Abstract
Studies in young rodents have shown that the transient receptor potential vanilloid-1 (TRPV1) channel plays a suppressive role in the systemic inflammatory response syndrome (SIRS) by inhibiting production of tumor necrosis factor (TNF)α and possibly by other mechanisms. We asked whether the anti-inflammatory role of TRPV1 changes with age. First, we studied the effect of AMG517, a selective and potent TRPV1 antagonist, on aseptic, lipopolysaccharide (LPS)-induced SIRS in young (12 wk) mice. In agreement with previous studies, AMG517 increased LPS-induced mortality in the young. We then studied the effects of TRPV1 antagonism (AMG517 or genetic deletion of TRPV1) on SIRS in middle-aged (43–44 wk) mice. Both types of TRPV1 antagonism delayed and decreased LPS-induced mortality, indicating a reversal of the anti-inflammatory role of TRPV1 with aging. In addition, deletion of TRPV1 decreased the serum TNFα response to LPS, suggesting that the suppressive control of TRPV1 on TNFα production is also reversed with aging. In contrast to aseptic SIRS, polymicrobial sepsis (induced by cecal ligation and puncture) caused accelerated mortality in aged TRPV1-deficient mice as compared with wild-type littermates. The recovery of TRPV1-deficient mice from hypothermia associated with the cecal ligation and puncture procedure was delayed. Hence, the reversal of the anti-inflammatory role of TRPV1 found in the aged and their decreased systemic inflammatory response are coupled with suppressed defense against microbial infection. These results caution that TRPV1 antagonists, widely viewed as new-generation painkillers, may decrease the resistance of older patients to infection and sepsis.
Introduction
Systemic inflammatory response syndrome (SIRS) is the leading cause of death in hospitalized patients.Citation1,Citation2 SIRS is considered a disease of the aged: its incidence and mortality are substantially higher in the older population.Citation3 SIRS can be either triggered by non-infectious insults, such as blunt trauma, or associated with an infection (in which case it is called sepsis). In the laboratory, systemic administration of lipopolysaccharide (LPS, a cell-wall constituent of Gram-negative bacteria) in mice and rats is often used to induce SIRS aseptically, whereas polymicrobial sepsis is often studied in rodents subjected to cecal ligation and puncture (CLP). In either model, shock and death can occur, largely as the result of the “cytokine storm,” an overt production of proinflammatory cytokines, including TNFα,Citation4 and other mediators, cumulatively referred to as the “inflammatory soup.”Citation5–Citation7 In both LPS-induced SIRS and CLP-induced sepsis, proinflammatory cytokine production and mortality rate are much lower in young animals.Citation8–Citation11 Furthermore, sepsis in young animals is much more responsive to treatment.Citation11
Recent studies have brought attention to the role that the transient receptor potential vanilloid-1 (TRPV1) channel may play in SIRS. Abundant on small-diameter sensory nerve fibers, TRPV1 is activated by diverse stimuli, including several ingredients of the inflammatory soup.Citation12,Citation13 Activation of TRPV1 on sensory nerves potently inhibits LPS-induced TNFα production.Citation14 Studies using either knockout (Trpv1−/−) mice, a pharmacological blockade with capsazepine (TRPV1 antagonist) or desensitization with resiniferatoxin (TRPV1 agonist) have shown that TRPV1 plays an anti-inflammatory role in LPS-induced SIRS by, among other mechanisms, limiting the production of TNFα, possibly via sensory nerves.Citation15–Citation17 However, all studies cited above were conducted in young rodents. Whether TRPV1 channels play a similarly prominent anti-inflammatory role in the aged is unknown.
Results and Discussion
Effects of a TRPV1 antagonist on LPS-induced systemic inflammation in young mice.
