To the Editor:
I read with great interest the study by Shen et al. titled “Alterations of high endothelial venules in primary and metastatic tumors are correlated with lymph node metastasis of oral and pharyngeal carcinoma and congratulate them on their insightful findings and results”.Citation1 They studied high endothelial venules (HEV) in 64 cases of primary oral and pharyngeal squamous cell carcinoma (OPSCC) and demonstrated morphological and functional changes of HEV during OPSCC progression which were correlated with LN metastasis. They also reported the HEV endothelial cell proliferation rate was the highest in metastatic LNs and concluded these tumor-associated remodeled HEV are correlated with LN metastasis of OPSCC and play a crucial role in the metastasis cascade.
This observation, in my opinion, is interesting but not novel. We have previously reported in 2012 very similar findings in 65 cases of tongue SCC and their LN basin.Citation2 In addition, we correlated the HEV immunohistochemistry findings and vessel density with the respective patients’ clinical outcome. We had previously proposed and defined the following HEV parameters as an objective and systematic method of analyzing HEV characteristics.Citation3
Definition of the HEV parameters and ratios ():
Figure 1. Definitions of the high endothelial venules (HEV) parameters and its ratios (A) and metamorphosis spectrum of HEV in a nodal microenvironment (B). (A) Venn diagram illustrating the relationship between HEV and their different morphological forms. (B) Immunohistochemistry of lymph nodes (LN) showing the different HEV morphology stained by MECA-79 and the spectrum from a normal appearing HEV (normal LN) to an abnormal/ modified HEV in a LN in the presence of cancer (but no LN mets) to lastly a LN with frank LN metastases.
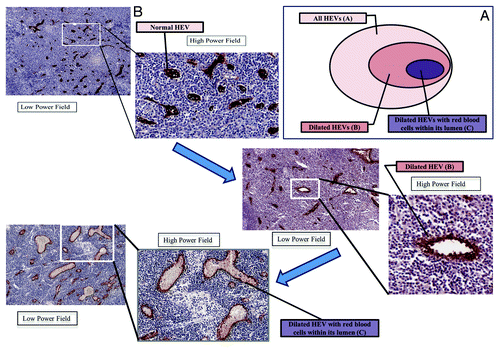
• number of all HEV: A
• number of dilated HEV (defined as lumen size more than 80 square micron): B
• number of dilated HEV with red blood cells (RBC) within its lumen: C
The ratios of these abnormal HEV (B and C) with respect to the total number of HEV (A).
• Ratio of dilated HEV to the total number of HEV: B/A
• Ratio of dilated HEV with RBC within its lumen to total number of dilated HEV: C/B
• Ratio of dilated HEV with RBC within its lumen with respect to total number HEV: C/A
We demonstrated the total number of HEV in the LN was found to be significantly associated to disease-free interval and there was a similar association comparing the above defined HEV parameters to overall survival. The density of abnormal HEV was significantly higher in patients with established metastases in their lymph nodes and HEV was shown to be a better prognosis factor than conventional tumor staging.Citation3 This is support of their findings in a few aspects, first the total MECA-79+ vessels (defined by us as “total number of HEV”) was higher in LN with metastasis (P = 0.051) and second HEV-like vessels (defined by us as “abnormal HEV”) was also higher in LN with metastasis (P = 0.037).
In their study, they expressed the different morphological forms of HEV as “classic HEVs” and “HEV-like vessels” according to their features. We think the description of “HEV-like vessels” may not be precise as we believe these vessels are in fact originally HEV which have transformed morphologically due to a functional change in the presence of cancer. In my opinion, the term “abnormal or modified HEV” is a more accurate description for these vessels. We also demonstrated similar findings but we believe that the HEV morphological metamorphosis is not in just 2 distinct forms but encompasses a whole morphological spectrum that correlates well with both disease progression and clinical outcome.Citation3 Our hypothesis is that the HEV transformation starts from a normal immunological mediator to a tumor metastasis mediator: this is reflected by morphological changes from a normal appearing HEV, to a dilated HEV, and then to a dilated HEV containing RBC. We believe that this metamorphosis occurs progressively as a spectrum—this process begins with the HEV increasing in absolute numbers, then each becoming more dilated before progressing into a functional vessel carrying blood ()
