Abstract
This phase 2 study assessed PF-3512676 plus erlotinib in patients with epidermal growth factor receptor-positive advanced non-small cell lung cancer after prior chemotherapy failure. Patients were randomized 1:1 to PF-3512676 (0.20 mg/kg injected subcutaneously once weekly) plus erlotinib (150 mg daily) or erlotinib alone. The primary objective was to estimate progression-free survival (PFS). Patients received PF-3512676 plus erlotinib (n = 18) or erlotinib alone (n = 21). The study was halted because an unplanned interim analysis indicated that large improvement in PFS with addition of PF-3512676 would be unlikely. In the PF-3512676-plus-erlotinib and erlotinib-alone arms, median PFS was 1.6 and 1.7 mo (hazard ratio, 1.00; 95% confidence interval, 0.5–2.0; P = 0.9335), respectively. Salient grade ≥ 3 adverse events in PF-3512676-plus-erlotinib and erlotinib-alone arms were diarrhea (5/0), dyspnea (5/6), fatigue (4/1), other flu-like symptoms (2/0), anemia (2/1), and lymphocytopenia (based on laboratory values, 1/4). Adding PF-3512676 to erlotinib did not show potential for increased progression-free survival over erlotinib alone in patients with advanced recurrent epidermal growth factor receptor-positive non-small cell lung cancer.
Introduction
Erlotinib (Tarceva®; Genentech) is a small-molecule inhibitor of epidermal growth factor receptor (EGFR) tyrosine kinase that is approved as a treatment for patients with advanced non-small cell lung cancer (NSCLC) who have failed prior chemotherapy. A phase 3 study (BR.21) in patients with NSCLC who had received 1 or 2 prior chemotherapy regimens demonstrated that erlotinib monotherapy prolonged both progression-free survival (PFS; 2.2 vs. 1.8 mo; P < 0.001) and overall survival (OS; 6.7 vs. 4.7 mo; P < 0.001) and improved the objective response rate (8.9% vs. < 1.0%; P < 0.001) compared with placebo.Citation1
Although the approval of erlotinib has provided a new treatment option with improved survival for patients with NSCLC who have failed prior chemotherapy, their overall prognosis and survival remain poor. Efforts to combine EGFR inhibitors with standard chemotherapy have not resulted in any improvement in efficacy in unselected patients with advanced NSCLC. Identifying activating mutations in the EGFR tyrosine kinase domain has led to preferential and predictive activity with EGFR tyrosine kinase inhibitors. However, many patients are still refractory to treatment, and most eventually develop resistance through a variety of mechanisms, including secondary mutation in exon 20 (T790M) of EGFR leading to a structural change that alters the binding pocket for the tyrosine kinase inhibitors or activation of alternate cell signaling through the c-Met pathway.Citation2
PF-3512676 (CPG 7909; Pfizer Inc.) is an oligodeoxynucleotide capable of binding specifically to the Toll-like receptor-9 (TLR9) and triggering innate and adaptive immune responses.Citation3,Citation4 It is possible that this enhanced immune activation may promote an antitumor immune response against cancer. Antitumor activity of single-agent PF-3512676 has been demonstrated in phase 1 and/or 2 studies in patients with melanoma,Citation5 cutaneous T-cell lymphoma,Citation6 and renal cell carcinoma.Citation7 Objective response rates after treatment with single-agent PF-3512676 have ranged from 5% to 32%.Citation5-Citation7 PF-3512676 also demonstrated potential antitumor activity in a phase 1 study of its combination with rituximab in patients with non-Hodgkin lymphomaCitation8 and in a phase 2 study in combination with chemotherapy in first-line treatment of patients with NSCLC.Citation9 When chemotherapy-naive patients with stage IIIB or IV NSCLC were treated with PF-3512676 0.2 mg/kg in combination with taxane/platinum chemotherapy (n = 74) or chemotherapy alone (n = 37), a higher proportion of patients in the PF-3512676 arm had an objective response to treatment (38% vs. 19%; P = 0.043), and median survival favored patients treated with PF-3512676 (12.3 vs. 6.8 mo for chemotherapy alone, P = 0.1876).Citation9 Common adverse events (AEs) associated with PF-3512676 were mild to moderate injection-site reactions and flu-like symptoms and occasional grade 3 or 4 hematologic AEs. Unfortunately, two phase 3 studies demonstrated that adding PF-3512676 to chemotherapy failed to provide added clinical benefit in chemotherapy-naive patients with NSCLC.Citation10,Citation11
PF-3512676 enhances immune system activation in multiple tumor types. It was hypothesized that the combination of immune activation with PF-3512676 and erlotinib, which does not suppress the immune system, might potentially provide additional benefit in patients with NSCLC. Because the safety profiles of PF-3512676 and erlotinib seemed to be non-overlapping, it was also hypothesized that the combination would be well tolerated. Therefore, the present phase 2 study was conducted to estimate the improvement in PFS by adding PF-3512676 to erlotinib over erlotinib alone while maintaining tolerability in second-line treatment of patients with EGFR-positive advanced NSCLC.