First, we verified whether pretreatment with AMG517, a potent and selective TRPV1 antagonist,Citation18,Citation19 decreases the mortality of young adult (12 wk) C57BL/6 mice in LPS-induced SIRS. Mice responded to LPS (40 mg/kg, ip) with a marked, rapidly progressing SIRS (). Pretreatment with AMG517 (210 µg/kg, sc) profoundly decreased the survival rate at multiple time points (e.g., from 50% to 5% at 26 h, p < 0.001), overall (48 h) survival rate (from 15% to 5%, p < 0.05) and increased the risk of mortality (hazard ratio of death of 0.9, p = 0.01, ). AMG517 pretreatment also shortened the mean time to death from 26 ± 2 to 19 ± 1 h (p = 0.003). All LPS-treated mice, both in this experiment and in other experiments within the present study, developed profound hypothermia: deep (abdominal) body temperature (Tb) decreased from ∼36°C to ∼33°C at 10 h. However, no inter-treatment differences in the hypothermic response occurred during the short time period before mice started dying (data not shown). Similar to our previous studies in references Citation18 and Citation19, AMG517 caused a short-lasting increase in Tb compared with the vehicle (p < 0.01, ), thus confirming an effective systemic blockade of TRPV1 channels. Overall, the results of our experiment show that pharmacological blockade of TRPV1 increases mortality of young mice in LPS-induced SIRS. Similar observations have been made in adolescent (6–8 wk) mice and in rats treated with capsazepine.Citation16,Citation17 It should be noted, however, that capsazepine is not a highly selective TRPV1 antagonist and has a low potency of blocking the proton mode of TRPV1 activation in the rat and mouse.Citation20 In fact, a non-TRPV1-mediated effect of capsazepine on the outcome of systemic inflammation has been proposed recently in reference Citation17. The present results also agree with the exaggerated symptoms of LPS-induced shock found in young mature (13–20 wk) Trpv1−/− mice.Citation15
As reviewed by Steiner et al.Citation21 and Guptill et al.,Citation17 many treatments affect mortality in LPS-induced aseptic inflammation and CLP-induced sepsis in opposite ways, confirming that the systemic inflammatory response per se can be harmful to the host, even though it is crucial for defending the host against infection.Citation6,Citation7 For example, mice with a dysfunctional toll-like receptor 4 are resistant to LPS but are highly susceptible to Gram-negative bacterial infection.Citation22–Citation24 This susceptibility to infection can be reserved by pretreating the toll-like receptor 4-deficient mice with TNF and interleukin-1α.Citation22 Interestingly, antibiotic treatment makes sepsis less different from aseptic SIRS, as it eradicates the infectious agent. For example, acute nicotine administration increases survival of mice in LPS-induced SIRSCitation21,Citation25–Citation27 and in antibiotic-treated CLP-induced sepsis,Citation25,Citation26 but it worsens the outcome of untreated CLP-induced sepsis in the same species.Citation21 From this point of view, two studies of the role of TRPV1 in CLP-induced sepsis in adolescent (5–8 wk) miceCitation17,Citation28 agree with the effects observed in LPS SIRS. The first studyCitation17 has found that antibiotic-treated CLP-induced sepsis causes a higher mortality when TRPV1 channels are absent (Trpv1−/− mice) or desensitized (with intrathecal resiniferatoxin). The second studyCitation28 has found that capsazepine increases survival in untreated CLP-induced sepsis. Overall, prior literature data obtained in young rodents (adolescents to mature adults) and our present experiment with AMG517 in young adult mice show that the effects of TRPV1 blockade on both LPS-induced SIRS and antibiotic-treated sepsis vary from none to strong exaggeration of severity and mortality,Citation15–Citation17 whereas the effect of TRPV1 blockade on mortality in untreated sepsis is the opposite: attenuation.Citation28
Effects of AMG517 on LPS-induced systemic inflammation in aged mice.
To study whether the anti-inflammatory role of TRPV1 in SIRS is preserved with aging, we conducted experiments in middle-aged (44 wk) C57BL/6 mice (). The outcome of LPS-induced SIRS in these older mice was more severe than in young mice (hazard ratio of 2.2, p < 0.001, ). The mean time to death in vehicle-pretreated aged mice was 16 ± 1 h, and none of the vehicle-pretreated aged mouse survived for longer than 24 h. Pretreatment of aged mice with AMG517 (210 µg/kg, sc) increased the survival rate (p < 0.05), delayed the mean time to death (19 ± 1 h, p < 0.05) and decreased the risk of mortality (hazard ratio of −1.0, p < 0.05)—effects directly opposite of those observed in the young. Survival rate of AMG517-pretreated aged mice at 18 h was 10 times higher than that of vehicle-pretreated aged mice (60% vs. 6%, p < 0.001). Confirming a systemic blockade of TRPV1 channels in this experiment, AMG517 increased Tb as compared with the vehicle (p < 0.05, ). Hence, whereas the effect of AMG517 on LPS-induced systemic inflammation in aged mice was the opposite to that found in young mice ( and ), the effect on Tb was qualitatively the same ( and ). It is possible that the role of TRPV1 in different functions changes with age in a different way. In the regulation of locomotor activityCitation29,Citation30 and inflammation (present results), the role of TRPV1 reverses with age. In the modulation of Tb (for mechanisms, review ref. Citation31), it does not. In the regulation of body mass, TRPV1 channels are either uninvolvedCitation29 or counteract obesityCitation32 in the young but promote obesity in the aged.Citation29,Citation30
Effects of genetic deletion of TRPV1 channels on LPS-induced systemic inflammation in aged mice.
