Abstract
High endothelial venules (HEVs) are special blood vessels in the paracortical region of lymph nodes (LNs) and govern lymphocyte recruitment. LN metastasis has similarity to circulating lymphocytes homing to LNs, but the role of HEVs in the progression of oral and pharyngeal squamous cell carcinoma (OPSCC) is unclear. In this study, we found that HEVs experienced a series of morphological and functional changes during OPSCC progression and were correlated with LN metastasis. In 9 cases of 73 metastatic LNs, tumor emboli were located adjacent to HEVs or just out of the vessels but not lymphatic channels. Gap junctions of tumor cells close to HEVs decreased or disappeared, and gaps were left at contact points where tumor cells attached to the HEVs. Moreover, the proliferation rate of endothelial cells of HEVs was the highest in metastatic LNs. Finally, L-selectin was detected in both primary and metastatic tumors, and it facilitated tumor cells adhering to LNs. In conclusion, our findings suggest that remodeled HEVs are correlated with LN metastasis of OPSCC and play important role in this process by preparing premetastatic soil for cancer cell metastasis.
Introduction
High endothelial venules (HEVs) are post-capillary venules with characteristically thick-walled and plump endothelial cells, and are generally located in the lymphoid organs to govern lymphocyte recruitment.Citation1-Citation3 In addition, HEV-like vessels have been observed in non-lymphoid tissues during medical conditions such as chronic inflammatory diseases.Citation1,Citation4,Citation5
Recently, the implications of HEVs in human cancers have gained much attention because accumulating evidence suggested that lymph node (LN) metastasis of human cancer may share similar mechanisms with lymphocyte trafficking.Citation6,Citation7 Martinet et al. found that the density of HEVs in primary solid tumors was a strong predictor of T- and B-lymphocyte infiltration, and high density of tumor HEVs was correlated with a favorable prognosis in breast carcinoma.Citation8 However, other studies reported vasculature reorganization in sentinel LNs without any evidence of metastasis.Citation9,Citation10 The remodeled functional blood vessels in such nodes develop from HEVs, which may help establish a premetastatic microenvironment before the arrival of cancer cells.Citation11 In addition, Stauder et al. demonstrated that specific-HEV recognition could determine the traffic and dissemination of tumor cells.Citation12 Obviously, these results seem contradictory, and the role of HEV remodeling in cancer biology need to be further characterized.
Oral and pharyngeal squamous cell carcinoma (OPSCC) ranks as the eighth most common cancer diagnosed in men in the United States, and pharynx and tongue are the most prevalent sites.Citation13 Despite efforts during the past several decades to improve clinical treatment, over 50% of OPSCC patients die within 5 y after diagnosis.Citation14,Citation15 LN metastasis is the most significant prognostic indicator influencing the survival outcome of OPSCC patients.Citation16-Citation18 To understand the role of HEVs in OPSCC progression, we made an overall examination on the profiles of HEV alteration in both primary tumors and different pathological status of cervical LNs. Our results showed that the remodeled HEVs in OPSCC were correlated with LN metastasis.
Results
HEV-like vessels are increased in primary OPSCCs and correlated with nodal metastases
We used blood vessel markers to detect the alterations of HEVs within 64 primary OPSCCs and 24 normal mucosa. MECA-79+ vessels were observed in the majority of tumor tissues analyzed (39/64), but not in normal control tissues. These endothelial cells were also labeled by pan-vascular markers CD31 () and CD34. Interestingly, MECA-79+ vessels in different tumor areas presented different morphological characteristics (), and were classified as classic HEVs and HEV-like vessels according to their morphological features. Classic HEVs were mainly located within lymphocyte-infiltrated tumor areas and kept the plump, cuboidal endothelial cells with closed lumen like in lymphoid tissues (); HEV-like vessels were found in areas adjacent to tumor cell cluster but without lymphocyte infiltration, and filled with endothelial cells with enlarged lumen containing numerous RBC (). In 39 of MECA-79+ primary OPSCCs, further analysis showed that there was an increase in the density of total MECA-79+ vessels, including classic HEVs and HEV-like vessels, in 14 OPSCC patients with LN metastasis. However, only the density of HEV-like vessels in metastasis group was significantly higher than in non-metastasis group (P = 0.037) (). These datat suggest that enhanced HEV-like vessels are increased in primary OPSCCs and correlated with nodal metastases.
Figure 1. Morphologic characterization of HEV in primary OPSCCs. (A and B) Immunohistochemical staining of CK (AE1/AE3) (blue), MECA-79 (green), and CD31 (red). The MECA-79+/CD31+ blood vessels were detected under the squamous endothelial cells (A) and approximate tumor cells (B). (C) Classic HEVs were located in the lymphocyte-rich areas. (E) high-magnification of the boxed region in (C), classic HEVs kept tall endothelial cells and narrow lumens. (D) HEV-like vessels were adjacent to tumor cells. (F and G) Two consecutive sections of high-magnification of the boxed region in (D). (F) Immunohistochemical staining of MECA-79, HEV-like vessels were dilated and the vessel wall transformed into thin-walled, containing RBC. (G) Immunohistochemical staining of CK (AE1/AE3), which indicated tumor cells. Bar, 100 μm (A and B); 200 μm (C and D), and 50 μm (E–G).