Results
Patient demographics and baseline characteristics
This study was conducted at 16 centers in the United States between August 2006 and August 2010. Forty-three patients were randomized to treatment with PF-3512676 plus erlotinib (n = 21) or erlotinib alone (n = 22). Of these, 39 received assigned treatment with PF-3512676 plus erlotinib (n = 18) or erlotinib alone (n = 21); 4 patients were randomized but not treated. Demographic data were well balanced between treatment arms ().Citation12 There were 22 men and 21 women in the study, ranging from 44 to 81 y of age. Most patients (39 [91%]) had good (Eastern Cooperative Oncology Group [ECOG] 0 or 1) performance status. Of the 43 randomized patients, 38 (88%) had been smokers and 27 (63%) had adenocarcinoma. Per inclusion criteria, all patients had received at least one prior chemotherapy regimen and 12 (30%) had received more than one regimen. Also per inclusion criteria, all patients were EGFR-positive; several patients also had EGFR mutations or amplifications ().Citation12
Table 1. Patient demographics and baseline disease characteristics
In the combination arm, 18 treated patients received PF-3512676 for a median of seven weeks (range, 2−37 weeks) and erlotinib for a median of six weeks (range, 1−81 weeks). Patients in the monotherapy arm received erlotinib for a median of seven weeks (range, 0−29 weeks).
Efficacy
An interim analysis was performed in June 2009 to evaluate the risk–benefit ratio for the patients on study. Study enrollment was halted because available efficacy data indicated that a large improvement in PFS with the addition of PF-3512676 was unlikely even if additional patients were to be enrolled.
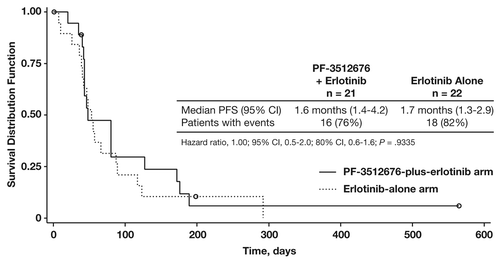
In the PF-3512676-plus-erlotinib and the erlotinib-alone arms, median PFS was 1.6 mo (95% confidence interval [CI], 1.4−4.2 mo) and 1.7 mo (95% CI, 1.3−2.9 mo), respectively (hazard ratio [HR] = 1.00; 95% CI, 0.5–2.0; P = 0.9335) ().Citation12 Sixteen patients (76%) in the PF-3512676-plus-erlotinib arm and 18 patients (82%) in the erlotinib-alone arm had PFS events.
Figure 1. Progression-free survival (PFS). Abbreviation: CI, confidence interval. Reprinted with permission from Belani et al.Citation12
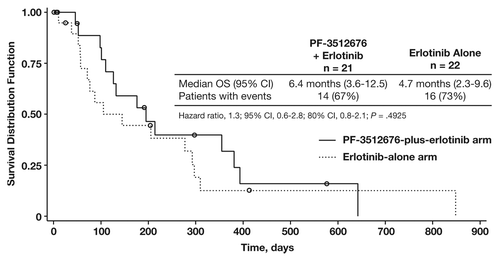
Kaplan-Meier estimates of median OS in the PF-3512676-plus-erlotinib arm and erlotinib-alone arms were 6.4 mo (95% CI, 3.6−12.5 mo) and 4.7 mo (95% CI, 2.3−9.6 mo), respectively (HR = 1.3; 95% CI, 0.6–2.8; P = 0.4925) ().Citation12 More than half of the patients in each arm had died at the time of final survival analysis (n = 14 [67%] and n = 16 [73%], respectively).
