Abstract
The low permeability of the BBB is largely responsible for the lack of effective systemic chemotherapy against primary and metastatic brain tumors. Kinin B1R and B2R have been shown to mediate reversible tumor-selective BBB disruption in preclinical animal models. We investigated whether co-administration of two novel potent kinin B1R and B2R agonists offers an advantage over administering each agonist alone for enhancing BBB permeability and tumor targeting of drugs in the malignant F98 glioma rat model. A new covalent kinin heterodimer that equally stimulates B1R and B2R was also constructed for the purpose of our study. We found that co-administration of B1R and B2R agonists, or alternatively administration of the kinin heterodimer more effectively delivered the MRI contrast agent Gd-DTPA and the anticancer drug carboplatin to brain tumors and surrounding tissues than the agonists alone (determined by MRI and ICP-MS methods). Importantly, the efficient delivery of carboplatin by the dual kinin receptor targeting on the BBB translated into increased survival of glioma-bearing rats. Thus, this report describes a potential strategy for maximizing the brain bioavailability and therapeutic efficacy of chemotherapeutic drugs.
Gliomas are the most common and deadly intrinsic tumors of the brain and are among the most aggressive of all tumors. Because no cure is available, the prognosis is very poor. Relatively little therapeutic progress has been made over the past three decades.Citation1 The complexity of treating malignant gliomas is compounded by their highly infiltrative phenotype and the existence of the BBB, a powerful physiological barrier that seriously impairs the delivery of numerous anticancer drugs to tumor sites.Citation2 An intact or only partially compromised BBB may also explain the particular refractoriness and poor responses to chemotherapy of brain metastases, mainly arising from lung, breast and skin cancers.Citation3,Citation4
Two basic types of experimental strategies have traditionaly been investigated to improve the effectiveness of anticancer drugs. The first strategy is aimed at physically bypassing the BBB by directly injecting drugs into the brain. The second relies on modulating the permeability of the BBB/BTB via transcellular or paracellular transport process. For the latter strategy, the systemic administration of vaso-acting agents, such as kinins, has been shown to be particularly useful and effective.Citation5 In this regard, our previous work using the highly malignant F98 rat glioma model, showed that the intravascular injection of peptidic kinin agonist analogs selective for the B2R ([Phe8ψ(CH2NH)Arg9]-BK; R523) or the B1R (SarLys[dPhe8]desArg9BK; NG29) induces greater tumor uptake (by 2- to 4-fold) of co-injected tracer molecules including contrast material, albumin and carboplatin.Citation6,Citation7 Interestingly, B1R and B2R recruit separate pathways and distinct chemical mediators to disrupt the BBB/BTB. B1R is linked to the cyclooxygenase pathway, which modulates the production of PGs while B2R is driven almost entirely by endogenous NO.Citation6,Citation7
Certain evidence supports an NO/PGs reciprocal interaction in the regulation of microvascular reactivity.Citation8-Citation10 However, the extent of this interaction at the BBB/BTB is unclear. Thus, a formulation or a compound that successfully targets B1R and B2R simultaneously would, in theory, combine the modulatory effects of endogenous NO and PGs on BBB/BTB permeability and integrity. In this study, we explored the possibility that the dual activation of both B1R and B2R by a combination treatment with potent agonists, selective for each receptor subtype, would be more effective than either receptor activated alone in improving the delivery to malignant brain tumors of drugs including MRI contrast agents and carboplatin. To strengthen our hypothesis and to simplify the BBB/BTB permeation procedure using single-molecule experiments, we designed a new kinin heterodimer capable of stimulating B1R and B2R equally (Scheme 1). To synthesize the bifunctional B1R/B2R kinin agonist using a straightforward, flexible and efficient strategy, we took advantage of CuAAC, widely refered to as the “click” reaction, to covalently link the two kinin agonists. For this purpose, we incorporated an N-terminal Boc-protected, azide-modified lysine residue to complete the preparation of the solid-phase attached B2R peptide agonist, [Hyp3,Thi5,NChg7,Thi8]-BK (NG291). This B2R agonist displayed potent in vitro and in vivo activity greater than that of BK.Citation11,Citation12 The highly potent B1R agonist, Lys-[dPhe8]desArg9BKCitation13, which was grown on an acid trityl resin, was N-terminally extended with an ε-Aca spacer followed by an Fmoc-protected (propargyl)glycine, as a CuAAC substrate. Efficient dimer formation was achieved by mixing the precursor resin-tethered B2R agonist containing azide with a solution of alkyne (propargyl)-containing B1R agonist under standard “click” conditions (see Scheme 1 for detailed synthesis conditions).
