Abstract
Despite the improvements in neoadjuvant chemotherapy, the outcome of patients with advanced bladder cancer has changed very little over the past 30 years. In the present study we tested and compared the in vitro antitumor activities of four different inhibitors of Polo-like kinase 1 (PLK1) (BI 2536, BI 6727, GW843682X, and GSK461364), against 3 bladder carcinoma cell lines RT4, 5637 and T24. The impact on radiosensitivity and drug interactions in simultaneous treatments with cisplatin, methotrexate, and doxorubicin were also investigated. Our results showed that PLK1 inhibition prevented cell proliferation and clonogenicity, causing significant inhibition of invasion of tumor cells, though modest differences were observed between drugs. Moreover, all PLK1 inhibitors induced G2/M arrest, with the subsequent induction of death in all 3 cell lines. Drug interactions studies showed auspicious results for all PLK1 inhibitors when combined with the commonly used cisplatin and methotrexate, though combinations with doxorubicin showed mostly antagonistic effects. Comparably, the four PLK1 inhibitors efficiently sensitized cells to ionizing radiation. Our findings demonstrate that irrespective of the inhibitor used, the pharmacological inhibition of PLK1 constrains bladder cancer growth and dissemination, providing new opportunities for future therapeutic intervention. However, further laboratorial and pre-clinical tests are still needed to corroborate the usefulness of using them in combination with other commonly used chemotherapeutic drugs.
Keywords: :
Introduction
Bladder carcinoma is a common cancer whose incidence continues to increase worldwide.Citation1 In up to 70% of patients, its diagnosis is performed at early stages and treated with transurethral resection.Citation2 For advanced cases, treatment is essentially palliative with chemotherapy based on methotrexate, vinblastine, doxorubicin, and cisplatin (MVAC) or radiotherapy.Citation2 However, over the past 30 years, the use of this combined chemotherapy has not improved survival. Of those patients who do not succumb to the disease, a substantial proportion will experience metastatic recurrence and die within 16 mo.Citation3 Therefore, novel therapeutic approaches to treat or even prevent bladder tumors propagation have been eagerly desired.
Recently, the Polo-like kinase 1 (PLK1) has shown to be highly expressed in urothelial cancer cells and correlated with higher pathological grade, pT stage, recurrence, and metastasis.Citation4,Citation5 PLK1 is a key cell cycle regulator promoting entry into mitosis, spindle formation, sister chromatid segregation, and cytokinesis.Citation6 Compelling evidence has shown a close association between high PLK1 expression and poor prognosis in various human malignant neoplasms. Remarkably, Fristrup and colleagues recently identified PLK1 as an independent prognostic marker in non-muscle invasive bladder cancer, corroborating its importance in tumor progression and its potential for therapeutic intervention.Citation7
PLK1 inhibition by BI 2536, the most intensively studied inhibitor has shown to decrease proliferation and induce mitotic arrest in several tumors.Citation8 However, its progress into clinical studies in patients with locally advanced or metastatic cancers has shown controversial results. Currently, there is no information available about the use of pharmacological PLK1 inhibitors against bladder cancer. Thus, the goal of this study was to study the antiproliferative effects of BI 2536 and to perform a comparison of its action with other three potent PLK1 inhibitors (BI 6727, GW843682X, and GSK461364) on three bladder cancer cell lines (RT4, 5637, and T24), providing strong support for future treatment of this tumor.
Results
PLK1 inhibition decreases cell proliferation in vitro
All PLK1 inhibitors tested effectively reduced the growth of the three bladder carcinoma cell lines (RT4, 5637, and T24) when compared with control (DMSO 0.1%) at all times tested (P < 0.05) (). However, IC50 values after 48 h of treatment varied considerably between inhibitors (). Dose and time dependency were observed for BI 2536, BI 6727, and GW843682X reaching a maximum of about 70% for RT4, 60% for 5637, and 50% for T24. In the case of GSK461364 growth inhibition of about 60% was achieved for all cell lines at 75 nM and maintained with increasing concentrations along time.
Figure 1. Characterization of the effects of PLK1 inhibition on cell growth in RT4, 5637, and T24 bladder carcinoma cells as detected by the XTT® assay after 24, 48, and 72 h of treatment. The number 1 corresponds to control and the numbers 2, 3, and 4 on the x-axis indicate increasing concentrations of each PLK1 inhibitor, being 10, 20, and 50 nM for BI 2536; 50, 100, and 150 nM for BI 6727; 300, 600, and 1200 nM for GW843682X; and 75, 150, and 300 nM for GSK461364, respectively. Statistically different (P < 0.05) results were obtained for all tests at all times tested except for treatment of 5637 cells with BI 2536 for 24 h and GW843682X 300 nM for 48 h and treatment of T24 cells with BI 2536 10 nM after 72 h. Asterisks were not included in order to avoid figure pollution.