Figure 2. Overall survival (OS). Abbreviation: CI, confidence interval. Reprinted with permission from Belani et al.Citation12
There were two partial responses (response rate 10%) and 2 patients with stable disease in the PF-3512676-plus-erlotinib arm; there was one partial response (5%) and three patients with stable disease in the erlotinib-alone arm. Among 13 patients in the PF-3512676-plus-erlotinib arm with tumor samples at baseline, four had EGFR mutations and two (both with mutations in exon 19: L747_P753 Del 7 INSQ in one patient and E746_A750 Del 5 in the other patient) of these four went on to have partial responses. In the erlotinib-alone arm, of the 16 available samples, two were positive for EGFR mutations and one of these (patient with mutation in exon 21, L858R) had a partial response.
Safety
All treated patients (n = 39) reported AEs; more than half of the patients in each arm [12 (67%) and 13 (62%) patients in the PF-3512676-plus-erlotinib arm and erlotinib-alone arm, respectively] reported grade 3 or 4 AEs of any causality. The most frequently reported AEs of any causality were diarrhea, fatigue, decreased appetite and rash ().Citation12 Salient grade ≥ 3 AEs reported in the PF-3512676-plus-erlotinib/erlotinib-alone arms were diarrhea (5/0), dyspnea (5/6), fatigue (4/1), other flu-like symptoms (2/0), anemia (2/1), and lymphocytopenia (based on laboratory values, 1/4). Injection-site reactions (72%), fatigue (44%), decreased appetite (33%), diarrhea (28%), and flu-like illness (28%) were the most common AEs considered related to PF-3512676.
Table 2. Treatment-emergent, all-causality adverse events reported in >2 patients
All treated patients in both study arms (n = 39) discontinued erlotinib. Most of these discontinuations (n = 27, 69%; 15 patients in the combination arm and 12 patients in the erlotinib-alone arm) were the result of progressive disease. One patient in the erlotinib-alone arm discontinued erlotinib as the result of a treatment-related AE (acneiform rash). All treated patients in the PF-3512676-plus-erlotinib arm discontinued PF-3512676. Similar to erlotinib, the most common reason for discontinuation of PF-3512676 was progressive disease (n = 15; 83%). One patient in the combination arm discontinued as a result of a treatment-emergent AE: grade 4 fatigue during cycle 1. No deaths were considered related to either study drug.
Immunopathology
Fourteen patients in the PF-3512676-plus-erlotinib arm (14/18, 78%) provided samples at baseline, and 11 of these patients also provided at least one sample after treatment with PF-3512676. Several patients were positive/abnormal for the presence of autoimmune antibodies at baseline: six for rheumatoid factor, five for antinuclear antibody (ANA) and two for antineutrophil cytoplasmic antibody (ANCA). The most common immunopathologic marker reported as positive after treatment with PF-3512676 was ANCA (n = 6).
Immune activation biomarkers
Levels of immune-activation biomarkers measured in patients treated with PF-3512676 plus erlotinib demonstrated an increase from baseline in serum interferon-inducible protein 10 (IP-10) by a mean of 667 pg/mL (90% CI, 391–944 pg/mL) at 24 h postdose in cycle 1 and remained elevated through day 8 ().Citation12 There was no change in the total number of white blood cells after treatment with PF-3512676 compared with baseline numbers. The absolute numbers of lymphocytes decreased after treatment; mean change was –2460 cells/mm3 at cycle 1, day 8. For percentage of natural killer cells relative to white blood cells, there was a decrease (–1.2%) at 24 h in cycle 1, but the same decrease was not seen relative to lymphocytes. For percentage of activated natural killer cells relative to lymphocytes, there was an increase at both 24 h in cycle 1 and cycle 1, day 8 (3.3% and 5.2%, respectively), but this increase was not observed relative to white blood cells. For percentage of T cells relative to white blood cells, there was a decrease at 24 h in cycle 1 (change −5.9%), but a similar decrease was not observed relative to lymphocytes. For percentage of activated T cells relative to both white blood cells and lymphocytes, there were no changes noted at any time point.
Table 3. Changes in Immune-Activation Biomarker Interferon-Inducible Protein 10 (IP-10) levels in the PF-3512676-Plus-Erlotinib Arm (n = 21)
Discussion
The present study in patients with EGFR-positive advanced NSCLC who had failed prior chemotherapy was halted because an interim analysis indicated that an improvement in PFS with the addition of PF-3512676 to erlotinib large enough to be discerned by the study was unlikely even if additional patients were enrolled.