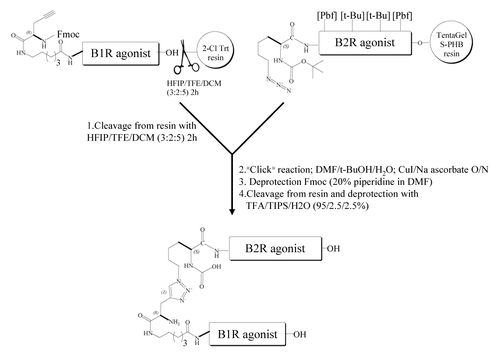
Scheme 1. Construction of a Nα-linked B1R/B2R agonist heterodimer utilizing “click” chemistry and Aca linker. Chemistry conjugation reaction between an alkyne-containing B1R agonist molecule (Fmoc-G(propargyl)-Aca-Lys-Arg-Pro-Pro-Gly-Phe-Ser-Pro-DPhe-OH) and an azide-containing B2R agonist molecule (Boc-Lys(N3)-Arg(Pdf)-Pro-Hyp(t-Bu)-Gly-Thi-Ser(t-Bu)-NChg-Thi-Arg(Pbf)). The peptide B1R agonist (in solution) and the peptide B2R agonist (immobilized on the solid-phase) were then joined at their N-terminal side via a triazole linker, made by “click” chemistry. Heterodimer was then deprotected, cleaved from resin and purified to homogeneity by using RP-HPLC and mass spectrometry. Abbreviations: Aca, ε-aminocaproyl; HFIP, 1,1,1,3,3,3-hexafluoroisopropanol; TFE, 2,2,2-trifluoroethanol; TIPS, triisopropylsilane; TFA, trifluoroacetic acid; Aca, 6-amino caproic acid; t-BuOH, tert-butanol. Hyp, trans-4-hydroxy-l-proline; Thi, β-(2-thienyl)-l-alanine; NChg, N-cyclohexyl-glycine.
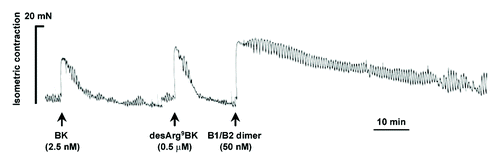
The ability of the novel heterodimer to bind and activate human kinin B1R and B2R was evaluated using in vitro assays (). Compared with the parental unconjugated agonists, the heterodimer showed moderate affinity in transiently transfected B1R- or B2R-HEK-293T cells and displayed full as well as ambivalent agonist activity on B1R and B2R, as determined in vasoconstriction tests using isolated human umbilical veins.Citation14 One peculiar characteristic of the heterodimer was its extended in vitro duration of action, which was at least 10-fold greater than that of naturally-occurring B1R and B2R agonists. Moreover, heterodimer action persisted, despite repeated washings of the human vessels (). Our previous work showed that the long-acting effect was similarly displayed by peptides NG291 and NG29.Citation11,Citation13 The prolonged in vitro duration effect may play an important role in the generation of long-lasting modulatory effects on BBB/BTB permeability in vivo. Co-administration of or pretreatment with HOE140 (B2R antagonist; dArg[Hyp3,Thi5,dTic7,Oic8]-BK) and R954 (B1R antagonist; AcOrn[Oic2,(αMe)Phe5,dβNal7,Ile8]desArg9-BK) (5 μM) fully antagonized the in vitro activity of the heterodimer, which is consistent with its non-selectivity and specificity of action (data not shown). Despite a significant loss of binding affinity/activity compared with the unmodified parent ligands, the heterodimeric agonist retained favorable pharmacodynamic properties (in the nanomolar affinity/activity range), which makes it an interesting candidate for further in vivo experiments.
Table 1. Receptor binding affinity and pharmacological activity of kinin derivatives at human B1R and B2R
Figure 1. Differential vasoconstrictor actions of natural B2R and B1R agonists, bradykinin (BK), and desArg9BK and the newly-developed heterodimeric B1R/B2R agonist in the isolated human (endothelium-denuded) umbilical veins. Representative tracings (of three replicates) of the vasoconstrictor responses induced by kinin agonists, are shown. Agonists were tested at concentrations near their EC50 value (see ). Note the important long, sustained duration of action of the heterodimer.