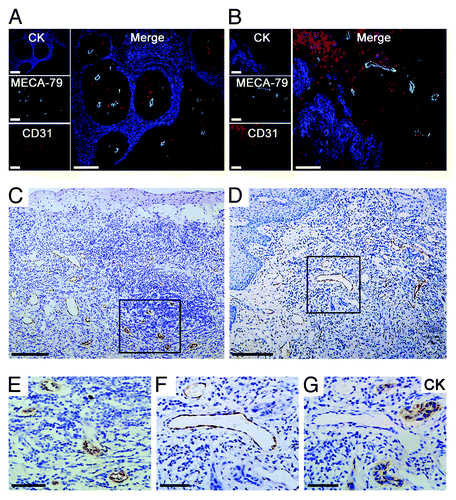
Table 1. Correlation of the density of MECA-79+ vessels and LN metastasis
Remodeling of HEVs in different pathological status of cervical LNs
All collected cervical LNs (n = 308) were classified into 5 groups: group I (46.01%; 142/308), group II (20.45%; 63/308), group III (14.29%; 44/308), group IV (11.36%; 35/308), and group V (7.79%; 24/308). To investigate the alterations of HEVs in different pathological status of LNs, CD34, and MECA-79 antibodies were used to recognize the endothelial cells of traditional blood vessels and/or HEVs. We found that MECA-79+ vessels experienced morphological alterations from classic HEVs to HEV-like vessels with the development of secondary tumor in LNs. In group II–V, MECA-79+ vessels showed lower number of endothelial cells as well as wider and fusiform lumens, and contained RBC, especially in reactively-enlarged LNs and macrometastatic LNs ().
Figure 2. Quantitative analyses of the remodeling of HEVs and blood vessels in LNs. (A) According to pathologic categories, LNs (n = 308) from OPSCC patients were classified into five groups: nearly-normal LNs (n = 142), reactively-enlarged LNs (n = 63), isolated tumor cells (ITCs) (n = 44), micrometastases (n = 35), and macrometastases (n = 24). Continuous sections from each LN were stained for HEVs (anti-MECA-79) and blood vessels (anti-CD34). Bar, 100 μm. (B) The increase of the mean number of MECA-79+ and CD34+ blood vessels in reactively-enlarged LNs was statistically significant, and the decrease of the mean number in ITCs, micrometastatic and macrometastatic groups was statistically significant. *P < 0.05 vs. normal; **P < 0.001 vs. enlarged. (C) The percentage of MECA-79+/CD34+ blood vessels increased in reactively-enlarged, micrometastatic and macrometastatic LNs, and decreased in ITCs group. *P < 0.001 vs. normal; **P < 0.01 vs. enlarged.
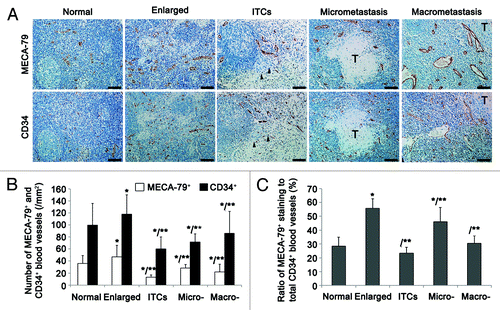
To quantitatively assess these alterations, 20 hotspots for each section were selected to count CD34+ and MECA-79+ vessels. We observed that the reactively enlarged group contained the maximum number of CD34+ and MECA-79+ vessels per field. In group III–V, the mean number of CD34+ vessels was increased following the enlargement of metastasis. However, the highest mean number of MECA-79+ vessels was found in the micrometastatic group, compared with the other two metastatic groups (vs. ITCs, P = 0.0034; vs. macrometastasis, P = 0.02) (). In addition, the proportion of MECA-79+ vessels in all CD34+ blood vessels in the reactively-enlarged group and micrometastatic group was much higher than that in other three groups (). Taken together, these results suggest that the changes of HEVs may help prepare premetastatic soil and provide nutrition for metastatic cancer cells.