The addition of PF-3512676 plus erlotinib was fairly well tolerated and resulted in expected safety profiles of the two individual agents. Although the incidence of diarrhea was 43% in the erlotinib-alone arm, no episodes were more severe than grade 2. Diarrhea has not typically been associated with PF-3512676; however, the incidence in the PF-3512676-plus-erlotinib arm (n = 13; 72%) was higher than in the erlotinib-alone arm, and five cases were grade ≥ 3. Unlike diarrhea, rash commonly associated with erlotinib was reported with similar frequencies in the PF-3512676-plus-erlotinib and erlotinib-alone arms (50% and 48%, respectively). Common AEs of any grade considered related to PF-3512676 were injection-site reactions (72%) and flu-like illness (28%). The frequencies of these events reported in this study were similar to the frequencies attributed to PF-3512676 in other clinical studies. Flu-like illness was not reported in the erlotinib-alone arm.
Patients in this study were required to be positive for EGFR by immunohistochemistry. Mutations in the EGFR gene encoding the tyrosine kinase domain may be activating or inactivating, and some mutations have been associated with resistance.Citation2 Interestingly, the presence of EGFR mutations was not predictive of survival benefit from erlotinib in the phase 3 study of erlotinib monotherapy.Citation1 In the present study, it seemed notable that all patients with partial responses had activating mutations in the EGFR tyrosine kinase domain (2 patients in the PF-3512676-plus-erlotinib arm and 1 in the erlotinib-alone arm).
Certain immune-activation biomarkers were assessed in the combination arm of the current study as potential indicators of PF-3512676-mediated immune activation. Similar to what has been noted in other PF-3512676 studies, the marked increase in IP-10 by 24 h postdose that was maintained for at least 8 d postdose indicated that some level of immune activation is possible in a patient population with advanced NSCLC treated with prior chemotherapy. However, data for interferon α were not interpretable because of the small number of available samples with results. No other soluble cytokines or chemokines were tested.
Although the addition of PF-3512676 did not provide antitumor benefits beyond those of erlotinib alone in patients positive for EGFR (by immunohistochemistry) with advanced NSCLC, other approaches to improve on the survival benefits of erlotinib are being investigated. Currently, erlotinib is being studied in second-line advanced NSCLC in combination with chemotherapy,Citation13,Citation14 c-Met tyrosine kinase inhibitor ARQ 197,Citation15 mitogen-activated protein kinase inhibitor AZD6244,Citation16 vinca alkaloid vinorelbine,Citation17 anti-HER3 monoclonal antibody U3–1287,Citation18 and hepatocyte growth factor/scatter factor antagonist AMG 102,Citation19 to name a few. In the first-line setting, erlotinib is also being explored in a subset of patients with NSCLC who have activating mutations in EGFR.Citation20
Patients and Methods
Patients
Adult patients (≥18 y of age) with measurable, histologically confirmed, advanced NSCLC who had received prior treatment with at least one platinum-based chemotherapy regimen and who were EGFR-positive were eligible for study participation. Eligible patients had an ECOG performance status of 0, 1, or 2. Included patients were required to have adequate organ function as indicated by the following: absolute neutrophil count ≥1.5 × 109 cells/L, platelets ≥100 × 109/L, serum creatinine ≤1.5 × upper limit of normal (ULN), aspartate and alanine aminotransferase levels ≤2 × ULN (or ≤5 × ULN if attributable to liver metastases), and total bilirubin ≤1.25 × ULN. Patients with evidence of small cell or carcinoid lung cancer, central nervous system metastases, uncontrolled cardiac disease, uncontrolled hypercalcemia or superior vena cava syndrome, allogeneic transplant, interstitial lung disease, active or chronic viral hepatitis, a history of other malignancies in the three years prior (except for nonmelanoma skin cancer, cervical cancer, or cured early prostate cancer), or chronic autoimmune or antibody-mediated diseases potentially requiring systemic corticosteroids or concurrent immunosuppressive therapy, were excluded. Patients requiring concomitant treatment with inhibitors or inducers of CYP3A4 were also excluded. Eligible patients were not allowed to have received prior treatment with PF-3512676 or any agent that targets the EGFR signaling pathway or to have received any investigational agents within four weeks before study entry.