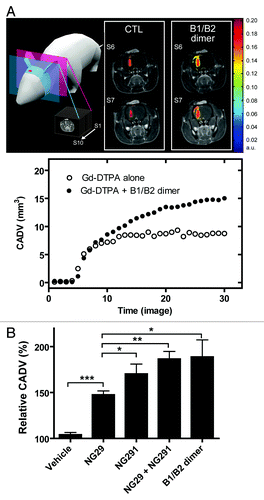
To examine the in vivo efficacy of the kinin B1R and B2R agonists at modulating the status of the BBB/BTB in Fischer rats bearing intracranial syngeneic F98 gliomas, we used DCE-MRI with intravenous Gd-DTPA (0.5 kDa; Magnevist). This technique has been shown to be suitable for real-time, non-invasive monitoring of the spatiotemporal processes of BBB/BTB opening and of the delivery of contrast agents into the brain.Citation6,Citation7 At 10 d post-implantation, i.e., at mid-stage of tumor development, the animals underwent baseline MRI and were randomized into 5 groups: (1) saline control; (2) BBB disruption by B1R agonist NG29; (3) BBB disruption by B2R agonist NG291; (4) BBB disruption by NG29 and NG291 given in conjunction; and (5) BBB disruption by the B1R/B2R heterodimer. Agonists were administered via the right internal carotid, directly into the tumor-bearing hemisphere at a high dose (50 nmol/min/kg for 5 min) that produces a robust increase in BBB/BTB permeability in gliomas. The Gd-DTPA was injected i.v. (caudal vein) 3 min after the beginning of BBB disruption maneuver.Citation6,Citation7 Malignant brain tumor cells easily infiltrate or invade nearby normal tissues. Thus, it is important to facilitate drug delivery/uptake in these regions for chemotherapy to be successful. Therefore, we investigated changes in the Gd-DTPA spatial distribution beyond tumor boundaries, following administration of kinin agonists. Compared with vehicle controls, all agonists significantly increased CADV at the tumor site (), which was likely due to the permeation of the vasculature feeding glioma. This is clearly illustrated for the B1R/B2R heterodimer, which produced a more marked signal enhancement of Gd-DTPA at the peripheral rim of the tumor relative to baseline (, upper panel). This led to a significantly wider CADV (, lower panel). Compared with the B1R agonist NG29, the B2R activating agonists produced a slightly higher increased CADV. Animal groups subjected to B1R/B2R co-stimulation, by both agonists administered individually or as a covalently-linked heterodimer, did not show any extended CADV as compared with the B2R agonist NG291 administered alone (), indicating that the co-stimulation of both B1R and B2R does not produce an additive effects upon modification of this parameter. In other words, spatial distribution of the contrast agent visualized as contrast enhancement in areas outside the tumor where BBB is disrupted, reached maximal amplitude after BBB disruption mediated through B2R. This is congruent with the widespread distribution of B2R throughout the cerebral circulatory system.
Figure 2. Comparison of contrast-agent distribution and uptake in the brain following intraarterial administration of B1R and B2R agonists. Brain tumors were induced by intracranial injection of 1 × 104 F98 glioma cells on day 0. MRI experiments were performed 10 d after tumor transplantation; whole set of acquired images consisted of 10 axial, contiguous slices with a thickness equal to 1.5 mm. The schematic diagram shows MRI slices in which measurements were taken. (A) Representative axial Gd-DTPA-enhanced T1-weighted MR images depicting the brain of an F98-implanted rat before and after intracarotid B1R/B2R dimer agonist treatment (50 nmol/kg/min for 5 min). Regions of contrast enhancement related to Gd-DTPA are highlighted in pseudo-colors. Contrast agent distribution volume (CADV) as a function of time calculated from the corresponding set of images (bottom panel). (B) Relative CADV in percent determined following the infusion of the vehicle (saline), B1R agonist NG29, B2R agonist NG291, NG29+NG291, or B1R/B2R dimer agonist. Agonists were given at the dose of 50 nmol/kg/min for 5 min (i.c.). Each bar represents the mean ± SEM for 4 to 6 animals. The differences between treatment groups were examined using the Student unpaired t test. *Statistical significance (P < 0.05). Methods of these experiments based on MRI are presented elsewhere.Citation6,Citation7
Unlike the B1R agonist NG29, the B2R interacting ligands NG291 and heterodimer were highly hypotensive at the selected BBB disrupting dose. This side effect was nevertheless reversible with recovery occurring within approximately 15–30 min after injection (not shown). Dosage adjustment will be required in order to reduce this unwanted effect.