We then tested whether genetic deletion of TRPV1 would have the same effects on SIRS in middle-aged mice as a pharmacological blockade. Experiments were conducted in 43–44 wk-old Trpv1−/− C57BL/6 × 129 mice of both sexes and in their age- and sex-matched Trpv1+/+ littermates. LPS caused death in all Trpv1+/+ mice but only in 80% of Trpv1−/− mice (p < 0.05, ). Survival rate of Trpv1−/− mice at 21 h was 3.5 times higher than of wild-type controls (70% vs. 20%, p < 0.001). Genetic deletion of TRPV1 channels decreased the risk of mortality (hazard ratio of −1.3, p < 0.05) and tended to delay death (p < 0.1, ). Importantly, Trpv1−/− mice exhibited a 70% suppression of the TNFα response at 12 h post-LPS (p < 0.05, ). It should be noted that aged Trpv1−/− mice in this study (see Materials and Methods) and in our previous studies in references Citation29 and Citation30 were overweight compared with their wild-type littermates. ObesityCitation33–Citation36 and hyperlipidemiaCitation37 associated with various mutations in rats do not seem to affect the febrile response to low, non-shock-inducing doses of LPS, and obesity does not seem to increase the risk of sepsis even though it increases the risk of an infection.Citation38 Nevertheless, systemic inflammation and obesity are intimately interconnected,Citation39,Citation40 and we cannot rule out that obesity could have been a contributing factor to at least some of the effects found in aged Trpv1−/− mice.
Mechanisms of the age-associated reversal of the anti-inflammatory role of TRPV1 are unknown. Our TNFα data suggest that the reversal occurs at initial stages of the pathogenesis of SIRS—at or upstream of TNFα production. The TNFα response to LPS has been shown to be under suppressive control of TRPV1 channels on sensory nerves.Citation14 Loss of TRPV1-mediated suppression of TNFα production in aged animals may reflect reduced translation of the TRPV1 protein and its reduced transport to the periphery,Citation41 possibly due to age-associated decline in neurotrophic support to ganglionic neurons.Citation42 Changes in TNFα production may be central to aging-related changes in the pathobiology of sepsis: elderly patients respond to infection, including septic shock, with higher TNFα,Citation43,Citation44 and inflammatory cytokine production in intensive-care-unit patients with sepsis is affected by TNFα-related genetic polymorphisms.Citation45
Effects of genetic deletion of TRPV1 channels on CLP-induced sepsis in aged mice.
Next, we tested whether the attenuation of aseptic SIRS (of LPS-induced TNFα response and mortality) observed in middle-aged Trpv1−/− mice would result in attenuation of the body's defense against CLP-induced polymicrobial infection. CLP sepsis caused substantial mortality in aged mice of both genotypes (). However, Trpv1−/− mice died significantly faster than their Trpv1+/+ littermates (). The mean time to death in Trpv1−/− mice was 20 ± 2 h, as compared with 52 ± 11 h in Trpv1+/+ mice (p < 0.01), and the 30 h survival rate in Trpv1+/+ mice was 3.9 times higher than in Trpv1−/− mice (86% vs. 22%, p < 0.001). Further confirming the higher severity of sepsis in Trpv1−/− mice, recovery of deep Tb after the CLP procedure (and the related anesthesia) was delayed (p < 0.001, ).
Conclusions.
The present study shows that the anti-inflammatory role firmly established for TRPV1 channels in young rodentsCitation15–Citation17 is reversed with aging. Whereas pharmacological or genetic TRPV1 antagonism decreases the survival rate in aseptic SIRS and in antibiotic-treated sepsis in the young, both types of TRPV1 antagonism have the opposite effect on aseptic SIRS in middle-aged mice. The age-dependent reversal of the anti-inflammatory role of TRPV1 to proinflammatory is likely due, at least in part, to a reversal of the suppressive control of TRPV1 on TNFα production. These pathobiological changes are highly important, as evident from the decreased ability of aged Trpv1−/− mice to resist polymicrobial sepsis. The reversal of the anti-inflammatory role of TRPV1 reported in this study is another example of profound effects of aging on the pathobiology of systemic inflammation.Citation11,Citation46,Citation47
Significance.