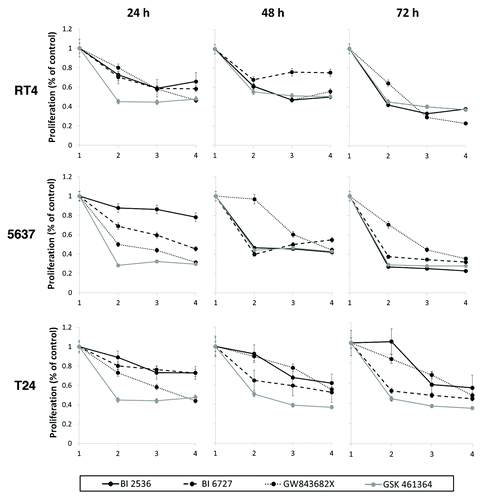
Table 1. Doses required to induce 50% inhibition of cell growth (IC50) in bladder carcinoma cell lines
PLK1 inhibition abrogates the clonogenic capacity of bladder carcinoma cell lines
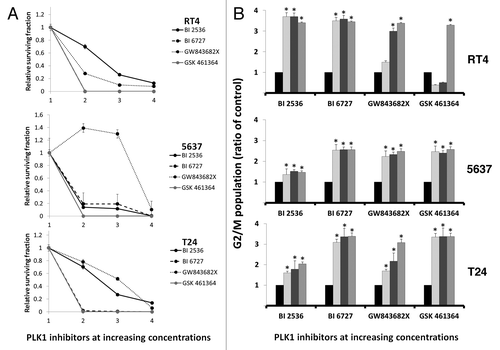
PLK1 inhibition by BI 6727 and GSK461364 strongly abolished the colony formation capacity for RT4 and T24 cell lines when compared with control (P < 0.05) at all concentrations tested (). The clonogenic capacity of 5637 cell line was also reduced in almost 80% with these drugs. BI 2536 and GW843682X, on the other hand, showed variable results between cell lines. Both drugs induced a dose-dependent inhibition for RT4 and T24 though results for 5637 varied greatly, while low concentrations of GW843682X increased the capacity of cells to form colonies, BI 2536 revealed a constant effect at all concentrations reducing colony formation in 90% ().
Figure 2. (A) PLK1 inhibition for 48 h abrogated the clonogenic capacity of RT4, 5637 and T24 bladder carcinoma cell lines. Note that in the case of 5637 cells, colony formation was significantly increased after treatment with low concentrations of GW843682X but drastically decresed to 90% after treatment with 1200 nM of this drug; (B) PLK1 inhibition induced cell cycle arrest with accumulation of G2/M populations for all drugs tested. Ratios of the proportion of G2/M subpopulation in cells treated with PLK1 inhibitors to that of vehicle-treated cells are shown as mean ± SD of 3 independent experiments.
PLK1 inhibitors induce cell cycle arrest of bladder carcinoma cell lines
Treatment of the cells with all inhibitors induced a prominent change in the cell cycle distribution within 24 h. During this period, treated cells significantly accumulated in the G2/M phase (up to 80% irrespective of the inhibitor tested) (). The percentage of the cells in G1 and S phases decreased in the same proportion as a result of treatment while untreated cells (control) were more evenly distributed throughout the cell cycle (data not shown).
PLK1 inhibition increases cell death in bladder carcinoma cells
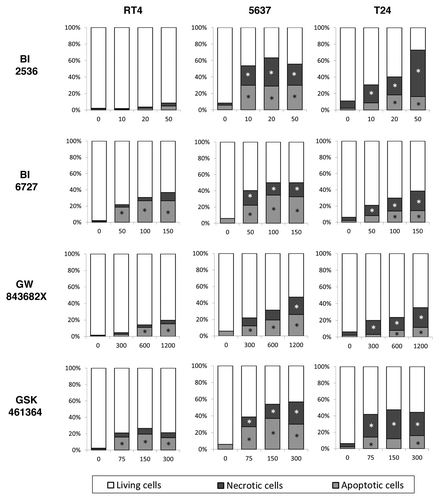
Compared with control, all PLK1 inhibitors induced a significant increase in the percentage of apoptotic cells (detected by caspase-3 activity) at all concentrations tested after 48 h (P < 0.05) for 5637 and T24 cells. For RT4 cells the effects of the drugs were drug was more moderate with no effects after treatment with BI 2536 and a maximum of about 20% after treatment with GSK461364 or GW843682X and 30% for after treatment with BI 6727. Additionally, the microscopical analysis of treated cells by differential staining with propidium iodide also demonstrated higher frequency of necrotic-like cells after treatment of 5637 and T24 cells with all PLK1 inhibitors tested. Alternatively, neither of these drugs was able to induce significant necrosis in the low grade cell line RT4 ().
Figure 3. After 48 h of treatment PLK1 inhibition increased apoptosis rates in bladder carcinoma cell lines as detected by caspase-3 activation (NucView 488®). Differential staining with propidium iodide also demonstrated a significant increase of necrotic-like cells in a dose dependent manner. *Statistically different P < 0.05
PLK1 significantly restrained cell invasion
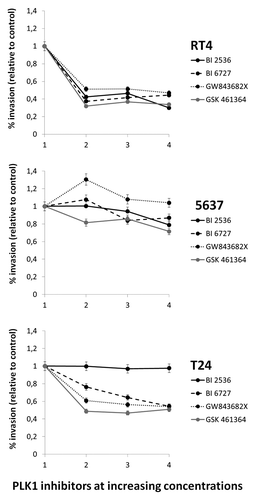
Invasion assay using transwell chambers coated with Matrigel® showed significant reductions of invasion compared with controls, in RT4 cells for all drugs at all concentrations tested, though the effect was not dose-dependent. For 5637, treatments were ineffective (GW843682X) or showed mild effects reaching a maximum reduction of 20% after treatment with the highest concentration of GSK461364. T24 cells on the other hand, did not suffer any invasion reduction after treatment with BI 2536 but suffered a dose-dependent reduction of the invasive capacity (maximum of about 60%) for the other three drugs tested ().
Combinatorial studies show different responses for each PLK1 inhibitor
For combinatorial studies, three test concentrations of each commonly used drug were chosen (corresponding with dilutions of the IC50) for association with IC50 concentrations for each PLK1 inhibitor and and analyzed by the CalcuSyn® software. As shown in , CI values for the simultaneous combinations of drugs showed dissimilar responses for each PLK1 inhibitor. BI 3526 showed synergistic effects when combined with CDDP irrespective of the cell line treated (CI > 1) when combined with MTX for RT4 and T24 cells and for combinations with DXR for 5637 (at the highest dose) and T24 cells. BI 6727 and GW843682X, efficiently sensitized the three cell lines to CDDP and MTX, though was highly antagonic when combined with DXR. GSK461364 on the other hand, synergistically increased the cytotoxicity of CDDP and MTX in RT4 and T24 cells but was highly antagonist (CI >>>> 1) for 5637 cells for all combinations tested ().