HEVs act as a potential metastatic pathway for OPSCC
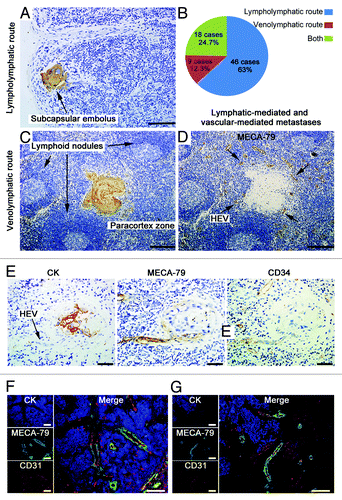
Immunohistochemical analysis showed three routes of metastasis in 73 of 103 metastatic LNs (cases with damaged LNs structures and extensive marginal sinus involvement were excluded). Forty-six cases (63%) met criteria for lymphatic-mediated metastases,Citation19 including intralymphatic emboli, subcapsular or intrasinusoidal tumor nest, interfollicular nest with sparing of germinal center (); 9 cases (12.3%) were classified as HEV-mediated venolymphatic metastases; and 18 cases (24.7%) presented evidence for both lympholymphatic and venolymphatic routes (). In 9 cases of HEV-mediated venolymphatic metastases, tumor emboli were situated in the paracortical regions, directly adjacent to HEVs or just out of the vessels, without any association with lymphatic channels or sinuses (). In total 103 cases of metastatic LNs detected by triple-labeling immunofluorescent staining, we observed that the tumor foci were surrounded by abundant HEVs. Moreover, MECA-79+ vessels were observed within the metastatic tumor nests (), and the tumor cells tended to infiltrate to the HEV-rich regions in the front of metastasis ().
Figure 3. HEVs are implicated in LN metastasis of OPSCC. Metastatic LNs of 30 OPSCC patients were stained for CK (AE1/AE3) (A and C) and MECA-79 antibody (D). (A) Image of a lymphatic-mediated metastatic LN, with a subcapsular tumor embolus (arrow). (C and D) Images of LN consecutive sections, showing tumor embolus in paracortical region (C) where HEVs were present (D). (B) The proportion of lympholymphatic and venolymphatic pathways in 73 metastatic LNs. (E) Images of LN consecutive sections, tumor cells were stained with CK (AE1/AE3) and HEVs stained with HEV-specific marker MECA-79 and pan-endothelial cell marker CD34. Tumor embolus were obverved adjacent to the vascular space lined by plump epithelial cells (arrow). (F and G) Immunohistochemical staining of CK (AE1/AE3) (blue), MECA-79 (green), and CD31 (red). The MECA-79+/CD31+ blood vessels were detected within the tumor lesion (F), and tumor cells migrated to the HEV-dense regions in the front of metastasis (G). Bar, 100 μm (A, F, and G); 200 μm (C and D), and 50 μm (E).
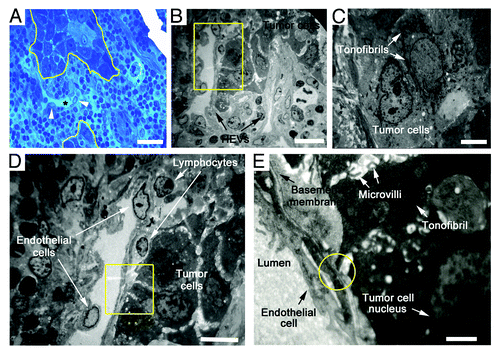
To further characterize the ultrastructural features of HEVs and the surrounding tumor cells, TEM analysis was performed on ultrathin sections of metastatic LNs. Typical HEVs contained characteristically cuboidal epithelial cells encircling the narrow lumen, with thick basal lamina and prominent perivascular sheath. However, the remodeled HEVs close to tumor nest displayed an extremely thin and enlarged lumen, containing red blood cells with loose structure and non-continuous basement membrane (). The tumor cells far away from the HEVs contacted firmly with each other through frequent gap junctions such as desmosomes and hemidesmosomes, and had abundant tonofibrils in the cytoplasm (). In contrast, the tumor nests adjacent to HEVs were randomly scattered by different-sized and shaped cells, and the gap junctions decreased or disappeared between adjacent cells. Extensive, short, and stubby microvilli could be observed on the tumor cell surface (). These alterations may help cancer cells fall off and spread easily. More importantly, when migrating tumor cells adhered to HEV luminal surfaces through microvilli, gaps were left at contact points between tumor cells and endothelial cell membranes (). These resembled the pattern of lymphocytes migrating into HEVs and suggest that HEVs may provide an alternative pathway for tumor cells entering into LNs.
Figure 4. Ultrastructure of HEVs and the surrounding tumor cells in metastatic LNs. (A) Methylene blue-stained epoxy section of a metastatic LN from an OPSCC patient, showing tumor cells (marked by yellow lines) adjacent to a dilated HEV (marked by *). Arrows, the cuboidal epithelial cells of HEVs. (B) Electron microscopy image. Metastatic tumor lesion was adjacent to two HEVs, which displayed thin and enlarged lumens with loose structure. (C) Tumor cells distant to HEVs in a close structure had mass tonofibrils and frequent gap junctions. (D) High-magnification of the yellow boxed region in (B), tumor cells were randomly scattered, and gap junctions decreased or disappeared between adjacent cells. (E) High-magnification of the yellow boxed region in (D), extensive microvilli was observed on the surface of tumor cells, and a few tonofibrils in the cytoplasm. Microvilli of tumor cell adhered to HEV luminal surfaces, and a gap was formed at contact point between tumor cells and endothelial cell membrane (yellow circle). Bar, 50 μm (A), 20 μm (B), 4 μm (C), 10 μm (D), and 2 μm (E).
