-specific ones or primer dimmers, by comparing the melting temperatures (Tm) of the formed PCR amplicons.
The relative changes in the transcriptional levels of the apoptosis-related genes BCL2L12, BAX, and BCL-2 between the untreated AGS cells and the same cells treated with the examined anti-neoplastic agents were measured using the comparative Ct method (2-ddCt).Citation41 This relative quantification method requires the use of a reference gene for the normalization of the results, which, in our case, was GAPDH and a calibrator sample, the gene expression levels of which are designated as reference points for the following comparisons between different samples. Another prerequisite of this method is that the qPCR amplification efficiencies of target and reference genes must be approximately equal. This condition was verified by conducting a validation experiment as described by Korbakis et al.Citation53 The interpretation of our data was achieved using the arbitrary RQ unit (Relative Quantification unit), which is calculated by the arithmetic equation 2-ddCt and represents the normalized-to-GAPDH amounts of BCL2L12, BAX, or BCL-2 mRNA levels of a given time-point anti-cancer drug-exposed AGS cell sample, relatively to the normalized gene expression of the untreated AGS cells, which served as the calibrator of the study. In the case of control (untreated) cells, the average expression from all time points was used, since similar expression levels for all target genes were observed in this cell population. The relevant equations that were utilized for this purpose were the following:
RQ units = 2-ddCt and
ddCt = [(Ct BFG – Ct GAPDH) sample – (Ct BFG – Ct GAPDH) calibrator], where BFG (BCL-2 Family Genes) denotes BCL2L12, BAX or BCL-2.
| Abbreviations: | ||
| BAX | = | BCL2-asociated X protein |
| BCL-2 | = | B-cell CLL/ lymphoma 2 |
| BCL2L12 | = | BCL2-like 12 |
| bp | = | base pairs |
| cDNA | = | complementary DNA |
| Ct | = | threshold cycle |
| GAPDH | = | glyceraldehyde-3-phosphate dehydrogenase |
| PBS | = | phosphate buffered saline |
| PCR | = | polymerase chain reaction |
| qPCR | = | quantitative real-time PCR |
| RPMI | = | Roswell Park Memorial Institute |
| RQ units | = | relative quantification units |
Acknowledgments
This work was supported with funds from the European Community through the INsPiRE project (EU-FP7-REGPOT-2011-1; proposal No. 284460). It was also co-funded by the Hellenic Society of Medical Oncology.
Disclosure of Potential Conflicts of Interest
No potential conflict of interest was disclosed.
References
- Bray F, Ren JS, Masuyer E, Ferlay J. Global estimates of cancer prevalence for 27 sites in the adult population in 2008. Int J Cancer 2013; 132:1133 - 45; http://dx.doi.org/10.1002/ijc.27711; PMID: 22752881
- Hohenberger P, Gretschel S. Gastric cancer. Lancet 2003; 362:305 - 15; http://dx.doi.org/10.1016/S0140-6736(03)13975-X; PMID: 12892963
- Lee JH, Kim KM, Cheong JH, Noh SH. Current management and future strategies of gastric cancer. Yonsei Med J 2012; 53:248 - 57; http://dx.doi.org/10.3349/ymj.2012.53.2.248; PMID: 22318810