Table 2. Median dose effect analysis was also employed to characterize the interactions between each PLK1 inhibitor with CDDP, MTX, or DXR
Figure 4. Invasion assay using transwell chambers coated with Matrigel® showed significant reductions of invasion compared with controls, in RT4 cells for all drugs at all concentrations tested. For 5637, treatments were ineffective (GW843682X) or showed mild effects. T24 cells on the other hand, did not suffer any invasion reduction after treatment with BI 2536 though dose-dependency was observed for the other three drugs tested (). Both cell lines presented a significant decrease in invasion rate after treatment with higher concentrations BI 2536; *Statistically different P < 0.05
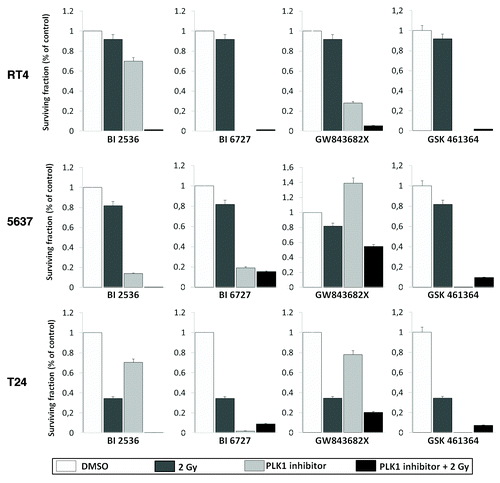
PLK1 inhibition sensitizes cells to ionizing radiation
To study the cytotoxic effects of the different PLK1 inhibitors in association with γ-radiation, RT4, 5637, and T24 cells were incubated with BI 2536 10 nM, BI 6727 50 nM, GW843682X 300 nM, or GSK461364 75 nM for 24h to induce G2 arrest. After the treatment, the cell culture medium was replaced and cells irradiated with a final dose of 2 Gy. The results showed that pre-treatment with any of the PLK1 inhibitors tested led to radiosensitization in all three human bladder carcinoma cell lines (DER > 1)() ().
Table 3. Radiosensitization induced by PLK1 inhibitors in RT4, 5637, and T24 bladder carcinoma cell lines
Figure 5. Clonogenic survival assay of bladder carcinoma cell lines exposed the different PLK1 inhibitors for 24 h and then irradiated with 2 Gy. Treatment significantly radio-sensitized cells. Each value represents the mean derived from at least three individual experiments in duplicate (mean ± SD). Dose enhancement ratios are described in .
Discussion
Since the 1980s, treatment of advanced and metastatic bladder cancer has been cisplatin-based. However, despite changes in surgical techniques and the development of new drugs and combinations, the outcomes have not improved to any great extent.Citation3
PLK1 is a serine/threonine kinase known to be one of the key players in the regulation of mitosis progression.Citation9 PLK1 is almost exclusively expressed in proliferating cells and its overexpression in human malignant neoplasms is associated with poor prognosis.Citation6-Citation10 Particularly, it has been demonstrated that PLK1 expression correlates with the progression of bladder cancer (not detected in normal and dysplastic bladder mucosa) and significantly shorter survival.Citation4-Citation11
The potential of directing against PLK1 in cancer therapy has been repeatedly demonstrated in different in vitro and in vivo models resulting in growth inhibition and apoptosis induction.Citation8 Targeting PLK1 in bladder cancer with intravesical siRNA effectively prevents growth of cancer cells and decreases tumor volumes.Citation11,Citation12 In the present study, we tested the effects of BI 2536, a synthetic PLK1 inhibitor on three bladder carcinoma cell lines, RT4, 5637, and T24 and compared its antiproliferative activities with other less studied ATP-competitive small-molecules: BI 6727, GW843682X, and GSK461364.
Our results showed that treatment with the four inhibitors significantly decreased cell proliferation, though IC50 values varied greatly between them. For instance, while low nanomolar (<100) concentrations of BI 2536 were effective, significantly higher concentrations (>1000 nM) of GW843682X were needed to achieve comparable results. Also, we show that PLK1 inhibition impairs clonogenicity an equally essential requisite for testing potential therapeutic targets. Abrogation of the capacity of forming colonies by BI 2536 has previously been reported for myeloid leukemiaCitation13 and in two osteosarcoma cell lines.Citation14,Citation15 Also GW843682X has shown to inhibit clonogenicity in melanoma cellsCitation16 and to effectively inhibit clonogenic growth of freshly isolated leukemia cells from patientsCitation17; however, in our experimental setting low concentrations of this drug were unable to reduce clonogenicity. Also, treatment with the PLK1 inhibitors provoked a clear disturbance of cell cycle phase distribution, with an accumulation of cells in G2/M after 24 h. This increase in doubled-DNA cells was previously reported after PLK1 inhibition by BI 2536 in osteosarcoma cell linesCitation14 and by scytonemin in T24 cells.Citation5 Moreover, GW843682X also induced accumulation of human leukemia cells in the G2/M phase of the cell cycle.Citation17
Earlier cytological studies demonstrated that PLK1 inhibition prompts mitotic catastropheCitation18 which has been defined as a stage preceding cell death that can occur through apoptosis or undergo slow death in a necrosis-like manner with the characteristic loss of nuclear and plasma membrane integrities.Citation19 Mitotic catastrophe is distinctly enhanced in cells lacking p53 function, which is the most common genetic alteration in bladder cancer associated with high grade and progressed disease.Citation20,Citation21 However, our results show that the status of p53 gene might be an important factor to determine the sensitivity of different cell lines to apoptosis caused by PLK1 inhibitors. Although increased caspase-3 activity was detected for all inhibitors, the level of cell death was significantly higher in 5637 and T24 cells which are p53-deficient, and RT4 cells were particularly unaffected by exposure to BI 2536. Differential staining with propidium iodide also demonstrated increased disruption of the plasmatic membrane integrity which is also an indicative of necrosis-like death in 5637 and T24 cells; though levels of necrosis were lower in the higher grade cell line. In osteosarcoma cell lines treatment with BI 2536 has previously shown to induce apoptosis in KHOS and U-2OS,Citation15 while HOS and MG-63 experience caspase-independent mitotic catastrophe followed by necrosis.Citation14 Thus, the outcome of mitotic catastrophe after PLK1 inhibition most likely depends on the molecular profile of the cells. Also, even though 5637 and T24 cell lines express high levels of PLK1,Citation5,Citation11 our results showed that PLK1 expression in T24 cells is 2.6 times higher, which might explain the different sensitivities observed. Thus, the more PLK1 present, the more drug concentration is needed to achieve growth inhibition.