Our findings may influence development of TRPV1 antagonists, widely viewed as new-generation painkillers.Citation18,Citation20,Citation48 If what we found for murine models applies to human sepsis, anti-TRPV1 therapy may suppress the systemic inflammatory response in the previously uninfected (untreated with antibiotics) elderly and, hence, decrease their resistance to bacterial infection and sepsis. This potential side effect is especially serious, because recognition of infection is often complicated in older patients by a variety of factors, including the absence of fever, which often delays treatment.Citation49,Citation50
Materials and Methods
The study was conducted in 168 adult mice (either young or middle-aged) of both sexes, including 101 Trpv1+/+ C57BL/6 mice (Charles River Laboratories) and 67 Trpv1−/− or Trpv1+/+ C57BL/6 × 129 mice (Amgen colony at Charles River Laboratories). Several phenotypic properties of Trpv1−/− mice from the Amgen colony have been characterized in our recent studies in references Citation29 and Citation30. At the time of experiments, young adult C57BL/6 mice were 12 wk-old, and their body mass was 25 ± 0 g (n = 55); middle-aged C57BL/6 mice were 44 wk-old, and their body mass was 32 ± 1 g (n = 46). The ages of C57BL/6 mice listed are approximate; the vendor filled orders for a specified age, but did not provide the date of birth for each mouse. Middle-aged Trpv1+/+ C57BL/6 × 129 mice were 43 ± 1 wk-old (n = 30), and their body mass was 40 ± 1 g. Middle-aged Trpv1−/− C57BL/6 × 129 mice were 44 ± 1 wk-old (n = 37) and significantly heavier (46 ± 1 g; p < 0.001) than their wild-type littermates. The ages of C57BL/6 × 129 mice were calculated based on the dates of birth, which were known for all mice. Mice were maintained, surgically prepared and habituated to experimental setups as described in our earlier studies.Citation21,Citation29 All surgeries were performed under ketamine-xylazine-acepromazine (81.7, 9.3 and 1.2 mg/kg, ip) anesthesia. Antibiotic protection (enrofloxacin, 1.1 mg/kg, sc) was provided, except for animals subjected to CLP. For deep Tb measurements, all mice were implanted intraperitoneally with telemetry transmitters (G2 E-Mitter series, Mini Mitter). For CLP, under the same anesthesia, the cecum was pulled out of the abdominal cavity, filled with the intestinal content (by gently squeezing the content from the ascending colon) and ligated with 3-0 silk just distal to the ileocecal junction. The cecal wall was punctured through at the antimesenteric side with a 26-gauge needle. All protocols were approved by the St. Joseph's Hospital and Medical Center Animal Care and Use Committee.
During all experiments, mice were housed singly in their home cages and placed inside a climatic chamber (model 3940, Forma Scientific) with an ambient temperature maintained at 28.0°C, i.e., within the thermoneutral zone for this experimental setup.Citation29,Citation51 Cages were kept on top of telemetry receivers (model ER-4000, Mini Mitter). In the experiments with LPS-induced SIRS, the survival rate and Tb were monitored for 48 h. To this end, mice were periodically examined for the presence of spontaneous movements and cardiac and respiratory activities. Because deep Tb decreases steeply toward the ambient temperature when an animal dies, Tb curves were also examined to identify mortality events. In the experiments with CLP-induced sepsis, the same parameters were monitored for 108 h. At the end of experiments, any survivors were euthanized with sodium pentobarbital (200 mg/kg, ip).
In a separate series of experiments (under the same anesthesia as for surgery), blood (1 ml) was collected by cardiac puncture at 12 h after LPS administration, and mice were euthanized with sodium pentobarbital. The 12 h time point for blood collection was chosen because aged mice in this model start dying shortly after this point (). Serum concentration of TNFα was determined by ELISA according to the manufacturer's instructions (SABiosciences, catalog number MEM-004A).
A suspension of E. coli 0111:B4 LPS (Sigma-Aldrich, L2630, 2.5 mg/ml) in saline was prepared in advance and stored at 4°C. To induce SIRS, LPS (40 mg/kg ip) was injected as a bolus; controls received saline.
AMG517 (gift from Amgen) was used to block TRPV1 receptors pharmacologically. This is a highly potent and selective TRPV1 antagonist that had been tested in human patients.Citation18 Aliquots of an ethanolic solution of AMG517 (3 mg/ml) were stored at −80°C. This stock was diluted with ethanol and saline ex tempore to achieve a 21 µg/ml final concentration of AMG517 in 3.3% ethanol. AMG517 (210 µg/kg sc) or its vehicle was administered as a bolus, 1 h before the administration of LPS (or saline).
Survival data were analyzed by the χ2 test for individual time points and by the logrank testCitation52 for the entire observation period. The Cox proportional hazard survival regression model was used to determine hazard ratios of death.Citation53 Unpaired student's t-test was used to compare times to death and TNFα concentrations. Deep Tb values were compared across treatments and time points by two-way ANOVA; p-values for the entire duration of response are reported. A difference was considered significant at p < 0.05. Results are reported in the format mean ± SE.