Proliferation of HEVs endothelial cells in different regions
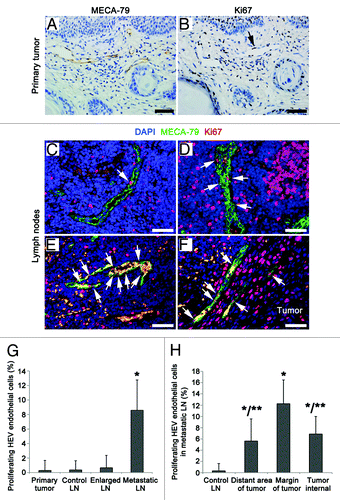
To determine whether HEVs have a functional shift in the process of morphological transformation, we detected the proliferation rates of HEVs endothelial cells in primary tumor (), nearly-normal, reactively-enlarged (), and metastatic LN (). We observed a significant increase in the proliferation of HEVs endothelial cells in metastatic group, compared with other three groups (). Furthermore, the proliferation rate of HEV endothelial cells was different in different areas within metastatic LNs (). The highest proliferation rate was found in HEVs around metastatic tumors, compared with HEVs distant from and/or within metastatic lesions (). These results indicate that HEVs with higher proliferation rate of endothelial cells may be correlated with LN metastasis of OPSCC.
Figure 5. Proliferation rates of the HEV endothelial cells in primary tumor and LNs from OPSCC patients. (A and B) Consecutive sections of a primary tumor, HEVs, and proliferating cells were detected by MECA-79 staining (A) and proliferating marker Ki67 staining (B), respectively. Arrow, the proliferating endothelial cells of HEVs. (C–F) Staining of the proliferating endothelial cells in HEVs (arrows) in reactively-enlarged LN (C), the distant region of metastasis (D), the margin of metastatic tumor lesion (E) and the internal of tumor lesion (F) in metastatic LNs by MECA-79 (green), Ki67 (red), and DAPI (blue). Bar, 50 μm (A–F). (G) The proliferating rates of HEV endothelial cells in primary tumor and LNs. *P < 0.001 vs. control. (H) The proliferating rates of HEV endothelial cells in metastatic LNs. *P < 0.05 vs. control; **P < 0.05 vs. the margin of metastatic tumor lesion.
L-selectin is involved in HEV-mediated metastasis to LNs
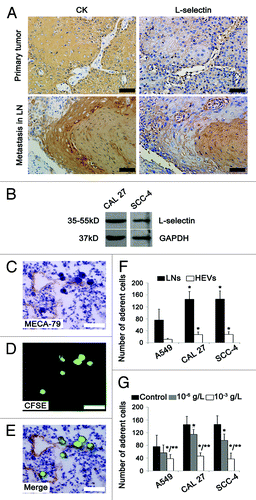
LN metastasis of cancer cells is similar to lymphocyte homing which is mediated by the interaction between lymphocyte L-selectin and PNAd ligands on HEVs.Citation20 Thus we investigated the possible role of L-selectin in HEV-mediated LN metastasis of OPSCC. Strong staining of L-selectin was observed in both primary and metastatic tumors (). The expression of L-selectin was also confirmed in two oral cancer cell lines CAL 27 and SCC-4 by western blot analysis (). In Stamper–Woodruff assay, SCC-4 cells were clustered within and around HEVs which were recognized by MECA-79 antibody (). Similar results were obtained in CAL-27 cells. Statistical analysis showed that there was a significant tendency for oral cancer cells to bind to LNs, compared with A549 cells (). After treatment with L-selectin antibody, the number of cancer cells adhering to LNs was reduced in an antibody concentration-dependent manner. Moreover, the inhibition effect of L-selectin antibody on the adhesion of CAL 27 and SCC-4 cells was much stronger than that of A549 cells (). These data suggest that L-selectin is involved in the adhesion of tumor cells to HEVs.
Figure 6. L-selectin is involved in HEV-mediated LN metastasis in OPSCC patients. (A) Immunohistochemical staining of primary and metastatic tumor sections by tumor cells marker CK (AE1/AE3) and adhesion molecule L-selectin. Strong staining of L-selectin was observed in primary and metastatic tumors with membranous and/or cytoplasmic pattern. (B) Western blot analysis of L-selectin expression in human oral cancer cells CAL 27 and SCC-4. GAPDH was loading control. (C–G) Stamper–Woodruff assay of the adherence of human lung adenocarcinoma (A549) and human oral SCC (CAL 27 and SCC-4) cells to LNs and HEVs. (C) HEVs were detected by MECA-79 staining. (D) SCC-4 tumor cells were detected by CFSE (green). (E) Merged images of (C and D), clustered tumor cells adhered to HEVs. Bar, 50 μm (A and C–E). (F) The number of tumor cells adhering to LNs and HEVs. *P < 0.001 vs. A549. (G) L-selectin antibody inhbited the adherence of tumor cells to LNs. Every 200 μl 2 × 106 cell suspension was added: PBS 1 μl (control); 10−6 g/L L-selectin antibody 1 μl; 10−3 g/L L-selectin antibody 1 μl. *P < 0.05 vs. control; **P < 0.05 vs. 10−6 g/L L-selectin antibody.