5637 and T24 cell lines have also been described as highly invasive. The ability of cells to cross through coated chambers and migrate was also significantly decreased (>60%) in T24 cells by treatment with BI 6727, GW843682X, and GSK461364. Similar results were reported by Zhang et al.Citation5 in these cells treated with the PLK1-inhibitor scytonemin. The invasive capacity of 5637 cells on the other hand was moderately affected, though the underlying mechanisms by which PLK1 inhibition might contribute to suppress cell migration or invasion are still unclear.
In this study, we also addressed concomitant combinations of BI 2536, BI 6727, GW843682X, and GSK461364 with MTX, CDDP, and DXR. Synergistic effects of PLK1 inhibition have been described for combinations of BI 2536 with imatinib and nilotinib in chronic myelogenous leukemia cells,Citation22 for combinations with NVP-AEW541 (a small molecule inhibitor of insulin-like growth factor-1 receptor) in biliary tract cancer,Citation23 and when combined with bortezomib and dexamethasone in multiple myeloma.Citation24 Moreover, GW843682X synergistically potentiated the growth inhibition and apoptosis of leukemia cells when combined with tubulin depolymerizing agent vincristineCitation17 and with VP-16.Citation25 In our experimental model, association with CDDP, showed synergistic effects for all cell lines when combined with BI 2536, BI 6727, and GW843682X at all concentrations tested (CI < 1). Though, combinations with GSK461364 only showed synergistic effects in T24 cells while for the other lower grade cell lines resulted highly antagonist. These sensitizing effects of PLK1 inhibitors to CDDP are particularly interesting considering that both 5637 and T24 cells were derived from tumors with unfavorable prognosis and have repeatedly demonstrated to be especially resistant to this platinum-containing drug.Citation20 Moreover, by combining both drugs, the substantial side effects of CDDP treatment experienced by patients might be mitigated. Combinations with MTX, showed similar synergistic effects, but were also antagonistic (CI > 1) in 5637 when combined GSK461364; and combinations with DXR showed antagonist effects when combined with PLK1 inhibitors except for combinations of BI 2536 for T24 cells.
On the other hand, our results showed that pre-treatment with the four inhibitors led to significant radiosensitization in human bladder carcinoma cell lines. Previous reports have shown that tumor cells overexpressing PLK1 exhibit a more radioresistant phenotype.Citation27 Conversely, compelling evidence has shown that combinatorial treatment with drugs that induce G2/M arrest could enhance the radiosensitivity of cells.Citation28,Citation29 Synergistic effects on clonogenicity have been demonstrated in rectal tumor cells using PLK1 inhibition by siRNA combined with radiation,Citation26 this approach has also been effective on head-and-neck squamous cell carcinoma.Citation30 Comparatively, the pharmacological inhibition of PLK1 by BI 2536 has shown to radiosensitize medulloblastoma cells,Citation31 whereas GSK461364A-sensitized tumor cells to radiation and prevented the growth of metastatic breast cancer cells.Citation32
The development of drug resistance is a common problem in treatment of bladder cancers. Moreover, standard chemotherapy with MVAC, or the alternative combination of cisplatin and gemcitabine are often associated with substantial toxicity.Citation33 Antimitotic chemotherapy remains a cornerstone of multimodality treatment for locally advanced and metastatic cancers. Recently, the pharmaceutical industry has initiated intensive efforts to develop potent and specific low molecular mass compounds that target PLK1 as a deadly weakness that can actually or potentially lead to tumor downfall. Even with chemical optimization, PLK1 inhibitors such as BI 2536 show high efficacy in cultured tumor cells and nude mice tumor xenografts. However, phase I clinical trials in patients with different tumor entities revealed that antitumor responses observed for BI 2536 as a monotherapy in advanced cancers seem to be modest and clinicians must face severe drawbacks such as hematotoxicityCitation34 and low intratumoral levels.Citation35
Preclinical studies with BI 6727 have shown that this dihydropteridinone derivative has better tissue distribution and penetration with manageable hematological toxicities.Citation36 Alternatively, patients who received escalating doses of GSK461364 in different schedules showed different dose-limiting toxicity effects, including sepsis and pulmonary embolism, though neutropenia was observed in only 18% of patientsCitation37 which differs considerably from the phase I trial of BI 2536, in which neutropenia was observed in 45% of patients.Citation34
This increasing clinical evidence supports the assessment of certain PLK1-specific inhibitors at the phase II/III level. However, it is still indispensable to monitor the early tumor response to these drugs in cell lines and primary tissues. In the present study we showed that PLK1 inhibition by different small-molecules has antiproliferative effects as a single agent with growth retardation and death of bladder cancer cells holding promising prospects for future therapy. However, even though the four compounds tested share the same mechanism of action (as ATP-competitors) their antiproliferative effects vary considerably, mainly when combinations with other commonly used drugs are considered. Additional laboratorial and pre-clinical trials are necessary to corroborate our data.