- Jackson C, Cunningham D, Oliveira J, ESMO Guidelines Working Group. Gastric cancer: ESMO clinical recommendations for diagnosis, treatment and follow-up. Ann Oncol 2009; 20:Suppl 4 34 - 6; http://dx.doi.org/10.1093/annonc/mdp122; PMID: 19454457
- Maconi G, Manes G, Porro GB. Role of symptoms in diagnosis and outcome of gastric cancer. World J Gastroenterol 2008; 14:1149 - 55; http://dx.doi.org/10.3748/wjg.14.1149; PMID: 18300338
- Gallo A, Cha C. Updates on esophageal and gastric cancers. World J Gastroenterol 2006; 12:3237 - 42; PMID: 16718845
- Jeong SH, Han JH, Kim JH, Ahn MS, Hwang YH, Lee HW, et al. Bax predicts outcome in gastric cancer patients treated with 5-fluorouracil, leucovorin, and oxaliplatin palliative chemotherapy. Dig Dis Sci 2011; 56:131 - 8; http://dx.doi.org/10.1007/s10620-010-1280-8; PMID: 20503071
- Rivera F, Vega-Villegas ME, López-Brea MF. Chemotherapy of advanced gastric cancer. Cancer Treat Rev 2007; 33:315 - 24; http://dx.doi.org/10.1016/j.ctrv.2007.01.004; PMID: 17376598
- Sastre J, Garcia-Saenz JA, Diaz-Rubio E. Chemotherapy for gastric cancer. World J Gastroenterol 2006; 12:204 - 13; PMID: 16482619
- Wagner AD, Grothe W, Haerting J, Kleber G, Grothey A, Fleig WE. Chemotherapy in advanced gastric cancer: a systematic review and meta-analysis based on aggregate data. J Clin Oncol 2006; 24:2903 - 9; http://dx.doi.org/10.1200/JCO.2005.05.0245; PMID: 16782930
- Brown JM, Wouters BG. Apoptosis, p53, and tumor cell sensitivity to anticancer agents. Cancer Res 1999; 59:1391 - 9; PMID: 10197600
- Gerl R, Vaux DL. Apoptosis in the development and treatment of cancer. Carcinogenesis 2005; 26:263 - 70; http://dx.doi.org/10.1093/carcin/bgh283; PMID: 15375012
- Wong RS. Apoptosis in cancer: from pathogenesis to treatment. Journal of experimental & clinical cancer research. CR (East Lansing, Mich) 2011; 30:87
- Hanahan D, Weinberg RA. The hallmarks of cancer. Cell 2000; 100:57 - 70; http://dx.doi.org/10.1016/S0092-8674(00)81683-9; PMID: 10647931
- Saikumar P, Dong Z, Mikhailov V, Denton M, Weinberg JM, Venkatachalam MA. Apoptosis: definition, mechanisms, and relevance to disease. Am J Med 1999; 107:489 - 506; http://dx.doi.org/10.1016/S0002-9343(99)00259-4; PMID: 10569305
- Kuchino Y, Kitanaka C. Apoptosis in cancer. Hum Cell 1996; 9:223 - 8; PMID: 9183653
- Nasif WA, El-Emshaty HM, Lotfy M, Zalata K, El-Hak NG. Apoptosis dysregulation in human gastric carcinomas: relationship to anti- and pro-apoptotic protein expression. Asian Pac J Cancer Prev 2007; 8:45 - 50; PMID: 17477770
- Thomadaki H, Scorilas A. BCL2 family of apoptosis-related genes: functions and clinical implications in cancer. Crit Rev Clin Lab Sci 2006; 43:1 - 67; http://dx.doi.org/10.1080/10408360500295626; PMID: 16531274
- Tsujimoto Y, Finger LR, Yunis J, Nowell PC, Croce CM. Cloning of the chromosome breakpoint of neoplastic B cells with the t(14;18) chromosome translocation. Science 1984; 226:1097 - 9; http://dx.doi.org/10.1126/science.6093263; PMID: 6093263
- Hockenbery D, Nuñez G, Milliman C, Schreiber RD, Korsmeyer SJ. Bcl-2 is an inner mitochondrial membrane protein that blocks programmed cell death. Nature 1990; 348:334 - 6; http://dx.doi.org/10.1038/348334a0; PMID: 2250705