The authors demonstrated that 12.3% of their cases have HEV-mediated venolymphatic metastases and more than a third (12.3% + 24.7%) of metastasis involves a venolymphatic route. More importantly they have observed that in the cases of HEV-mediated venolymphatic metastases, tumor emboli were situated in the paracortical regions, adjacent to HEVs or just out of the vessels, without any association with lymphatic channels or sinuses. These findings, in addition to the observations that tumor foci were surrounded by abundant HEV, MECA-79+ vessels were observed within the metastatic tumor and that the tumor cells tended to infiltrate to the HEV-rich regions in the front of metastasis, are strongly supportive of our hypothesis that HEV serves as a physical shortcut in the LN for cancer cell metastasis to the systematic vascular system—thereby bypassing the long traditional lymphatic vessel to thoracic duct to subclavian vein route. This is further evident from our observations of the presence of significant abundance of red blood cells within the HEV and significant increase size of its lumen in the presence of a tumor. Our combined findings support the hypothesis that HEV presumably functions like a blood vessel in anticipation to supply the needs for an accelerating growth of a soon-to-arrive tumor deposit, i.e., “preparing the soil”.Citation4,Citation5
As a possible reason for the apparent contradictory findings of high HEV density in primary tumors associated with good prognosis but vice versa when high HEV density are observed in LNs,Citation6 we believe that there is a “point of no return” in this process. HEV proliferate in the primary tumor in response to the cancer, as part of their native and normal immune role, recruiting tumor-associated lymphocytes to combat the tumor and hence, this is reflected and consistent with the findings that high density of HEV and high level of cytotoxic lymphocytes in the primary tumor are often associated with good clinical prognosis.Citation7,Citation8 Once the tumor proliferates beyond the immunotherapy capacity of the tumor-associated T lymphocytes and overwhelms it, tumor cytokines or even single tumor cells escape via lymphatics or tumor HEV themselves to the LN and induces the metamorphosis of the HEV in the LNs from slit-like empty lymphocyte carrier to a large lumen functioning blood vessel. In fact, it was shown that in the presence of cancer, the HEV becomes the predominant blood vessels in the LN.Citation4
Hence, the findings and conclusions of the authors consistently and highly support our previous hypothesis and also provided more evidence that HEV plays a central role in the cancer metastasis cascade. Akin to HEV functioning as a gateway for recruiting lymphocytes in immunological conditions (“lymphocyte trafficking”); in the presence of a cancer, they transformed to that of “tumor cell trafficking” role instead.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- Shen H, Wang X, Shao Z, Liu K, Xia XY, Zhang HZ, Song K, Song Y, Shang ZJ. Alterations of high endothelial venules in primary and metastatic tumors are correlated with lymph node metastasis of oral and pharyngeal carcinoma. Cancer Biol Ther 2014; 15:342 - 9; http://dx.doi.org/10.4161/cbt.27328; PMID: 24351553
- Lee SY, Qian CN, Ooi AS, Chen P, Tan VK, Chia CS, Hwang JS, Teh BT, Soo KC. 2011 Young Surgeon’s Award Winner: high endothelial venules: a novel prognostic marker in cancer metastasis and the missing link?. Ann Acad Med Singapore 2012; 41:21 - 8; PMID: 22499477
- Lee SY, Chao-Nan Q, Seng OA, Peiyi C, Bernice WH, Swe MS, Chii WJ, Jacqueline HS, Chee SK. Changes in specialized blood vessels in lymph nodes and their role in cancer metastasis. J Transl Med 2012; 10:206; http://dx.doi.org/10.1186/1479-5876-10-206; PMID: 23035663
- Qian CN, Berghuis B, Tsarfaty G, Bruch M, Kort EJ, Ditlev J, Tsarfaty I, Hudson E, Jackson DG, Petillo D, et al. Preparing the “soil”: the primary tumor induces vasculature reorganization in the sentinel lymph node before the arrival of metastatic cancer cells. Cancer Res 2006; 66:10365 - 76; http://dx.doi.org/10.1158/0008-5472.CAN-06-2977; PMID: 17062557
- Qian CN, Resau JH, Teh BT. Prospects for vasculature reorganization in sentinel lymph nodes. Cell Cycle 2007; 6:514 - 7; http://dx.doi.org/10.4161/cc.6.5.3931; PMID: 17329977
- Gallimore A, Godkin A, Ager A. High endothelial venules: Help or hindrance in the quest for antitumor immunity?. Oncoimmunology 2013; 2:e24272; http://dx.doi.org/10.4161/onci.24272; PMID: 23762806
- Martinet L, Garrido I, Filleron T, Le Guellec S, Bellard E, Fournie JJ, Rochaix P, Girard JP. Human solid tumors contain high endothelial venules: association with T- and B-lymphocyte infiltration and favorable prognosis in breast cancer. Cancer Res 2011; 71:5678 - 87; http://dx.doi.org/10.1158/0008-5472.CAN-11-0431; PMID: 21846823
- Martinet L, Le Guellec S, Filleron T, Lamant L, Meyer N, Rochaix P, Garrido I, Girard JP. High endothelial venules (HEVs) in human melanoma lesions: Major gateways for tumor-infiltrating lymphocytes. Oncoimmunology 2012; 1:829 - 39; http://dx.doi.org/10.4161/onci.20492; PMID: 23162750