Methods
Cell culture
The established bladder carcinoma cell lines RT4 (low-grade papillary tumor), 5637 (moderately differentiated tumor, grade II), and T24 (grade III) were obtained from the Cell Bank of the Federal University of Rio de Janeiro, Brazil. Cells were cultured in HAM F10 (T24) (Life Technologies, 11550043) or RPMI 1640 (RT4 and 5637) (Life Technologies, 11875119) supplemented with 10% fetal bovine serum (Life Technologies, A12618DG), penicillin (Sigma-Aldrich, P3032) (100 U/mL) and streptomycin (Sigma-Aldrich, S9137) (100 ug/mL) at 37 °C in a humidified 5% CO2 incubator.
Quantitative real time RT-PCR
For validation of the hyperexpression of PLK1 in all cell lines, quantitative real time RT-PCR was used. Total RNA was isolated using the Trizol Reagent (Life Technologies, 15596018) following the manufacturer's instructions and reverse transcribed using the HighCapacity kit (Applied Biosystems, P/N 4322171).
Real time RT-PCR reaction were performed in triplicate in 10 μl reactions using the inventoried TaqMan® probe for PLK1 (Hs00153444_m1, Applied Biosystems), on a ABI Prism 7500 Sequence Detector (Applied Biosystems). As endogenous controls β-actin and GUS genes were used. Peripheral lymphocytes were used as calibrator. The relative quantification was performed by the 2−ΔΔCT method.Citation38 Results confirmed PLK1 expression being 136, 172.7, and 353.6 times higher for 5637, RT4, and T24, respectively, when compared with calibrator.
Drug and treatments
BI 2536, BI 6727, GW843682X, and GSK461364 were acquired from Axon Medchem (1129, 1473, 1688 and 1131) and diluted in dimethyl sulfoxide (DMSO) (Sigma-Aldrich, D8418) according to the manufacturer’s instructions. For all experiments, cells were treated with nanomolar concentrations based on previous tests: 10, 20, and 50 nM for BI 2536; 50, 100, and 150 nM for BI 6727; 300, 600, and 1200 nM for GW843682X, and 75, 150, and 300 nM for GSK461364. Corresponding control cultures received an equal volume of solvent. For combinatorial treatments cisplatin (CDDP), methotrexate (MTX), and doxorubicin (DXR) were purchased from Sigma-Aldrich (C2210000, M9929 and D1515) and diluted in DMSO or in 0.9% NaCl accordingly.
Measurement of cell growth
Cell survival was assessed using the XTT assay (XTT II; Roche Molecular Biochemicals, 11465015001). Briefly, equal amounts of cells were seeded in 96-well flat-bottom plates (2500 cells/well) and allowed to attach. Subsequently, cells were treated with different concentrations of PLK1 inhibitors, or combinations of each inhibitor with CDDP, MTX, and VBL, incubated for 24, 48, and 72 h. After treatment, the culture medium was removed and replaced with medium containing 10 μL of XTT dye (3 mg/mL) in each well. The plates were incubated for 2 h at 37 °C and results interpreted by using an iMark microplate reader (Bio-Rad Laboratories®).
Colony formation assay
Clonogenic assays were performed according to Franken et al.Citation39 Single cell suspensions of 300 cells were seeded in 6-well plates and treated with the different PLK1 inhibitors concentrations for 48 h and then allowed to grow in drug-free medium. After 10 d of growth the medium was aspirated, the wells were washed in PBS and then the colonies fixed with methanol and stained with Giemsa (Merk, 109204). The number of colonies per well were counted using a dissection microscope with of threshold of >50 cells.
Analysis of caspase activation
For apoptosis 3 × 104 cells were seeded on 6-well plates containing 3 mL of culture medium. After 24 h, the medium was replaced and cells treated with the different concentrations of PLK1 inhibitors or vehicle only and cultured for additional 48 h. Caspase activation was determined using the NucView™ 488 Caspase-3 Detection in Living Cells kit (Biotium Inc., 30029) according to the manufacturer’s instructions. Five hundred nuclei were analyzed by fluorescence microscopy per treatment.
Detection of necrotic cells by differential staining
Necrotic cells were recognized by differential staining with bisbenzimide (Hoechst, 33342), propidium iodide and fluorescein diacetate (Sigma Aldrich, 14533, P4864, and F7378) according to Lee and Shacter.Citation40 Cells were analyzed by fluorescence microscopy and categorized as follows: (1) normal: blue nucleus and green cytoplasm, (2) apoptotic: fragmented blue nucleus and green cytoplasm and (3) necrotic: red nucleus. Five hundred nuclei were analyzed per treatment.
Cell cycle analysis
After drug treatment, cells were trypsinized, fixed in 70% ethanol, stained with propidium iodide (Sigma Aldrich, 14533) and analyzed on a Guava Personal Cell Analysis system (Guava Technologies) according to the standard protocol provided by the manufacturer. Percentages of cells in G0/G1, S, or G2/M phase were scored using the GUAVA Cytosoft 4.2.1 version Software.