- Tzifi F, Economopoulou C, Gourgiotis D, Ardavanis A, Papageorgiou S, Scorilas A. The Role of BCL2 Family of Apoptosis Regulator Proteins in Acute and Chronic Leukemias. Adv Hematol 2012; 2012:524308; http://dx.doi.org/10.1155/2012/524308; PMID: 21941553
- Yang J, Liu X, Bhalla K, Kim CN, Ibrado AM, Cai J, et al. Prevention of apoptosis by Bcl-2: release of cytochrome c from mitochondria blocked. Science 1997; 275:1129 - 32; http://dx.doi.org/10.1126/science.275.5303.1129; PMID: 9027314
- Apte SS, Mattei MG, Olsen BR. Mapping of the human BAX gene to chromosome 19q13.3-q13.4 and isolation of a novel alternatively spliced transcript, BAX delta. Genomics 1995; 26:592 - 4; http://dx.doi.org/10.1016/0888-7543(95)80180-T; PMID: 7607685
- Green DR, Reed JC. Mitochondria and apoptosis. Science 1998; 281:1309 - 12; http://dx.doi.org/10.1126/science.281.5381.1309; PMID: 9721092
- Gross A, Jockel J, Wei MC, Korsmeyer SJ. Enforced dimerization of BAX results in its translocation, mitochondrial dysfunction and apoptosis. EMBO J 1998; 17:3878 - 85; http://dx.doi.org/10.1093/emboj/17.14.3878; PMID: 9670005
- Yin C, Knudson CM, Korsmeyer SJ, Van Dyke T. Bax suppresses tumorigenesis and stimulates apoptosis in vivo. Nature 1997; 385:637 - 40; http://dx.doi.org/10.1038/385637a0; PMID: 9024662
- Jeong SH, Lee HW, Han JH, Kang SY, Choi JH, Jung YM, et al. Low expression of Bax predicts poor prognosis in resected non-small cell lung cancer patients with non-squamous histology. Jpn J Clin Oncol 2008; 38:661 - 9; http://dx.doi.org/10.1093/jjco/hyn089; PMID: 18772168
- Krajewska M, Fenoglio-Preiser CM, Krajewski S, Song K, Macdonald JS, Stemmerman G, et al. Immunohistochemical analysis of Bcl-2 family proteins in adenocarcinomas of the stomach. Am J Pathol 1996; 149:1449 - 57; PMID: 8909234
- Laudanski J, Chyczewski L, Niklińska WE, Kretowska M, Furman M, Sawicki B, et al. Expression of bcl-2 protein in non-small cell lung cancer: correlation with clinicopathology and patient survival. Neoplasma 1999; 46:25 - 30; PMID: 10355530
- Scorilas A, Kyriakopoulou L, Yousef GM, Ashworth LK, Kwamie A, Diamandis EP. Molecular cloning, physical mapping, and expression analysis of a novel gene, BCL2L12, encoding a proline-rich protein with a highly conserved BH2 domain of the Bcl-2 family. Genomics 2001; 72:217 - 21; http://dx.doi.org/10.1006/geno.2000.6455; PMID: 11401436
- Stegh AH, Kim H, Bachoo RM, Forloney KL, Zhang J, Schulze H, et al. Bcl2L12 inhibits post-mitochondrial apoptosis signaling in glioblastoma. Genes Dev 2007; 21:98 - 111; http://dx.doi.org/10.1101/gad.1480007; PMID: 17210792
- Stegh AH, Kesari S, Mahoney JE, Jenq HT, Forloney KL, Protopopov A, et al. Bcl2L12-mediated inhibition of effector caspase-3 and caspase-7 via distinct mechanisms in glioblastoma. Proc Natl Acad Sci U S A 2008; 105:10703 - 8; http://dx.doi.org/10.1073/pnas.0712034105; PMID: 18669646
- Hong Y, Yang J, Wu W, Wang W, Kong X, Wang Y, et al. Knockdown of BCL2L12 leads to cisplatin resistance in MDA-MB-231 breast cancer cells. Biochim Biophys Acta 2008; 1782:649 - 57; http://dx.doi.org/10.1016/j.bbadis.2008.09.008; PMID: 18930135