Disclosure of Potential Conflicts of Interest
N.R.G. is employed by Amgen Inc. A.A.R. has consulted for TRP programs at Amgen, Inc. and several other pharmaceutical companies, and his TRP-related research has been supported by Amgen, Inc. and Abbott Laboratories.
Abbreviations
| CLP | = | cecal ligation and puncture |
| LPS | = | lipopolysaccharide |
| SIRS | = | systemic inflammatory response syndrome |
| Tb | = | body temperature |
| TNF | = | tumor necrosis factor |
| TRPV1 | = | transient receptor potential vanilloid-1 |
Figures and Tables
Figure 1 Systemic pretreatment with AMG517 (dose indicated) decreases survival of young mice in LPS-induced SIRS (A). Confirming an effective blockade of TRPV1 channels, the AMG517 pretreatment increases deep Tb in young mice (B).
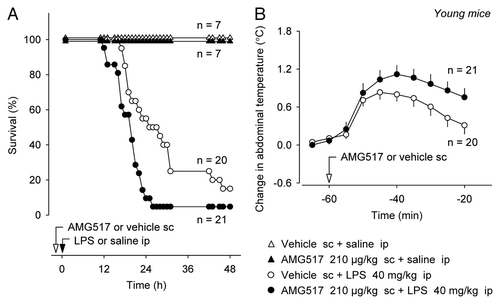
Figure 2 Systemic pretreatment with AMG517 (dose indicated) increases survival of aged mice in LPS-induced SIRS (A). Confirming an effective blockade of TRPV1 channels, the AMG517 pretreatment increases deep Tb in aged mice (B).
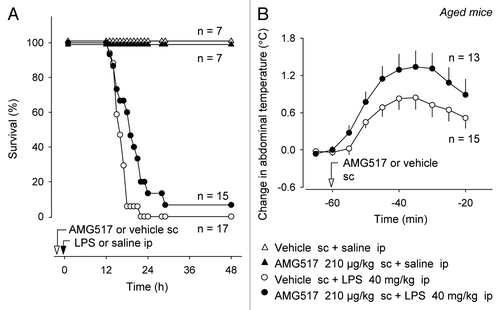
Figure 3 Compared with their age-matched wild-type littermates, middle-aged Trpv1−/− mice have a higher survival rate (A) and a lower serum TNFα concentration (B) during LPS-induced SIRS.
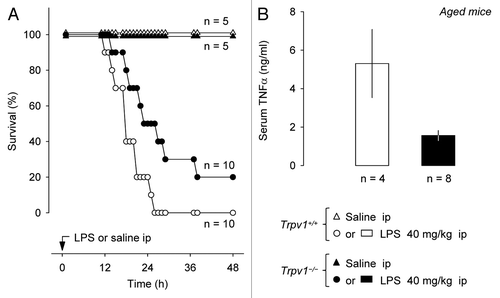
Figure 4 Compared with their age-matched wild-type littermates, middle-aged Trpv1−/− mice have a shorter survival time (A) and a slower Tb recovery (B) during CLP-induced sepsis.
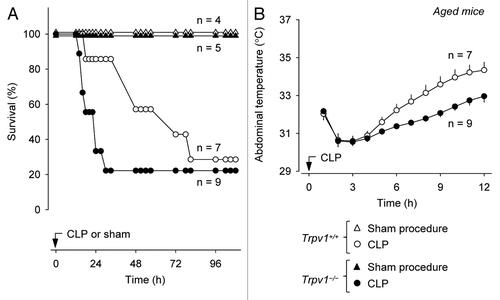
Table 1 Effects of age and TRPV1 antagonism on mortality in LPS-induced SIRS and CLP-induced sepsis
Acknowledgements
We thank Tatiane B. Nucci and Justin Eales for their help with animal work, Drs. Junwei Hao and Fu-Dong Shi for help with the ELISA assay, and Catherine M. Krall and Julie M. Turko for editing the manuscript. A.G.'s and E.P.'s present address is University of Pécs Medical School, Pécs, Hungary. This research has been supported in part by the National Institutes of Health (grant R01NS41233 to A.A.R.) and Amgen, Inc., (study agreements with A.A.R.). S.P.W. was a Fellow of the National Council for Scientific and Technological Development, Brazil. Author contributions: S.P.W., C.C.C. and A.A.R. designed the study. S.P.W., A.G., E.P. and D.L.O. performed experiments. N.R.G. provided reagents and materials. S.P.W. and A.A.R. analyzed data and wrote the manuscript.