This study was conducted in accordance with the Declaration of Helsinki and in compliance with all International Conference on Harmonisation Good Clinical Practice Guidelines. Study conduct had approval from the Institutional Review Boards at each study site. Patients provided written informed consent before study participation. This trial is registered with clinicaltrials.gov, number NCT00321815.
Study design
This study was an open-label, multicenter, randomized (1:1) phase 2 study. Patients were stratified by ECOG performance status and smoking history before randomization. Patients randomized to the PF-3512676-plus-erlotinib arm were treated with 150 mg oral erlotinib once daily on days 1 through 21 of each cycle and 0.20 mg/kg PF-3512676 by subcutaneous injection once weekly (days 1, 8, and 15 of each cycle). Patients randomized to monotherapy received 150 mg oral erlotinib once daily on days 1 through 21. PF-3512676 injection sites in the upper body were preferred, and the site of injection was changed at each administration. If the injection volume exceeded 1 mL (15 mg), the injection was divided between two sites. Maximum dose was 2 mL (30 mg), divided between two injection sites. Dose reductions of erlotinib (≤2 occasions) or PF-3512676 were allowed in the event of specific treatment-related toxicities.
The primary objective of the study was to estimate PFS. Secondary objectives included assessment of secondary measures of efficacy, safety and tolerability, and, as an exploratory analysis, detection of specific immune-activation biomarkers.
Assessments
Baseline assessments, including medical history, vital signs, height, weight, ECOG performance status, baseline signs and symptoms, 12-lead resting electrocardiogram, hematology, serum chemistry, urinalysis, and an evaluation of concomitant medications, were performed within 7 d before first treatment. Efficacy assessments including radiologic imaging were performed at baseline (within 28 d before treatment initiation) and every other treatment cycle. Objective tumor responses were measured using the Response Evaluation Criteria in Solid Tumors (RECIST) version 1.0.Citation21 After discontinuation for reasons other than progression, imaging was repeated every 6 weeks until disease progression or initiation of another cancer treatment. Safety was assessed throughout the trial. Adverse events were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events, version 3.0.Citation22 Patients in the combination arm also provided blood samples at baseline and periodically after dosing for immunopathology, pharmacokinetics and biomarker assays. In the experimental arm, serum and plasma samples for immunopathology testing were collected at baseline, day 1 of every cycle except cycle 1 and at final visit. Potential immunopathologic tests included but were not limited to antibodies specific for double-stranded or single-stranded DNA, ANA, ANCA, and anti-rheumatoid factor antibodies.
During screening before randomization, EGFR expression was confirmed by immunohistochemistry (using a kit from Dako) on either archived or new formalin-fixed, paraffin-embedded tumor tissue. When possible, EGFR genotype was further assessed (1) as mutant or wild type and (2) with or without amplification by fluorescence in situ hybridization.
Statistical analysis
All primary analyses were based on the population as randomized. Safety analyses were conducted on the as-treated (with ≥1 dose) population. The planned enrollment was 60 patients, with 50 events over the study course. Assuming 50 events (disease progression or death), the standard error for estimating the log HR is approximately 0.28, assuming a 1-sided α of 0.10, corresponding to approximately 80% power to detect an HR of 0.55, representing a true improvement in median PFS. Progression-free survival was measured from the date of randomization to the date of disease progression or death and OS was measured from the date of randomization to the date of death. The HR and CI of the investigational treatment arm over the control treatment for PFS and OS were estimated using a Cox regression model with stratification factors: disease stage and smoking history. Progression-free survival and OS were also evaluated by Kaplan–Meier methods using a 2-sided stratified log-rank test with these stratification factors. The Brookmeyer–Crowley method was used to determine the 95% CI for median times. Descriptive statistics were used to summarize patient demographics and baseline characteristics, treatment administration, immune-activation biomarker data and safety parameters. Confidence intervals for the mean change from baseline for the immune-activation biomarker data were computed using the t-distribution. No interim analyses were planned.
| Abbreviations: | ||
| AE | = | adverse event |
| ANA | = | antinuclear antibody |
| CI | = | confidence interval |
| ECOG | = | Eastern Cooperative Oncology Group |
| EGFR | = | epidermal growth factor receptor |
| HR | = | hazard ratio |
| IP-10 | = | interferon-inducible protein 10 |
| NA | = | not available |
| NSCLC | = | non-small cell lung cancer |
| OS | = | overall survival |
| PFS | = | progression-free survival |
| RECIST | = | Response Evaluation Criteria in Solid Tumors |
| ULN | = | upper limit of normal |
Acknowledgments
This study (NCT00321815) was sponsored by Pfizer Inc. Medical editorial support was provided by Tamara Fink, PhD, of Accuverus Inc., a division of ProEd Communications, Inc.® and was funded by Pfizer Inc.