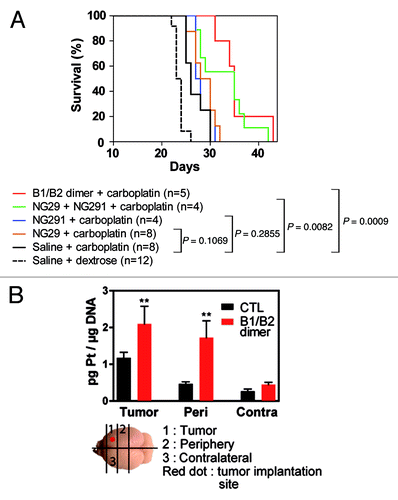
To determine whether dual kinin B1R/B2R activation provides enhanced BBB/BTB permeability and, as a result, increased anticancer drug levels in tumors, we used the highly sensitive, validated ICP-MS method.Citation6 Specifically, this method was used to directly and simultaneously determine the concentrations of platinum (Pt) and gadolinium (Gd), originating from carboplatin and Gd-DTPA (used as a reference), respectively, in tissue extracts from tumors, peripheral brain tissues and cortical matched contralateral tissues of control and experimental animal groups (). Administration of individual agonists led to a marked and rapid increase in transvascular delivery as well as an incremental increase of circulating Gd-DTPA and carboplatin in the tumor and its periphery compared with the controls (1.5- to 4-fold). It should be stressed that tissue Pt and Gd concentrations were measured soon (5 min) after i.c. administration of the agonist/drug combination and probably underestimated the true magnitude of the effect of kinin agonist treatment. Notably, NG29 combined with NG291 caused significantly greater penetration and accumulation (5- to 10-fold) of the molecules in the tumors and surrounding normal tissues, suggesting an additive effect of the agonists. Co-stimulation of B1R and B2R using the novel heterodimer also led to a dominant uptake of Pt and Gd within tumors. Concentrations at contralateral sites remained low or unchanged (with some exceptions) following BBB disruption with the agonists. Most importantly, the greater efficacy of the B1R/B2R agonist combination over agonists alone at increasing delivery of carboplatin across BBB translated to increased survival of F98 glioma-bearing rats (). This effect may be directly related to increased formation of Pt-DNA adducts in tumors as shown in initial results obtained with the B1R/B2R heterodimer (). A trend toward therapeutic benefit with single-agonist therapy was also evident, but did not reach statistical significance. These results suggest that BBB targeting with selective kinin receptor modulators used in combination or as non-selective forms (heterodimer) contribute to the cytotoxic efficacy of carboplatin against F98 rat-glioma. The promising survival data warrants further preclinical investigations in additional animal models.