- Nakajima A, Nishimura K, Nakaima Y, Oh T, Noguchi S, Taniguchi T, et al. Cell type-dependent proapoptotic role of Bcl2L12 revealed by a mutation concomitant with the disruption of the juxtaposed Irf3 gene. Proc Natl Acad Sci U S A 2009; 106:12448 - 52; http://dx.doi.org/10.1073/pnas.0905702106; PMID: 19617565
- Stegh AH, Brennan C, Mahoney JA, Forloney KL, Jenq HT, Luciano JP, et al. Glioma oncoprotein Bcl2L12 inhibits the p53 tumor suppressor. Genes Dev 2010; 24:2194 - 204; http://dx.doi.org/10.1101/gad.1924710; PMID: 20837658
- Florou D, Papadopoulos IN, Scorilas A. Molecular analysis and prognostic impact of the novel apoptotic gene BCL2L12 in gastric cancer. Biochem Biophys Res Commun 2010; 391:214 - 8; http://dx.doi.org/10.1016/j.bbrc.2009.11.034; PMID: 19903463
- Kontos CK, Papadopoulos IN, Scorilas A. Quantitative expression analysis and prognostic significance of the novel apoptosis-related gene BCL2L12 in colon cancer. Biol Chem 2008; 389:1467 - 75; http://dx.doi.org/10.1515/BC.2008.173; PMID: 18844453
- Talieri M, Diamandis EP, Katsaros N, Gourgiotis D, Scorilas A. Expression of BCL2L12, a new member of apoptosis-related genes, in breast tumors. Thromb Haemost 2003; 89:1081 - 8; PMID: 12783122
- Barranco SC, Townsend CM Jr., Casartelli C, Macik BG, Burger NL, Boerwinkle WR, et al. Establishment and characterization of an in vitro model system for human adenocarcinoma of the stomach. Cancer Res 1983; 43:1703 - 9; PMID: 6831414
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods 1983; 65:55 - 63; http://dx.doi.org/10.1016/0022-1759(83)90303-4; PMID: 6606682
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001; 25:402 - 8; http://dx.doi.org/10.1006/meth.2001.1262; PMID: 11846609
- Imazawa M, Kojima T, Boku N, Onozawa Y, Hironaka S, Fukutomi A, et al. Efficacy of sequential methotrexate and 5-fluorouracil (MTX/5FU) in improving oral intake in patients with advanced gastric cancer with severe peritoneal dissemination. Gastric Cancer 2009; 12:153 - 7; http://dx.doi.org/10.1007/s10120-009-0517-8; PMID: 19890695
- Kim HJ, Eun JY, Jeon YW, Yun J, Kim KH, Kim SH, et al. Efficacy and safety of oxaliplatin, 5-Fluorouracil, and folinic Acid combination chemotherapy as first-line treatment in metastatic or recurrent gastric cancer. Cancer Res Treat 2011; 43:154 - 9; http://dx.doi.org/10.4143/crt.2011.43.3.154; PMID: 22022292
- Kim SY, Park HC, Yoon C, Yoon HJ, Choi YM, Cho KS. OK-432 and 5-fluorouracil, doxorubicin, and mitomycin C (FAM-P) versus FAM chemotherapy in patients with curatively resected gastric carcinoma: a randomized Phase III trial. Cancer 1998; 83:2054 - 9; http://dx.doi.org/10.1002/(SICI)1097-0142(19981115)83:10<2054::AID-CNCR2>3.0.CO;2-1; PMID: 9827708
- Viudez-Berral A, Miranda-Murua C, Arias-de-la-Vega F, Hernández-García I, Artajona-Rosino A, Díaz-de-Liaño A, et al. Current management of gastric cancer. Rev Esp Enferm Dig 2012; 104:134 - 41; http://dx.doi.org/10.4321/S1130-01082012000300006; PMID: 22449155
- Adams JM, Cory S. The Bcl-2 apoptotic switch in cancer development and therapy. Oncogene 2007; 26:1324 - 37; http://dx.doi.org/10.1038/sj.onc.1210220; PMID: 17322918