Disclosure of Potential Conflicts of Interest
SJM is employed by Pfizer Inc.; RJB and CBM are Pfizer Inc. employees and stockholders; JJN is the Chief Medical Officer of and holds stock in Gradalis, Inc.; AC has received speakers bureau honoraria from Eli Lilly and Genentech; LER has been a consultant/advisor and received speakers bureau honoraria from Genentech and OSI and has received research grants/support from Pfizer and Genentech; PDE has received research grants/support from Pfizer and Genentech. All remaining authors have declared no conflicts of interest.
References
- Shepherd FA, Rodrigues Pereira J, Ciuleanu T, Tan EH, Hirsh V, Thongprasert S, et al, National Cancer Institute of Canada Clinical Trials Group. Erlotinib in previously treated non-small-cell lung cancer. N Engl J Med 2005; 353:123 - 32; http://dx.doi.org/10.1056/NEJMoa050753; PMID: 16014882
- Ray M, Salgia R, Vokes EE. The role of EGFR inhibition in the treatment of non-small cell lung cancer. Oncologist 2009; 14:1116 - 30; http://dx.doi.org/10.1634/theoncologist.2009-0054; PMID: 19892771
- Krieg AM. Development of TLR9 agonists for cancer therapy. J Clin Invest 2007; 117:1184 - 94; http://dx.doi.org/10.1172/JCI31414; PMID: 17476348
- Krieg AM. Toll-like receptor 9 (TLR9) agonists in the treatment of cancer. Oncogene 2008; 27:161 - 7; http://dx.doi.org/10.1038/sj.onc.1210911; PMID: 18176597
- Pashenkov M, Goëss G, Wagner C, Hörmann M, Jandl T, Moser A, et al. Phase II trial of a Toll-like receptor 9-activating oligonucleotide in patients with metastatic melanoma. J Clin Oncol 2006; 24:5716 - 24; http://dx.doi.org/10.1200/JCO.2006.07.9129; PMID: 17179105
- Kim YH, Girardi M, Duvic M, Kuzel T, Link BK, Pinter-Brown L, et al. Phase I trial of a Toll-like receptor 9 agonist, PF-3512676 (CPG 7909), in patients with treatment-refractory, cutaneous T-cell lymphoma. J Am Acad Dermatol 2010; 63:975 - 83; http://dx.doi.org/10.1016/j.jaad.2009.12.052; PMID: 20888065
- Thompson JA, Kuzel T, Drucker BJ, Urba WJ, Bukowski RM. Safety and efficacy of PF-3512676 for the treatment of stage IV renal cell carcinoma: an open-label, multicenter phase I/II study. Clin Genitourin Cancer 2009; 7:E58 - 65; http://dx.doi.org/10.3816/CGC.2009.n.025; PMID: 19815483
- Leonard JP, Link BK, Emmanouilides C, Gregory SA, Weisdorf D, Andrey J, et al. Phase I trial of Toll-like receptor 9 agonist PF-3512676 with and following rituximab in patients with recurrent indolent and aggressive non Hodgkin’s lymphoma. Clin Cancer Res 2007; 13:6168 - 74; http://dx.doi.org/10.1158/1078-0432.CCR-07-0815; PMID: 17947483
- Manegold C, Gravenor D, Woytowitz D, Mezger J, Hirsh V, Albert G, et al. Randomized phase II trial of a Toll-like receptor 9 agonist oligodeoxynucleotide, PF-3512676, in combination with first-line taxane plus platinum chemotherapy for advanced-stage non-small-cell lung cancer. J Clin Oncol 2008; 26:3979 - 86; http://dx.doi.org/10.1200/JCO.2007.12.5807; PMID: 18711188
- Hirsh V, Paz-Ares L, Boyer M, Rosell R, Middleton G, Eberhardt WE, et al. Randomized phase III trial of paclitaxel/carboplatin with or without PF-3512676 (Toll-like receptor 9 agonist) as first-line treatment for advanced non-small-cell lung cancer. J Clin Oncol 2011; 29:2667 - 74; http://dx.doi.org/10.1200/JCO.2010.32.8971; PMID: 21632509
- Manegold C, van Zandwijk N, Szczesna A, Zatloukal P, Au JSK, Blasinska-Morawiec M, et al. A phase III randomized study of gemcitabine and cisplatin with or without PF-3512676 (TLR9 agonist) as first-line treatment of advanced non-small-cell lung cancer. Ann Oncol 2012; 23:72 - 7; http://dx.doi.org/10.1093/annonc/mdr030; PMID: 21464154
- Belani CP, Nemunaitis JJ, Chachoua A, Eisenberg PD, Raez LE, Cuevas JD, et al. Randomized phase II trial of erlotinib with or without PF-3512676 (CPG 7909, a Toll-like receptor 9 [TLR9] agonist) in patients with advanced recurrent epidermal growth factor receptor (EGFR)-positive non-small cell lung cancer (NSCLC). Presented at: 14th Annual World Conference on Lung Cancer (WCLC) of the International Association for the Study of Lung Cancer (IASLC); July 3-7, 2011; Amsterdam, The Netherlands. Abstract 1167.