Discussion
HEVs are post-capillary venules with specialized endothelial cells, and govern lymphocyte recruitment in physiologic conditions.Citation1-Citation3 Accumulating evidence suggested that tumor cell migration and metastasis may share similar mechanisms with lymphocyte trafficking.Citation6,Citation7 Therefore, in the current study we investigated HEV remodeling in primary and metastatic OPSCC, and its possible correlation with neck LN metastasis.
Our results showed that MECA-79+ vessels were consistently observed in primary OPSCCs. Different from previous reports, however, we found that total MECA-79+ vessels existed in two forms: classic HEVs were mainly located within inflammatory cell-infiltrated regions and kept thick-walled and closed vessels like in lymphoid tissues; HEV-like vessels approximated tumor cells without lymphocyte infiltration and presented thin-walled, enlarged vessels containing numerous RBC. Martinet et al. reported that HEVs present in primary tumors may facilitate lymphocytes infiltration into tumors and limit metastasis establishment in distant organs, and high density of tumor-HEVs was correlated with a favorable prognosis in breast carcinoma.Citation8 Our results showed that the density of total MECA-79+ vessels had no significant difference between metastasis and non-metastasis groups. However, the patients with LN metastasis had higher density of HEV-like vessels in primary tumors than patients without metastasis. Therefore, we speculated that while classic HEVs may function as a gateway for recruiting lymphocytes to exhibit anti-tumor effects, HEV-like vessels with wider and thinner lumen may participate in metastatic process through providing blood channels for nutrition supply and tumor metastasis.
Previous studies have shown that remodeled functional blood vessels develop from HEVs in sentinel LNs without any evidence of metastasis, and the vascular reorganization is quite different from endotoxin-induced inflammatory vasculature alteration.Citation9-Citation11 In the present study, we examined the changes of HEVs in OPSCC-related cervical LNs with different pathologic status. We found that MECA-79+ vessels experienced morphological alterations from classic HEVs to HEV-like vessels with the development of secondary tumor in LNs. Before tumor cells migrated into LNs, blood vessels and HEVs density were significantly increased. Following tumor cells settlement and metastatic growth, the vascular density became higher, but the most HEV-enriched stage was when the nest grew to 0.2–2.0 mm. Moreover, the HEV endothelial cells displayed different proliferation rates in primary and metastatic tumors. In metastatic LNs, the proliferation rate of HEV endothelial cells was the highest, especially the HEVs surrounding the metastatic tumor lesions. Taken together, our results suggest that the dynamic HEV remodeling may play different roles in different metastatic phases, first prepare the premetastatic soil before the arrival of cancer cells, and then provide necessary nutrition for tumor cells when secondary tumor is established.
Notably, our results provided several lines of evidence that the HEV is a potential metastatic pathway for cancer cells to metastasize to neck LNs. First, in some cases metastatic tumor clusters were located in the paracortical areas without any relationship with lymphatic channels, consistent with previous study.Citation21 Second, isolated tumor embolus could be found just migrated from the vessels or directly adjacent to HEVs. Third, the MECA-79+ vessels were observed within the metastatic tumors. Fourth, the tumor cells in the front of metastasis seemed to transfer into HEV-rich regions. Fifth, TEM examination showed that the tumor nest adjacent to HEVs had looser structure, and the gap junctions between the cells were decreased, indicating that tumor cells may be easily shed from nest. When microvilli of tumor cells adhered to HEV luminal surfaces, gaps were left at the contact points suggesting the tendency of tumor cells to enter into HEVs, in a manner similar to the migration of lymphocytes into HEVs. Finally, the Stamper–Woodruff assay confirmed that oral cancer cells displayed preferential binding to cervical LN sections, and had more cells adherent to HEV structures than adenocarcinoma cells. The adherence could be obviously inhibited by L-selectin antibody. L-selectin is normally expressed in all myeloid cells, naïve and activated memory T cells, and it plays an essential role in the initial steps of lymphocytes homing and also facilitates LN metastasis.Citation22-Citation24 In our study, the expression of L-selectin was detected in both primary and metastatic OPSCC tumors. Taken together, these results suggest that HEVs may provide an alternative route for OPSCC tumor cells to enter into LNs for metastasis, and this process is L-selectin-dependent.