Invasion assay
5 × 105 cells were treated with different concentrations of PLK1 inhibitors and transferred to the top of Matrigel-coated invasion chambers (24-well insert, 8-µm pore size; Becton Dickinson and Co., 353097) and placed in a plate with medium supplemented with 10% FBS as a chemoattractant. After 22 h incubation, non-invading cells were removed from the upper surface of the membrane by scrubbing with moistened swabs. The invasive cells attached to the lower surface of the membrane insert were fixed in 100% methanol for 10 min and stained with Giemsa (Merk). Membranes were then removed from the insert housing with scalpel blade, placed on a microscope slide, mounted with Entellan, and coverslipped. Invading cells were photographed under the microscope at 100× magnification and counted with the CytolabView software (Applied Spectral Imaging).
Cell irradiation
To test the effect of individual PLK1 inhibitors on radioresistance a proliferation-based assay (XTT assay) was used, which is highly comparable to the clonogenic assay when the cells are allowed to undergo six cell divisions.Citation41 Cell cultures were treated for 24 h and then were irradiated with γ-rays from 60Cobalt at a dose rate of about 0.47 Gy/min, using a Gammatron S-80 equipment (Siemens Medical Systems Inc.) at the University Clinical Hospital (FMRP-USP). After irradiation with 2 Gy, the cells were plated in 96-well plates (100 µL cell suspension, 500 cells/well) and the number of living cells was determined after 7 d by the proliferation XTT assay as described above. The radiation dose enhancement ratio (DER) by each inhibitor was calculated using the following formula: DER = (surviving fraction at an indicated dose of radiation alone)/(surviving fraction at an indicated dose of radiation + PLK1 inhibitor). Dose enhancement ratio = 1 suggests an additive radiation effect and DER > 1 a supra-additive effect as against a sub-additive effect in the case of DER < 1.Citation42
Statistical analysis
Statistical analyses were performed by using the SigmaStat software (Jandel Scientific Company). Two way repeated measures analysis of variance (ANOVA) followed by the Holm-Sidak pairwise multiple comparison was used to establish whether significant differences existed between groups. All tests were performed for α = 0.05. Effective concentrations (IC50) were analyzed using the CalcuSyn software v2.0 (Biosoft). This program provides a measure of the combined drug interaction by the generation of a combination index (CI) value. The CI value is based on the multiple drug-effect equation of Chou and TalalayCitation43 and defines the drug interactions as synergistic CI value < 1, CI value = 1 for additive, and CI value > 1 for antagonism. Calcusyn® software was also used to calculate the dose reduction index (DRI) of drug combinations which estimates the extent to which the dose of one or more agents in the combination can be reduced to achieve effect levels that are comparable with those achieved with single agents.
Acknowledgments
We are grateful to Leonardo L Amaral for his cooperation on the irradiation of cell cultures. This research was supported by CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico, Brazil) under grant number 471952/2011-7.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- Jemal A, Thun MJ, Ries LA, Howe HL, Weir HK, Center MM, et al. Annual report to the nation on the status of cancer, 1975-2005, featuring trends in lung cancer, tobacco use, and tobacco control. J Natl Cancer Inst 2008; 100:1672 - 94; http://dx.doi.org/10.1093/jnci/djn389; PMID: 19033571
- Sternberg CN, Yagoda A, Scher HI, Watson RC, Herr HW, Morse MJ, et al. M-VAC (methotrexate, vinblastine, doxorubicin and cisplatin) for advanced transitional cell carcinoma of the urothelium. J Urol 1988; 139:461 - 9; PMID: 3343727
- Kaufman DS. Challenges in the treatment of bladder cancer. Ann Oncol 2006; 17:Suppl 5 v106 - 12; http://dx.doi.org/10.1093/annonc/mdj963; PMID: 16807436
- Yamamoto Y, Matsuyama H, Kawauchi S, Matsumoto H, Nagao K, Ohmi C, et al. Overexpression of polo-like kinase 1 (PLK1) and chromosomal instability in bladder cancer. Oncology 2006; 70:231 - 7; http://dx.doi.org/10.1159/000094416; PMID: 16837776
- Zhang Z, Zhang G, Kong C. High expression of polo-like kinase 1 is associated with the metastasis and recurrence in urothelial carcinoma of bladder. Urol Oncol 2011; PMID: 22192978
- Strebhardt K. Multifaceted polo-like kinases: drug targets and antitargets for cancer therapy. Nat Rev Drug Discov 2010; 9:643 - 60; http://dx.doi.org/10.1038/nrd3184; PMID: 20671765
- Fristrup N, Ulhøi BP, Birkenkamp-Demtröder K, Mansilla F, Sanchez-Carbayo M, Segersten U, et al. Cathepsin E, maspin, Plk1, and survivin are promising prognostic protein markers for progression in non-muscle invasive bladder cancer. Am J Pathol 2012; 180:1824 - 34; http://dx.doi.org/10.1016/j.ajpath.2012.01.023; PMID: 22449953