- U.S. National Institutes of Health. 2nd line erlotinib treatment with (out) chemotherapy of advanced non small cell lung cancer (NSCLC) (NVALT10) (NCT00835471). Available at: http://www.clinicaltrials.gov/ct2/show/NCT00835471?term=erlotinib&recr=Open&rank=13term=erlotinib&recr=Open&rank=3. Accessed December 3, 2012.
- U.S. National Institutes of Health. Erlotinib and docetaxel in patients with locally advanced or metastatic non-small cell lung cancer (NSCLC) after failure of one chemotherapy regimen (NCT00908336). Available at: http://www.clinicaltrials.gov/ct2/show/NCT00908336?term=erlotinib&recr=Open&rank=21. Accessed December 3, 2012.
- U.S. National Institutes of Health. ARQ 197 plus erlotinib versus placebo plus erlotinib for the treatment of non-squamous, non-small-cell lung cancer (NCT01244191). Available at: http://www.clinicaltrials.gov/ct2/show/NCT01244191?term=erlotinib&recr=Open&rank=27. Accessed December 3, 2012.
- U.S. National Institutes of Health. Randomized phase II study of AZD6244 MEK-inhibitor with erlotinib in KRAS wild type and KRAS mutant advanced non-small cell lung cancer (NCT01229150). Available at: http://www.clinicaltrials.gov/ct2/show/NCT01229150?term=erlotinib&recr=Open&rank=28. Accessed December 3, 2012.
- U.S. National Institutes of Health. Study of oral vinorelbine and erlotinib in non-small cell lung cancer (NCT00702182). Available at: http://www.clinicaltrials.gov/ct2/show/NCT00702182?term=erlotinib&recr=Open&rank=41. Accessed December 3, 2012.
- U.S. National Institutes of Health. Study of erlotinib with or without investigational drug (U3-1287) in subjects with advanced non-small cell lung cancer (NCT01211483). Available at: http://www.clinicaltrials.gov/ct2/show/NCT01211483?term=erlotinib&recr=Open&rank=44. Accessed December 3, 2012.
- U.S. National Institutes of Health. AMG 102 and erlotinib for advanced non-small cell lung cancer (NCT01233687). Available at: http://www.clinicaltrials.gov/ct2/show/NCT01233687?term=erlotinib&recr=Open&rank=52. Accessed December 3, 2012.
- U.S. National Institutes of Health. Study of erlotinib (Tarceva®) in combination with OSI-906 in patients with advanced non-small cell lung carcinoma (NSCLC) with activating mutations of the epidermal growth factor receptor (EGFR) gene (NCT01221077). Available at: http://www.clinicaltrials.gov/ct2/show/NCT01221077?term=erlotinib&recr=Open&rank=3. Accessed December 3, 2012.
- Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 2000; 92:205 - 16; http://dx.doi.org/10.1093/jnci/92.3.205; PMID: 10655437
- U.S. National Institutes of Health. Common terminology criteria for adverse events v3.0 (CTCAE). Available at: http://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/ctcaev3.pdf. Accessed December 3, 2012.