Abstract
Comment on: Soliman GA, et al. Lipids 2010; 45:1089-100.
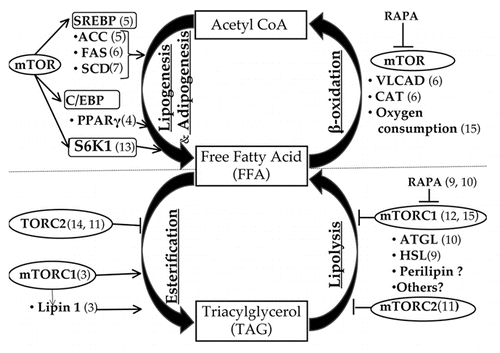
The mammalian Target of Rapamycin (mTOR) is comprised of two complexes, mTOR complex 1 (mTORC1) and mTOR complex 2 (mTORC2), that have shared as well as distinct partners (reviewed in ref. Citation1). mTOR serves as a controller of both anabolic and catabolic pathways. Anabolic processes of lipid metabolism include lipogenesis (fatty acid synthesis), adipogenesis and esterification. Fatty acid synthesis is mediated by Acetyl CoA Carboxylase (ACC) and Fatty Acid Synthase (FAS) enzymes. Subsequent desaturation by steroyl CoA desaturase (SCD) and chain elongation yields polyunsaturated fatty acids. Consequently, esterification of free fatty acids (FFA) forms triacylglycerol (TAG). The catabolic processes of lipid metabolism include hydrolysis of TAG (lipolysis) to yield glycerol and FFA, followed by β-oxidation of FFA to yield Acetyl CoA (). While the roles of mTOR in lipid biosynthesis have been addressed,Citation2–Citation7 the effects of mTOR on TAG lipolysis and FFA breakdown via β-oxidation are not understood.Citation6,Citation8–Citation12
mTOR and de novo Fatty Acid Synthesis
mTOR has been shown to activate the transcription factor, SREBP (Sterol Regulatory Element Binding Protein) in rat hepatocytes,Citation5 which in turn activates ACC,Citation5 FAS,Citation6 and SCDCitation7 enzymes involved in lipogenesis (). Additionally, rapamycin, a specific mTORC1 inhibitor, has been shown to block the expression of SREBP1 target genes ACC, FAS and SCD, indicating a role for mTORC1 in fatty acid biosynthesis.Citation2,Citation5,Citation6 Rapamycin also inhibited the transactivation of transcription factors PPARγ (Peroxisome Proliferator-Activated Receptor-gamma, an adipocyte specific nuclear receptor) and C/EBP (CCAAT-enhancer-binding proteins).Citation4 C/EBPβ and C/EBPγ induce the expression of C/EBPα that in turn stimulates PPARγ transactivation driving the process of adipogenesis. Subsequently, PPARγ activates fatty acid uptake, synthesis, esterification and storage in the newly synthesized adipocytes. Using the S6K1 knockout mouse model, a recent study documented the role of S6K1, an mTOR downstream target, in de novo adipogenesis and affirmed that S6K1 is involved in the commitment of multipotent stem cells to the adipocyte lineage.Citation13
mTOR and Triacylglycerol Synthesis
FFA are esterified by glycerol to generate TAG, which are the major storage fuels in the adipose tissue. By promoting both lipogenesis and adipogenesis, mTOR is well poised to enhance FFA esterification to yield TAG. Indeed, studies have shown that mTOR increases TAG synthesis via phosphorylation of Lipin 1.Citation3 Lipin 1, a phosphatidic acid phosphatase involved in the cleavage of phosphatidic acid, an integral step in TAG synthesis, has been shown to be phosphorylated by mTOR in an insulin sensitive manner.Citation3 In C. elegans, a rictor (TORC2 partner) mutant phenotype exhibited increased fat accumulation, implying that TORC2 serves as a negative regulator of fatty acid esterification and TAG formation.Citation14 Taken together, it appears that there is a balance between mTORC1 and mTORC2 activities that functions to maintain energy homeostasis.