Figure 3. Differential effects of synthetic B1R and B2R agonists on tumor and tissue uptake of the chemotherapy drug carboplatin and the contrast agent Gd-DTPA. Direct measures of drug concentration (ng/g of wet tissue) by ICP-MS in three different tissue extracts (tumor, tumor periphery and contralateral) from the brains of F98 glioma-bearing Fischer rats were made following intra-arterial (internal carotid) injections of Gd-DTPA (Gd) (143 mM i.v.) and carboplatin (Pt) (20 mg/kg) with various agonists (250 nmol/kg) or saline (CTRL) in a single 1-ml infusion over 10 min. Five minutes after the end of the infusion, rats were euthanized and the brain rapidly removed, dissected into regions, and prepared for ICP-MS analysis. Differences were examined by the Student unpaired t test, considering P < 0.05 siginificant. General methods as in Côté et al.Citation6
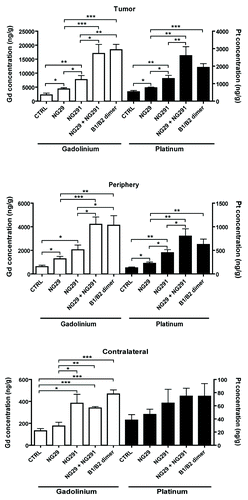
Figure 4. Dual B1R/B2R agonist treatment enhances the in vivo antitumor activity of carboplatin. (A) Kaplan–Meier survival curves of F98 glioma-bearing rats. F98 glioma cells (1 × 104 cells) were inoculated on day 0. MRI scans were performed on day 9 in the animal groups (2−3 rats/group) to confirm the presence and size of tumors before the beginning of the treatments. On day 10, animals were treated with the vehicle, agonists alone or in combination (50 nmol/kg/min i.c; 0.5 ml over 5 min) followed by carboplatin (20 mg/kg; 1 ml over 10 min). Survival curves were compared with the log-rank test. (B) Pt-DNA adducts in tumors, tumor periphery and contralateral tissues from F98 glioma-bearing Fischer rats treated with carboplatin in combination or not with the B1R/B2R dimer agonist as above. Brain slices (100−200 mg) were collected 24h after the administration of the drugs and immediately snap frozen in liquid nitrogen. A diagram depicting how the schematic slices were taken is shown. Frozen rat brain tissues were cut into pieces and digested with RNase A and Proteinase K in the presence of SDS detergent. Genomic DNA was extracted by the phenol/chloroform method and its purity estimated from the 260/280 nm-absorbance ratio. DNA samples were then analyzed for their Pt content by ICP-MS as described in Côté et al.Citation5P < 0.05 as determined by the Dunnett test; n = 3−4 animals per group.
In summary, the increased uptake of platinum by malignant brain tumors and surrounding tissues (showing invasion of cancer cells), induced by the concerted actions of B1R and B2R agonist analogs, indicate the potential usefulness of this pharmacological approach to increase dose-intensity and treatment efficacy of chemotherapy in patients with brain tumors. These findings are significant given that the best method for delivering higher concentrations of intracerebral anticancer drugs remains elusive.Citation2,Citation15 In addition, aside from being highly potent modulators of BBB/BTB, single molecules exhibiting mixed B1R/B2R agonist activity, such as the heterodimer described here, may provide a number of advantages over combination therapy with individual agonists. These include a single pharmacokinetic profile, a uniform ratio of activities at the cellular level and simpler in vivo preclinical testing in animal models. Heterodimeric B1R/B2R agonists also show good potential for the development of more efficient chemotherapeutic treatments for various classes of brain tumors that display variable patterns of expression of their cognate kinin receptors.Citation16
| Abbreviations: | ||
| BBB | = | blood–brain barrier |
| BTB | = | blood–tumor barrier |
| GPCR | = | G protein-coupled receptors |
| B1R | = | B1 receptor |
| B2R | = | B2 receptor |
| BK | = | bradykinin |
| DCE-MRI | = | dynamic contrast-enhanced magnetic resonance imaging |
| Gd-DTPA | = | gadolinium diethylenetriamine-pentaacetic acid (Magnevist) |
| ICP-MS | = | inductively coupled plasma-mass spectrometry |
| NO | = | nitric oxide |
| PGs | = | prostaglandins |
| CuAAC | = | copper (I)-catalysed azide–alkyne cycloaddition |
| ε-Aca | = | ε-aminocaproyl |
| CADV | = | contrast agent distribution volume |
Acknowledgments
FG is a senior scholar from the Fonds de la recherche en santé du Québec (FRSQ) and a researcher of the Canada Foundation for Innovation (CFI). ML is the Canada Research Chair in Magnetic Resonance Imaging. FG, DF, and ML are members of the FRQS-funded Centre de recherche clinique Étienne-Le Bel. This work was supported by a Canadian Institutes for Health Research Proof of Principle grant (PPP-120206).
Disclosure of Potential Conflicts of Interest
No potential conflict of interest was disclosed.