- Steegmaier M, Hoffmann M, Baum A, Lénárt P, Petronczki M, Krssák M, et al. BI 2536, a potent and selective inhibitor of polo-like kinase 1, inhibits tumor growth in vivo. Curr Biol 2007; 17:316 - 22; http://dx.doi.org/10.1016/j.cub.2006.12.037; PMID: 17291758
- Schmit TL, Zhong W, Setaluri V, Spiegelman VS, Ahmad N. Targeted depletion of Polo-like kinase (Plk) 1 through lentiviral shRNA or a small-molecule inhibitor causes mitotic catastrophe and induction of apoptosis in human melanoma cells. J Invest Dermatol 2009; 129:2843 - 53; http://dx.doi.org/10.1038/jid.2009.172; PMID: 19554017
- Winkles JA, Alberts GF. Differential regulation of polo-like kinase 1, 2, 3, and 4 gene expression in mammalian cells and tissues. Oncogene 2005; 24:260 - 6; http://dx.doi.org/10.1038/sj.onc.1208219; PMID: 15640841
- Nogawa M, Yuasa T, Kimura S, Tanaka M, Kuroda J, Sato K, et al. Intravesical administration of small interfering RNA targeting PLK-1 successfully prevents the growth of bladder cancer. J Clin Invest 2005; 115:978 - 85; PMID: 15761500
- Seth S, Matsui Y, Fosnaugh K, Liu Y, Vaish N, Adami R, et al. RNAi-based therapeutics targeting survivin and PLK1 for treatment of bladder cancer. Mol Ther 2011; 19:928 - 35; http://dx.doi.org/10.1038/mt.2011.21; PMID: 21364537
- Renner AG, Dos Santos C, Recher C, Bailly C, Créancier L, Kruczynski A, et al. Polo-like kinase 1 is overexpressed in acute myeloid leukemia and its inhibition preferentially targets the proliferation of leukemic cells. Blood 2009; 114:659 - 62; http://dx.doi.org/10.1182/blood-2008-12-195867; PMID: 19458358
- Morales AG, Brassesco MS, Pezuk JA, Oliveira JC, Montaldi AP, Sakamoto-Hojo ET, et al. BI 2536-mediated PLK1 inhibition suppresses HOS and MG-63 osteosarcoma cell line growth and clonogenicity. Anticancer Drugs 2011; 22:995 - 1001; PMID: 21822121
- Liu X, Choy E, Harmon D, Yang S, Yang C, Mankin H, et al. Inhibition of polo-like kinase 1 leads to the suppression of osteosarcoma cell growth in vitro and in vivo. Anticancer Drugs 2011; 22:444 - 53; http://dx.doi.org/10.1097/CAD.0b013e32834513f4; PMID: 21399492
- Schmit TL, Zhong W, Setaluri V, Spiegelman VS, Ahmad N. Targeted depletion of Polo-like kinase (Plk) 1 through lentiviral shRNA or a small-molecule inhibitor causes mitotic catastrophe and induction of apoptosis in human melanoma cells. J Invest Dermatol 2009; 129:2843 - 53; http://dx.doi.org/10.1038/jid.2009.172; PMID: 19554017
- Ikezoe T, Yang J, Nishioka C, Takezaki Y, Tasaka T, Togitani K, et al. A novel treatment strategy targeting polo-like kinase 1 in hematological malignancies. Leukemia 2009; 23:1564 - 76; http://dx.doi.org/10.1038/leu.2009.94; PMID: 19421227
- Plyte S, Musacchio A. PLK1 inhibitors: setting the mitotic death trap. Curr Biol 2007; 17:R280 - 3; http://dx.doi.org/10.1016/j.cub.2007.02.018; PMID: 17437704
- Vakifahmetoglu H, Olsson M, Zhivotovsky B. Death through a tragedy: mitotic catastrophe. Cell Death Differ 2008; 15:1153 - 62; http://dx.doi.org/10.1038/cdd.2008.47; PMID: 18404154
- Reznikoff CA, Belair CD, Yeager TR, Savelieva E, Blelloch RH, Puthenveettil JA, et al. A molecular genetic model of human bladder cancer pathogenesis. Semin Oncol 1996; 23:571 - 84; PMID: 8893868
- da Silva GN, de Castro Marcondes JP, de Camargo EA, da Silva Passos Júnior GA, Sakamoto-Hojo ET, Salvadori DM. Cell cycle arrest and apoptosis in TP53 subtypes of bladder carcinoma cell lines treated with cisplatin and gemcitabine. Exp Biol Med (Maywood) 2010; 235:814 - 24; http://dx.doi.org/10.1258/ebm.2010.009322; PMID: 20558835
- Gleixner KV, Ferenc V, Peter B, Gruze A, Meyer RA, Hadzijusufovic E, et al. Polo-like kinase 1 (Plk1) as a novel drug target in chronic myeloid leukemia: overriding imatinib resistance with the Plk1 inhibitor BI 2536. Cancer Res 2010; 70:1513 - 23; http://dx.doi.org/10.1158/0008-5472.CAN-09-2181; PMID: 20145140
- Wolf S, Lorenz J, Mössner J, Wiedmann M. Treatment of biliary tract cancer with NVP-AEW541: mechanisms of action and resistance. World J Gastroenterol 2010; 16:156 - 66; http://dx.doi.org/10.3748/wjg.v16.i2.156; PMID: 20066734
- Stewart HJ, Kishikova L, Powell FL, Wheatley SP, Chevassut TJ. The polo-like kinase inhibitor BI 2536 exhibits potent activity against malignant plasma cells and represents a novel therapy in multiple myeloma. Exp Hematol 2011; 39:330 - 8; http://dx.doi.org/10.1016/j.exphem.2010.12.006; PMID: 21184800
- Didier C, Cavelier C, Quaranta M, Demur C, Ducommun B. Evaluation of Polo-like Kinase 1 inhibition on the G2/M checkpoint in Acute Myelocytic Leukaemia. Eur J Pharmacol 2008; 591:102 - 5; http://dx.doi.org/10.1016/j.ejphar.2008.06.062; PMID: 18616938