mTOR and β-Oxidation
FFA are transferred to the inner mitochondrial membrane by Carnitine Acyl Transferase (CAT) and are subsequently oxidized to yield acetyl CoA, which enters the citric acid cycle to generate energy. Using human BJAB B-lymphoma cell lines and murine CTLL-2 T lymphocytes, Peng et al. (2002) reported that rapamycin treatment (12 and 24 hours) increases VLACD (Very Long Acetyl CoA Dehydrogenase) enzyme gene expression, as well as CAT expression, indicating that inhibition of mTOR leads to accelerated β-oxidation and increased catabolism of FFA.Citation6 Similarly, Um et al. (2004) found that mice deficient in S6K1, an mTOR downstream target, showed enhanced β-oxidation, which led to a lean mouse phenotype.Citation15
mTOR and Lipolysis
Mobilization of stored TAG via the lipolytic pathway is necessary to provide FFA to be used as an energy source. Studies of Adipose Triglyceride Lipase (ATGL) deficient mice documented the role of ATGL in initiating lipolysis.Citation16 Knockdown of 4E-BP1 and 4E-BP2 genes, which lie downstream of mTOR, led to decreased lipolysis and increased TAG accumulation.Citation12 While in S6K1 knockout mice, increased lipolysis was observed, which contributed to a lean phenotype and resistance to obesity.Citation15 We showed that rapamycin treatment of 3T3-L1 adipocytes led to increased β-adrenergic agonist induced lipolysis without altering basal lipolysis,Citation9 an effect that is partially attributed to increased phosphorylation of Hormone Sensitive Lipase (HSL) at serine-563. Additionally, Charabati et al. reported that overexpression of the mTOR activator Rheb in 3T3-L1 cells led to decreased ATGL (Adipose Triacylglycerol lipase) expression, increased de novo lipogenesis and suppressed lipolysis. Using western blot analysis, we did not find changes in ATGL expression after rapamycin treatment.Citation9 The apparent discrepancy may be explained by different experimental conditions and possible increase in ATGL enzymatic activity that may have contributed to increased rates of lipolysis. We observed that rapamycin augments the PKA-mediated phosphorylation of HSL on Serine 563, which suggests a cooperative role of mTORC1 inhibition with a β-adrenergic PKA-activated response. Whether mTORC1 inhibition and PKA activation converge on the same lipolytic targets or via parallel pathways, remains to be elucidated. Polak et al.Citation8 reported that adipose tissue specific raptor (mTORC1 partner) knockout mice showed a lean phenotype, which was related to increased energy expenditure and increased mitochondrial uncoupling proteins. Further, adipose tissue-specific rictor knockout mice showed a phenotype of increased FFA and glycerol, which implies that mTORC2, also plays a role in suppressing lipolysis.Citation11
Taken together, mTOR signaling plays an integral role in lipid metabolism including anabolic pathways, which encompass lipogenesis, adipogenesis and fatty acid esterification, and catabolic pathways, which include lipolysis and β-oxidation (). These findings implicate mTOR as an attractive target for intervention in chronic diseases with deregulation of lipid homeostasis such as obesity and diabetes. Further research is warranted to understand the impact of mTOR signaling on lipolysis, β-oxidation and energy homeostasis.
Acknowledgements
This work was funded in part by NIH (K01 DK60654) and AHA (0750060Z) grants to G.S.
Comment on: Soliman GA, et al. Lipids 2010; 45:1089 - 1100
References
- Zoncu R, et al. Nat Rev Mol Cell Biol 2011; 12:21 - 35
- Laplante M, et al. Curr Biol 2009; 19:1046 - 1052
- Huffman TA, et al. Proc Natl Acad Sci USA 2002; 99:1047 - 1052
- Kim JE, et al. Diabetes 2004; 53:2748 - 2756
- Brown NF, et al. Metabolism 2007; 56:1500 - 1507
- Peng T, et al. Mol Cell Biol 2002; 22:5575 - 5584
- Mauvoisin D, et al. J Cell Commun Signal 2007; 1:113 - 125
- Polak P, et al. Cell Metab 2008; 8:399 - 410
- Soliman GA, et al. Lipids 2010; 45:1089 - 1100
- Chakrabarti P, et al. Diabetes 2010; 59:775 - 781
- Kumar A, et al. Diabetes 2010; 59:1397 - 1406
- Le Bacquer O, et al. J Clin Invest 2007; 117:387 - 396
- Carnevalli LS, et al. Dev Cell 2010; 18:763 - 774
- Jones KT, et al. PLoS Biol 2009; 7:60
- Um SH, et al. Nature 2004; 431:200 - 205
- Zimmermann R, et al. Science 2004; 306:1383 - 1386