- Fendri A, Kontos CK, Khabir A, Mokdad-Gargouri R, Ardavanis A, Scorilas A. Quantitative analysis of BCL2 mRNA expression in nasopharyngeal carcinoma: an unfavorable and independent prognostic factor. Tumour Biol 2010; 31:391 - 9; http://dx.doi.org/10.1007/s13277-010-0047-3; PMID: 20514538
- McDonnell TJ, Troncoso P, Brisbay SM, Logothetis C, Chung LW, Hsieh JT, et al. Expression of the protooncogene bcl-2 in the prostate and its association with emergence of androgen-independent prostate cancer. Cancer Res 1992; 52:6940 - 4; PMID: 1458483
- Lee HK, Lee HS, Yang HK, Kim WH, Lee KU, Choe KJ, et al. Prognostic significance of Bcl-2 and p53 expression in gastric cancer. Int J Colorectal Dis 2003; 18:518 - 25; http://dx.doi.org/10.1007/s00384-003-0491-2; PMID: 12811476
- Anagnostopoulos GK, Stefanou D, Arkoumani E, Chalkley L, Karagiannis J, Paraskeva K, et al. Expression of Bax protein in gastric carcinomas. A clinicopathological and immunohistochemical study. Acta Gastroenterol Belg 2007; 70:285 - 9; PMID: 18074738
- Fendri A, Kontos CK, Khabir A, Mokdad-Gargouri R, Scorilas A. BCL2L12 is a novel biomarker for the prediction of short-term relapse in nasopharyngeal carcinoma. Mol Med 2011; 17:163 - 71; http://dx.doi.org/10.2119/molmed.2010.00056; PMID: 21152697
- Thomadaki H, Talieri M, Scorilas A. Prognostic value of the apoptosis related genes BCL2 and BCL2L12 in breast cancer. Cancer Lett 2007; 247:48 - 55; http://dx.doi.org/10.1016/j.canlet.2006.03.016; PMID: 16647810
- Korbakis D, Scorilas A. Quantitative expression analysis of the apoptosis-related genes BCL2, BAX and BCL2L12 in gastric adenocarcinoma cells following treatment with the anticancer drugs cisplatin, etoposide and taxol. Tumour Biol 2012; 33:865 - 75; http://dx.doi.org/10.1007/s13277-011-0313-z; PMID: 22238053
- Thomadaki H, Scorilas A. Breast cancer cells response to the antineoplastic agents cisplatin, carboplatin, and doxorubicin at the mRNA expression levels of distinct apoptosis-related genes, including the new member, BCL2L12. Ann N Y Acad Sci 2007; 1095:35 - 44; http://dx.doi.org/10.1196/annals.1397.005; PMID: 17404015
- Thomadaki H, Scorilas A. Molecular profile of breast versus ovarian cancer cells in response to treatment with the anticancer drugs cisplatin, carboplatin, doxorubicin, etoposide and taxol. Biol Chem 2008; 389:1427 - 34; http://dx.doi.org/10.1515/BC.2008.161; PMID: 18783338
- Komatsu K, Suzuki S, Shimosegawa T, Miyazaki JI, Toyota T. Cre-loxP-mediated bax gene activation reduces growth rate and increases sensitivity to chemotherapeutic agents in human gastric cancer cells. Cancer Gene Ther 2000; 7:885 - 92; http://dx.doi.org/10.1038/sj.cgt.7700181; PMID: 10880019
- Cheng EH, Kirsch DG, Clem RJ, Ravi R, Kastan MB, Bedi A, et al. Conversion of Bcl-2 to a Bax-like death effector by caspases. Science 1997; 278:1966 - 8; http://dx.doi.org/10.1126/science.278.5345.1966; PMID: 9395403
- Wang NS, Unkila MT, Reineks EZ, Distelhorst CW. Transient expression of wild-type or mitochondrially targeted Bcl-2 induces apoptosis, whereas transient expression of endoplasmic reticulum-targeted Bcl-2 is protective against Bax-induced cell death. J Biol Chem 2001; 276:44117 - 28; http://dx.doi.org/10.1074/jbc.M101958200; PMID: 11546793