In conclusion, our data show that HEVs exist in primary OPSCCs and OPSCC-related LNs, and play important role in OPSCC progression and metastasis through providing nutrition for tumor growth, preparing premetastatic soil, and acting as a back door for cancer cell metastasis. In addition, our results provide supporting evidences that LN metastasis of human cancer is similar to lymphocyte homing.
Materials and Methods
Tissue samples and cell lines
Human OPSCC primary tumors (n = 64) and cervical LNs (n = 308) were collected at Hospital of Stomatology, Wuhan University, between 2009 and 2011. None of the patients in our study received chemotherapy or radiotherapy, or had previous cancer history before surgery. This study was approved by Ethics Committee of Hospital of Stomatology, Wuhan University, and informed consent was obtained from all patients. Twenty-four of normal mucosa tissues were chosen as controls.
Human oral SCC cell lines CAL 27, SCC-4, and human lung adenocarcinoma cell line A549 were purchased from American Type Culture Collection and maintained in DMEM medium (DMEM: F12 medium for SCC-4 cells) supplemented with 10% (v/v) fetal bovine serum (FBS) at 37 °C in a humidified atmosphere of 95% air and 5% CO2.
Immunohistochemical and triple-labeling immunofluorescent staining
For immunohistochemical staining, sections from paraffin-embedded specimens were incubated with primary antibodies at 4 °C overnight and detected by peroxidase-conjugated secondary antibody using diminobenzidine substrate kit (Dako). For immunofluorescence detection, sections were treated with a mixture of secondary antibodies coupled to AMCA, Cy3, and Alexa Fluor-488 for 1 h at 37 °C. The stained slides were scanned with high-resolution microscope (Olympus) and fluorescence microscope (Leica).
Quantification of MECA-79+ vessels in primary tumor
Absolute numbers of MECA-79+ vessels in the tumor area (mm2) were quantified from each primary tumor slide, and the densities of HEVs (HEV/mm2) were calculated. MECA-79+ vessels were classified into two groups. Classic HEVs were present in the lymphocyte infiltration regions with closed and thicker lumen like in lymphoid tissues. HEV-like vessels approximated tumor cells without correlation with lymphocyte, which had enlarged and thinner lumen containing numerous red blood cells (RBC).
Histological classification and evaluation of LNs
According to the diameter of LN and the dimension of metastasis, all collected LNs were classified into five groups.Citation25 Histologically verified non-metastasis: (1) nearly-normal LNs (the diameter ≤ 4 mm); (2) reactively-enlarged LNs (the diameter > 4 mm). Histologically verified metastasis: (3) isolated tumor cells (ITCs) (solitary tumor cells ≤ 0.2 mm in greatest dimension); (4) micrometastases (0.2 mm < the greatest dimension ≤ 2 mm); (5) macrometastases (the greatest dimension > 2 mm). Several LN sections (3 μm) were chosen for immunohistochemical staining, and other sections were subjected to H&E staining and histological examination to exclude micrometastasis.Citation26
Histological criteria were used to define metastatic route:Citation27 (1) lymphatic pathway (tumor emboli located in lymphatic channels, subcapsular or intrasinusoidal sinuses, and interfollicular nests with sparing of germinal centers); (2) HEV-mediated venolymphatic pathway (tumor cells in the paracortical regions, directly contiguous to HEVs but not lymph vessels or sinuses). Cases with damaged LNs structures and extensive marginal sinus involvement were excluded.
Evaluation of HEV endothelial cell proliferation
Sections were incubated with a mixture of proliferating cell nuclear antigen Ki67 antibody and MECA-79 antibody, and then with a mixture of secondary antibodies coupled to Cy3 and Alexa Flour-488. The nuclei were stained with 4’,6-diamidino-2-henylindole (DAPI).
Transmission electron microscopy
Twenty fresh metastatic LNs from 10 OPSCC patients were detected. Briefly, pieces of tissue < 2 mm3 were fixed in 2.5% glutaraldehyde in phosphate buffer for 4 h and post fixed in 1.0% osmic acid for 2 h, followed by dehydration in graded alcohols, and finally embedded in Lemix epoxy resin (TAAB Laboratories Equipment Ltd.). Ultrathin sections (60–90 nm) were stained with uranyl acetate and Reynold’s lead citrate, and viewed using an H-600 transmission electron microscope (Hitachi).
Western blot analysis
Cells were lysed and the supernatants were collected. Equal amounts of proteins were separated using 10% sodium dodecyl sulfate-PAGE and then transferred onto polyvinylidene fluoride membranes (Millipore). The membranes were cultured at 4 °C overnight with the corresponding primary antibodies in blocking solutions, and incubated with peroxidase-conjugated secondary antibody (Pierce). The membranes were developed using an enhanced chemiluminescence kit and exposed to X-ray film. GAPDH was used as a loading control.