References
- Brun-Buisson C. The epidemiology of the systemic inflammatory response. Intensive Care Med 2000; 26:64 - 74; PMID: 10786961; http://dx.doi.org/10.1007/s001340051121
- Martin GS, Mannino DM, Eaton S, Moss M. The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med 2003; 348:1546 - 1554; PMID: 12700374; http://dx.doi.org/10.1056/NEJMoa022139
- Martin GS, Mannino DM, Moss M. The effect of age on the development and outcome of adult sepsis. Crit Care Med 2006; 34:15 - 21; PMID: 16374151; http://dx.doi.org/10.1097/01.CCM.0000194535.82812.BA
- Töllner B, Roth J, Storr B, Martin D, Voigt K, Zeisberger E. The role of tumor necrosis factor (TNF) in the febrile and metabolic responses of rats to intraperitoneal injection of a high dose of lipopolysaccharide. Pflugers Arch 2000; 440:925 - 932; PMID: 11041560; http://dx.doi.org/10.1007/s004240000386
- Cohen J. The immunopathogenesis of sepsis. Nature 2002; 420:885 - 891; PMID: 12490963; http://dx.doi.org/10.1038/nature01326
- Rittirsch D, Flierl MA, Ward PA. Harmful molecular mechanisms in sepsis. Nat Rev Immunol 2008; 8:776 - 787; PMID: 18802444; http://dx.doi.org/10.1038/nri2402
- Stearns-Kurosawa DJ, Osuchowski MF, Valentine C, Kurosawa S, Remick DG. The pathogenesis of sepsis. Annu Rev Pathol 2011; 6:19 - 48; PMID: 20887193; http://dx.doi.org/10.1146/annurevpathol-011110-130327
- Hyde SR, Stith RD, McCallum RE. Mortality and bacteriology of sepsis following cecal ligation and puncture in aged mice. Infect Immun 1990; 58:619 - 624; PMID: 2307515
- Chorinchath BB, Kong LY, Mao L, McCallum RE. Age-associated differences in TNF-alpha and nitric oxide production in endotoxic mice. J Immunol 1996; 156:1525 - 1530; PMID: 8568256
- Tateda K, Matsumoto T, Miyazaki S, Yamaguchi K. Lipopolysaccharide-induced lethality and cytokine production in aged mice. Infect Immun 1996; 64:769 - 774; PMID: 8641780
- Turnbull IR, Wlzorek JJ, Osborne D, Hotchkiss RS, Coopersmith CM, Buchman TG. Effects of age on mortality and antibiotic efficacy in cecal ligation and puncture. Shock 2003; 19:310 - 313; PMID: 12688540; http://dx.doi.org/10.1097/00024382-200304000-00003
- Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature 1997; 389:816 - 824; PMID: 9349813; http://dx.doi.org/10.1038/39807
- Tominaga M, Caterina MJ, Malmberg AB, Rosen TA, Gilbert H, Skinner K, et al. The cloned capsaicin receptor integrates multiple pain-producing stimuli. Neuron 1998; 21:531 - 543; PMID: 9768840; http://dx.doi.org/10.1016/S0896-6273(00)80564-4
- Murai M, Tsuji F, Nose M, Seki I, Oki K, Setoguchi C, et al. SA13353 (1-[2-(1-Adamantyl)ethyl]-1-pentyl-3-[3-(4-pyridyl)propyl]urea) inhibits TNF-alpha production through the activation of capsaicin-sensitive afferent neurons mediated via transient receptor potential vanilloid 1 in vivo. Eur J Pharmacol 2008; 588:309 - 315; PMID: 18508045; http://dx.doi.org/10.1016/j.ejphar.2008.04.037
- Clark N, Keeble J, Fernandes ES, Starr A, Liang L, Sugden D, et al. The transient receptor potential vanilloid 1 (TRPV1) receptor protects against the onset of sepsis after endotoxin. FASEB J 2007; 21:3747 - 3755; PMID: 17601984; http://dx.doi.org/10.1096/fj.067460com
- Wang Y, Novotny M, Quaiserova-Mocko V, Swain GM, Wang DH. TRPV1-mediated protection against endotoxin-induced hypotension and mortality in rats. Am J Physiol Regul Integr Comp Physiol 2008; 294:1517 - 1523; PMID: 18337316; http://dx.doi.org/10.1152/ajpregu.00005.2008
- Guptill V, Cui X, Khaibullina A, Keller JM, Spornick N, Mannes A, et al. Disruption of the transient receptor potential vannilloid 1 can affect survival, bacterial clearance and cytokine gene expression during murine sepsis. Anesthesiology 2011; 114:1190 - 1199; PMID: 21383614; http://dx.doi.org/10.1097/ALN.0b013e318212515b