- Deckbar D, Jeggo PA, Löbrich M. Understanding the limitations of radiation-induced cell cycle checkpoints. Crit Rev Biochem Mol Biol 2011; 46:271 - 83; http://dx.doi.org/10.3109/10409238.2011.575764; PMID: 21524151
- Rödel F, Keppner S, Capalbo G, Bashary R, Kaufmann M, Rödel C, et al. Polo-like kinase 1 as predictive marker and therapeutic target for radiotherapy in rectal cancer. Am J Pathol 2010; 177:918 - 29; http://dx.doi.org/10.2353/ajpath.2010.100040; PMID: 20581060
- Bordon E, Leon LG, Rios-Luci C, Lara PC, Padron JM. In vitro synergistic interaction between DTA0100 and radiation in human cancer cell lines. Anticancer Agents Med Chem 2012; 12:988 - 93; http://dx.doi.org/10.2174/187152012802650057; PMID: 22339062
- Heravi M, Tomic N, Liang L, Devic S, Holmes J, Deblois F, et al. Sorafenib in combination with ionizing radiation has a greater anti-tumour activity in a breast cancer model. Anticancer Drugs 2012; 23:525 - 33; http://dx.doi.org/10.1097/CAD.0b013e32834ea5b3; PMID: 22357220
- Gerster K, Shi W, Ng B, Yue S, Ito E, Waldron J, et al. Targeting polo-like kinase 1 enhances radiation efficacy for head-and-neck squamous cell carcinoma. Int J Radiat Oncol Biol Phys 2010; 77:253 - 60; http://dx.doi.org/10.1016/j.ijrobp.2009.11.027; PMID: 20394857
- Harris PS, Venkataraman S, Alimova I, Birks DK, Donson AM, Knipstein J, et al. Polo-like kinase 1 (PLK1) inhibition suppresses cell growth and enhances radiation sensitivity in medulloblastoma cells. BMC Cancer 2012; 12:80; http://dx.doi.org/10.1186/1471-2407-12-80; PMID: 22390279
- Qian Y, Hua E, Bisht K, Woditschka S, Skordos KW, Liewehr DJ, et al. Inhibition of Polo-like kinase 1 prevents the growth of metastatic breast cancer cells in the brain. Clin Exp Metastasis 2011; 28:899 - 908; http://dx.doi.org/10.1007/s10585-011-9421-9; PMID: 21953073
- De Santis M, Bellmunt J, Mead G, Kerst JM, Leahy M, Maroto P, et al. Randomized phase II/III trial assessing gemcitabine/ carboplatin and methotrexate/carboplatin/vinblastine in patients with advanced urothelial cancer “unfit” for cisplatin-based chemotherapy: phase II--results of EORTC study 30986. J Clin Oncol 2009; 27:5634 - 9; http://dx.doi.org/10.1200/JCO.2008.21.4924; PMID: 19786668
- Schöffski P, Blay JY, De Greve J, Brain E, Machiels JP, Soria JC, et al. Multicentric parallel phase II trial of the polo-like kinase 1 inhibitor BI 2536 in patients with advanced head and neck cancer, breast cancer, ovarian cancer, soft tissue sarcoma and melanoma. The first protocol of the European Organization for Research and Treatment of Cancer (EORTC) Network Of Core Institutes (NOCI). Eur J Cancer 2010; 46:2206 - 15; http://dx.doi.org/10.1016/j.ejca.2010.03.039; PMID: 20471824
- Haupenthal J, Bihrer V, Korkusuz H, Kollmar O, Schmithals C, Kriener S, et al. Reduced efficacy of the Plk1 inhibitor BI 2536 on the progression of hepatocellular carcinoma due to low intratumoral drug levels. Neoplasia 2012; 14:410 - 9; PMID: 22745587
- Schöffski P, Awada A, Dumez H, Gil T, Bartholomeus S, Wolter P, et al. A phase I, dose-escalation study of the novel Polo-like kinase inhibitor volasertib (BI 6727) in patients with advanced solid tumours. Eur J Cancer 2012; 48:179 - 86; http://dx.doi.org/10.1016/j.ejca.2011.11.001; PMID: 22119200
- Olmos D, Barker D, Sharma R, Brunetto AT, Yap TA, Taegtmeyer AB, et al. Phase I study of GSK461364, a specific and competitive Polo-like kinase 1 inhibitor, in patients with advanced solid malignancies. Clin Cancer Res 2011; 17:3420 - 30; http://dx.doi.org/10.1158/1078-0432.CCR-10-2946; PMID: 21459796
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001; 25:402 - 8; http://dx.doi.org/10.1006/meth.2001.1262; PMID: 11846609
- Franken NA, Rodermond HM, Stap J, Haveman J, van Bree C. Clonogenic assay of cells in vitro. Nat Protoc 2006; 1:2315 - 9; http://dx.doi.org/10.1038/nprot.2006.339; PMID: 17406473
- Lee YJ, Shacter E. Oxidative stress inhibits apoptosis in human lymphoma cells. J Biol Chem 1999; 274:19792 - 8; http://dx.doi.org/10.1074/jbc.274.28.19792; PMID: 10391922
- Pauwels B, Korst AE, de Pooter CM, Pattyn GG, Lambrechts HA, Baay MF, et al. Comparison of the sulforhodamine B assay and the clonogenic assay for in vitro chemoradiation studies. Cancer Chemother Pharmacol 2003; 51:221 - 6; PMID: 12655440
- Tao Y, Zhang P, Frascogna V, Lecluse Y, Auperin A, Bourhis J, et al. Enhancement of radiation response by inhibition of Aurora-A kinase using siRNA or a selective Aurora kinase inhibitor PHA680632 in p53-deficient cancer cells. Br J Cancer 2007; 97:1664 - 72; http://dx.doi.org/10.1038/sj.bjc.6604083; PMID: 18026198
- Chou TC, Talalay P. Quantitative analysis of dose-effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Adv Enzyme Regul 1984; 22:27 - 55; http://dx.doi.org/10.1016/0065-2571(84)90007-4; PMID: 6382953