References
- Rich JN, Bigner DD. Development of novel targeted therapies in the treatment of malignant glioma. Nat Rev Drug Discov 2004; 3:430 - 46; http://dx.doi.org/10.1038/nrd1380; PMID: 15136790
- Muldoon LL, Soussain C, Jahnke K, Johanson C, Siegal T, Smith QR, et al. Chemotherapy delivery issues in central nervous system malignancy: a reality check. J Clin Oncol 2007; 25:2295 - 305; http://dx.doi.org/10.1200/JCO.2006.09.9861; PMID: 17538176
- Fortin D. The blood-brain barrier: its influence in the treatment of brain tumors metastases. Curr Cancer Drug Targets 2012; 12:247 - 59; http://dx.doi.org/10.2174/156800912799277511; PMID: 22229251
- Misra A, Ganesh S, Shahiwala A, Shah SP. Drug delivery to the central nervous system: a review. J Pharm Pharm Sci 2003; 6:252 - 73; PMID: 12935438
- Black KL, Ningaraj NS. Modulation of brain tumor capillaries for enhanced drug delivery selectively to brain tumor. Cancer Control 2004; 11:165 - 73; PMID: 15153840
- Côté J, Bovenzi V, Savard M, Dubuc C, Fortier A, Neugebauer W, et al. Induction of selective blood-tumor barrier permeability and macromolecular transport by a biostable kinin B1 receptor agonist in a glioma rat model. PLoS One 2012; 7:e37485; http://dx.doi.org/10.1371/journal.pone.0037485; PMID: 22629405
- Côté J, Savard M, Bovenzi V, Dubuc C, Tremblay L, Tsanaclis AM, et al. Selective tumor blood-brain barrier opening with the kinin B2 receptor agonist [Phe(8)psi(CH(2)NH)Arg(9)]-BK in a F98 glioma rat model: an MRI study. Neuropeptides 2010; 44:177 - 85; http://dx.doi.org/10.1016/j.npep.2009.12.009; PMID: 20080302
- Emerich DF, Dean RL, Snodgrass P, Lafreniere D, Agostino M, Wiens T, et al. Bradykinin modulation of tumor vasculature: II. activation of nitric oxide and phospholipase A2/prostaglandin signaling pathways synergistically modifies vascular physiology and morphology to enhance delivery of chemotherapeutic agents to tumors. J Pharmacol Exp Ther 2001; 296:632 - 41; PMID: 11160652
- Maeda H, Fang J, Inutsuka T, Kitamoto Y. Vascular permeability enhancement in solid tumor: various factors, mechanisms involved and its implications. Int Immunopharmacol 2003; 3:319 - 28; http://dx.doi.org/10.1016/S1567-5769(02)00271-0; PMID: 12639809
- Mollace V, Muscoli C, Masini E, Cuzzocrea S, Salvemini D. Modulation of prostaglandin biosynthesis by nitric oxide and nitric oxide donors. Pharmacol Rev 2005; 57:217 - 52; http://dx.doi.org/10.1124/pr.57.2.1; PMID: 15914468
- Bélanger S, Bovenzi V, Côté J, Neugebauer W, Amblard M, Martinez J, et al. Structure-activity relationships of novel peptide agonists of the human bradykinin B2 receptor. Peptides 2009; 30:777 - 87; http://dx.doi.org/10.1016/j.peptides.2008.12.003; PMID: 19111586
- Savard M, Labonté J, Dubuc C, Neugebauer W, D’Orléans-Juste P, Gobeil F Jr.. Further pharmacological evaluation of a novel synthetic peptide bradykinin B2 receptor agonist. Biol Chem 2013; 394:353 - 60; http://dx.doi.org/10.1515/hsz-2012-0295; PMID: 23362191
- Côté J, Savard M, Bovenzi V, Bélanger S, Morin J, Neugebauer W, et al. Novel kinin B1 receptor agonists with improved pharmacological profiles. Peptides 2009; 30:788 - 95; http://dx.doi.org/10.1016/j.peptides.2008.12.018; PMID: 19150636
- Gobeil F, Pheng LH, Badini I, Nguyen-Le XK, Pizard A, Rizzi A, et al. Receptors for kinins in the human isolated umbilical vein. Br J Pharmacol 1996; 118:289 - 94; http://dx.doi.org/10.1111/j.1476-5381.1996.tb15401.x; PMID: 8735629
- Neuwelt E, Abbott NJ, Abrey L, Banks WA, Blakley B, Davis T, et al. Strategies to advance translational research into brain barriers. Lancet Neurol 2008; 7:84 - 96; http://dx.doi.org/10.1016/S1474-4422(07)70326-5; PMID: 18093565
- Zhao Y, Xue Y, Liu Y, Fu W, Jiang N, An P, et al. Study of correlation between expression of bradykinin B2 receptor and pathological grade in human gliomas. Br J Neurosurg 2005; 19:322 - 6; http://dx.doi.org/10.1080/02688690500305555; PMID: 16455538