- Gavva NR, Treanor JJ, Garami A, Fang L, Surapaneni S, Akrami A, et al. Pharmacological blockade of the vanilloid receptor TRPV1 elicits marked hyperthermia in humans. Pain 2008; 136:202 - 210; PMID: 18337008; http://dx.doi.org/10.1016/j.pain.2008.01.024
- Garami A, Shimansky YP, Pakai E, Oliveira DL, Gavva NR, Romanovsky AA. Contributions of different modes of TRPV1 activation to TRPV1 antagonist-induced hyperthermia. J Neurosci 2010; 30:1435 - 1440; PMID: 20107070; http://dx.doi.org/10.1523/JNEUROSCI.5150-09.2010
- Romanovsky AA, Almeida MC, Garami A, Steiner AA, Norman MH, Morrison SF, et al. The transient receptor potential vanilloid-1 channel in thermoregulation: a thermosensor it is not. Pharmacol Rev 2009; 61:228 - 261; PMID: 19749171; http://dx.doi.org/10.1124/pr.109.001263
- Steiner AA, Oliveira DL, Roberts JL, Petersen SR, Romanovsky AA. Nicotine administration and withdrawal affect survival in systemic inflammation models. J Appl Physiol 2008; 105:1028 - 1034; PMID: 18617624; http://dx.doi.org/10.1152/japplphysiol.90619.2008
- Cross AS, Sadoff JC, Kelly N, Bernton E, Gemski P. Pretreatment with recombinant murine tumor necrosis factor alpha/cachectin and murine interleukin 1-alpha protects mice from lethal bacterial infection. J Exp Med 1989; 169:2021 - 2027; PMID: 2659724; http://dx.doi.org/10.1084/jem.169.6.2021
- Cross A, Asher L, Seguin M, Yuan L, Kelly N, Hammack C, et al. The importance of a lipopolysaccharide-initiated, cytokine-mediated host defense mechanism in mice against extraintestinally invasive Escherichia coli. J Clin Invest 1995; 96:676 - 686; PMID: 7635960; http://dx.doi.org/10.1172/JCI118110
- Vazquez-Torres A, Vallance BA, Bergman MA, Finlay BB, Cookson BT, Jones-Carson J, et al. Toll-like receptor 4 dependence of innate and adaptive immunity to Salmonella: importance of the Kupffer cell network. J Immunol 2004; 172:6202 - 6208; PMID: 15128808
- Wang H, Liao H, Ochani M, Justiniani M, Lin X, Yang L, et al. Cholinergic agonists inhibit HMGB1 release and improve survival in experimental sepsis. Nat Med 2004; 10:1216 - 1221; PMID: 15502843; http://dx.doi.org/10.1038/nm1124
- Pavlov VA, Ochani M, Yang LH, Gallowitsch-Puerta M, Ochani K, Lin X, et al. Selective alpha7-nicotinic acetylcholine receptor agonist GTS-21 improves survival in murine endotoxemia and severe sepsis. Crit Care Med 2007; 35:1139 - 1144; PMID: 17334244; http://dx.doi.org/10.1097/01.CCM.0000259381.56526.96
- Hofer S, Eisenbach C, Lukic IK, Schneider L, Bode K, Brueckmann M, et al. Pharmacologic cholinesterase inhibition improves survival in experimental sepsis. Crit Care Med 2008; 36:404 - 408; PMID: 18091537; http://dx.doi.org/10.1097/01.CCM.0B013E31816208B3
- Ang SF, Moochhala SM, Bhatia M. Hydrogen sulfide promotes transient receptor potential vanilloid 1-mediated neurogenic inflammation in polymicrobial sepsis. Crit Care Med 2010; 38:619 - 628; PMID: 19851090; http://dx.doi.org/10.1097/CCM.0b013e3181c0df00
- Garami A, Pakai E, Oliveira DL, Steiner AA, Wanner SP, Almeida MC, et al. Thermoregulatory phenotype of the Trpv1 knockout mouse: thermoeffector dysbalance with hyperkinesis. J Neurosci 2011; 31:1721 - 1733; PMID: 21289181; http://dx.doi.org/10.1523/JNEUROSCI.4671-10.2011
- Wanner SP, Garami A, Romanovsky AA. Hyperactive when young, hypoactive and overweight when aged: connecting the dots in the story about locomotor activity, body mass and aging in Trpv1 knockout mice. Aging (Albany NY) 2011; 3:450 - 454; PMID: 21483038