Stamper–Woodruff assay
Stamper–Woodruff assay was performed to evaluate cell adhesion.Citation28 The cells were labeled by a vital fluorescent dye CFSE (Sigma) and incubated with fresh-frozen sections of normal LN (8 μm). The slides were lightly rotated at 40–60 rpm for 30 min at 4 °C. Medium and non-adherent cells were removed and gently washed twice in phosphate-buffered saline (PBS) for 30 min. Subsequently, slides were fixed in 1% paraformaldehyde overnight, incubated with MECA-79 antibody, then stained with hematoxylin and counted under microscope (Olympus). Five slides were examined for every cell lines, and 20 fields per slide were counted by two independent observers. In addition, cells were treated with different concentrations of L-selectin antibody, and HEV binding assay was performed as described above.
Statistical analysis
Data were analyzed by using SPSS 16.0 software (SPSS Inc.). The correlation between the density of HEVs within primary and metastatic tumors was assessed by using the Student t test. The comparisons between groups were analyzed by one-way analysis of variance. P values less than 0.05 were considered statistically significant.
Disclosure of Potential Conflicts of Interest
The authors declare that they have no competing interests.
Acknowledgments
We thank Prof Xin-Ming Chen, Bao-Ping Chen, Ping Fang, and Sen-Lin Lei for their support. This work was supported by the National Natural Science Foundation of China (30872893 and 81172570) and PhD Candidates Self-research (including 1 + 4) Program of Wuhan University in 2010 (20103040101000165). The funding source had no any role in the study design; in the collection, analysis, and interpretation of data; in the writing of the report; and in the decision to submit the article for publication.
References
- Girard J-P, Springer TA. High endothelial venules (HEVs): specialized endothelium for lymphocyte migration. Immunol Today 1995; 16:449 - 57; http://dx.doi.org/10.1016/0167-5699(95)80023-9; PMID: 7546210
- von Andrian UH, Mempel TR. Homing and cellular traffic in lymph nodes. Nat Rev Immunol 2003; 3:867 - 78; http://dx.doi.org/10.1038/nri1222; PMID: 14668803
- von Andrian UH, M’Rini C. In situ analysis of lymphocyte migration to lymph nodes. Cell Adhes Commun 1998; 6:85 - 96; http://dx.doi.org/10.3109/15419069809004463; PMID: 9823458
- Aloisi F, Pujol-Borrell R. Lymphoid neogenesis in chronic inflammatory diseases. Nat Rev Immunol 2006; 6:205 - 17; http://dx.doi.org/10.1038/nri1786; PMID: 16498451
- Kobayashi M, Mitoma J, Nakamura N, Katsuyama T, Nakayama J, Fukuda M. Induction of peripheral lymph node addressin in human gastric mucosa infected by Helicobacter pylori. Proc Natl Acad Sci U S A 2004; 101:17807 - 12; http://dx.doi.org/10.1073/pnas.0407503101; PMID: 15591109
- Müller A, Homey B, Soto H, Ge N, Catron D, Buchanan ME, McClanahan T, Murphy E, Yuan W, Wagner SN, et al. Involvement of chemokine receptors in breast cancer metastasis. Nature 2001; 410:50 - 6; http://dx.doi.org/10.1038/35065016; PMID: 11242036
- Herrlich P, Zöller M, Pals ST, Ponta H. CD44 splice variants: metastases meet lymphocytes. Immunol Today 1993; 14:395 - 9; http://dx.doi.org/10.1016/0167-5699(93)90141-7; PMID: 7691067
- Martinet L, Garrido I, Filleron T, Le Guellec S, Bellard E, Fournie JJ, Rochaix P, Girard JP. Human solid tumors contain high endothelial venules: association with T- and B-lymphocyte infiltration and favorable prognosis in breast cancer. Cancer Res 2011; 71:5678 - 87; http://dx.doi.org/10.1158/0008-5472.CAN-11-0431; PMID: 21846823
- Qian CN, Berghuis B, Tsarfaty G, Bruch M, Kort EJ, Ditlev J, Tsarfaty I, Hudson E, Jackson DG, Petillo D, et al. Preparing the “soil”: the primary tumor induces vasculature reorganization in the sentinel lymph node before the arrival of metastatic cancer cells. Cancer Res 2006; 66:10365 - 76; http://dx.doi.org/10.1158/0008-5472.CAN-06-2977; PMID: 17062557
- Chung MK, Do I-G, Jung E, Son Y-I, Jeong H-S, Baek C-H. Lymphatic vessels and high endothelial venules are increased in the sentinel lymph nodes of patients with oral squamous cell carcinoma before the arrival of tumor cells. Ann Surg Oncol 2012; 19:1595 - 601; http://dx.doi.org/10.1245/s10434-011-2154-9; PMID: 22124758
- Qian CN, Resau JH, Teh BT. Prospects for vasculature reorganization in sentinel lymph nodes. Cell Cycle 2007; 6:514 - 7; http://dx.doi.org/10.4161/cc.6.5.3931; PMID: 17329977
- Stauder R, Hamader S, Fasching B, Kemmler G, Thaler J, Huber H. Adhesion to high endothelial venules: a model for dissemination mechanisms in non-Hodgkin’s lymphoma. Blood 1993; 82:262 - 7; PMID: 7686787
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin 2012; 62:10 - 29; http://dx.doi.org/10.3322/caac.20138; PMID: 22237781
- Silverman S Jr.. Demographics and occurrence of oral and pharyngeal cancers. The outcomes, the trends, the challenge. J Am Dent Assoc 2001; 132:Suppl 7S - 11S; PMID: 11803655
- Eheman C, Henley SJ, Ballard-Barbash R, Jacobs EJ, Schymura MJ, Noone AM, Pan L, Anderson RN, Fulton JE, Kohler BA, et al. Annual Report to the Nation on the status of cancer, 1975-2008, featuring cancers associated with excess weight and lack of sufficient physical activity. Cancer 2012; 118:2338 - 66; http://dx.doi.org/10.1002/cncr.27514; PMID: 22460733
- Grandi C, Alloisio M, Moglia D, Podrecca S, Sala L, Salvatori P, Molinari R. Prognostic significance of lymphatic spread in head and neck carcinomas: therapeutic implications. Head Neck Surg 1985; 8:67 - 73; http://dx.doi.org/10.1002/hed.2890080202; PMID: 4077553
- Fan S, Müller S, Chen ZG, Pan L, Tighiouart M, Shin DM, Khuri FR, Sun SY. Prognostic impact of Fas-associated death domain, a key component in death receptor signaling, is dependent on the presence of lymph node metastasis in head and neck squamous cell carcinoma. Cancer Biol Ther 2013; 14:365 - 9; http://dx.doi.org/10.4161/cbt.23636; PMID: 23358467
- Sano D, Myers JN. Metastasis of squamous cell carcinoma of the oral tongue. Cancer Metastasis Rev 2007; 26:645 - 62; http://dx.doi.org/10.1007/s10555-007-9082-y; PMID: 17768600
- Zhang Z, Helman JI, Li LJ. Lymphangiogenesis, lymphatic endothelial cells and lymphatic metastasis in head and neck cancer--a review of mechanisms. Int J Oral Sci 2010; 2:5 - 14; http://dx.doi.org/10.4248/IJOS10006; PMID: 20690413
- Rosen SD. Ligands for L-selectin: homing, inflammation, and beyond. Annu Rev Immunol 2004; 22:129 - 56; http://dx.doi.org/10.1146/annurev.immunol.21.090501.080131; PMID: 15032576
- Ivanov K, Som P, Wolf R, Misilim K, Li SP, Urken ML, Zhang D, Brandwein M. The back door: venous-mediated metastasis into cervical lymph nodes as an alternative metastatic pathway for oropharyngeal squamous cell carcinoma. Mod Pathol 1999; 12:683 - 8; PMID: 10430272
- Qian F, Hanahan D, Weissman IL. L-selectin can facilitate metastasis to lymph nodes in a transgenic mouse model of carcinogenesis. Proc Natl Acad Sci U S A 2001; 98:3976 - 81; http://dx.doi.org/10.1073/pnas.061633698; PMID: 11274419
- Sperandio M. Selectins and glycosyltransferases in leukocyte rolling in vivo. FEBS J 2006; 273:4377 - 89; http://dx.doi.org/10.1111/j.1742-4658.2006.05437.x; PMID: 16956372
- Läubli H, Borsig L. Selectins promote tumor metastasis. Semin Cancer Biol 2010; 20:169 - 77; http://dx.doi.org/10.1016/j.semcancer.2010.04.005; PMID: 20452433
- Ferlito A, Rinaldo A, Devaney KO, Nakashiro K, Hamakawa H. Detection of lymph node micrometastases in patients with squamous carcinoma of the head and neck. Eur Arch Otorhinolaryngol 2008; 265:1147 - 53; http://dx.doi.org/10.1007/s00405-008-0715-8; PMID: 18523794
- Weaver DL. Pathology evaluation of sentinel lymph nodes in breast cancer: protocol recommendations and rationale. Mod Pathol 2010; 23:Suppl 2 S26 - 32; http://dx.doi.org/10.1038/modpathol.2010.36; PMID: 20436499
- Leong SP, Cady B, Jablons DM, Garcia-Aguilar J, Reintgen D, Jakub J, Pendas S, Duhaime L, Cassell R, Gardner M, et al. Clinical patterns of metastasis. Cancer Metastasis Rev 2006; 25:221 - 32; http://dx.doi.org/10.1007/s10555-006-8502-8; PMID: 16770534
- Stamper HB Jr., Woodruff JJ. Lymphocyte homing into lymph nodes: in vitro demonstration of the selective affinity of recirculating lymphocytes for high-endothelial venules. J Exp Med 1976; 144:828 - 33; http://dx.doi.org/10.1084/jem.144.3.828; PMID: 956727