- Steiner AA, Turek VF, Almeida MC, Burmeister JJ, Oliveira DL, Roberts JL, et al. Nonthermal activation of transient receptor potential vanilloid-1 channels in abdominal viscera tonically inhibits autonomic cold-defense effectors. J Neurosci 2007; 27:7459 - 7468; PMID: 17626206; http://dx.doi.org/10.1523/JNEUROSCI.1483-07.2007
- Motter AL, Ahern GP. TRPV1-null mice are protected from diet-induced obesity. FEBS Lett 2008; 582:2257 - 2262; PMID: 18503767; http://dx.doi.org/10.1016/j.febslet.2008.05.021
- Ivanov AI, Kulchitsky VA, Romanovsky AA. Does obesity affect febrile responsiveness?. Int J Obes Relat Metab Disord 2001; 25:586 - 589; PMID: 11319666; http://dx.doi.org/10.1038/sj.ijo.0801523
- Ivanov AI, Romanovsky AA. Fever responses of Zucker rats with and without fatty mutation of the leptin receptor. Am J Physiol Regul Integr Comp Physiol 2002; 282:311 - 316; PMID: 11742853
- Ivanov AI, Kulchitsky VA, Romanovsky AA. Role for the cholecystokinin-A receptor in fever: a study of a mutant rat strain and a pharmacological analysis. J Physiol 2003; 547:941 - 949; PMID: 12562931; http://dx.doi.org/10.1113/jphysiol.2002.033183
- Steiner AA, Dogan MD, Ivanov AI, Patel S, Rudaya AY, Jennings DH, et al. A new function of the leptin receptor: mediation of the recovery from lipopolysaccharide-induced hypothermia. FASEB J 2004; 18:1949 - 1951; PMID: 15388670
- Ivanov AI, Steiner AA, Patel S, Rudaya AY, Romanovsky AA. Albumin is not an irreplaceable carrier for amphipathic mediators of thermoregulatory responses to LPS: compensatory role of α1-acid glycoprotein. Am J Physiol Regul Integr Comp Physiol 2005; 288:872 - 878; PMID: 15576666; http://dx.doi.org/10.1152/ajpregu.00514.2004
- Miehsler W. Mortality, morbidity and special issues of obese ICU patients. Wien Med Wochenschr 2010; 160:124 - 128; PMID: 20364415; http://dx.doi.org/10.1007/s10354-010-0767-4
- Steiner AA, Romanovsky AA. Leptin: at the crossroads of energy balance and systemic inflammation. Prog Lipid Res 2007; 46:89 - 107; PMID: 17275915; http://dx.doi.org/10.1016/j.plipres.2006.11.001
- Ye J, Keller JN. Regulation of energy metabolism by inflammation: a feedback response in obesity and calorie restriction. Aging (Albany NY) 2010; 2:361 - 368; PMID: 20606248
- Wang S, Davis BM, Zwick M, Waxman SG, Albers KM. Reduced thermal sensitivity and Nav1.8 and TRPV1 channel expression in sensory neurons of aged mice. Neurobiol Aging 2006; 27:895 - 903; PMID: 15979214; http://dx.doi.org/10.1016/j.neurobiolaging.2005.04.009
- Bergman E, Fundin BT, Ulfhake B. Effects of aging and axotomy on the expression of neurotrophin receptors in primary sensory neurons. J Comp Neurol 1999; 410:368 - 386; PMID: 10404406; http://dx.doi.org/10.1002/(SICI)1096-9861(19990802)410:3<368::AID-CNE2>3.0.CO;2-I
- Marik PE, Zaloga GP. The effect of aging on circulating levels of proinflammatory cytokines during septic shock. Norasept II Study Investigators. J Am Geriatr Soc 2001; 49:5 - 9; PMID: 11207836; http://dx.doi.org/10.1046/j.1532-5415.2001.49003.x
- Stewart L, Grifiss JM, Jarvis GA, Way LW. Elderly patients have more severe biliary infections: influence of complement-killing and induction of TNFalpha production. Surgery 2008; 143:103 - 112; PMID: 18154938; http://dx.doi.org/10.1016/j.surg.2007.06.035
- Watanabe E, Hirasawa H, Oda S, Matsuda K, Hatano M, Tokuhisa T. Extremely high interleukin-6 blood levels and outcome in the critically ill are associated with tumor necrosis factor- and interleukin-1-related gene polymorphisms. Crit Care Med 2005; 33:89 - 97; PMID: 15644653; http://dx.doi.org/10.1097/01.CCM.0000150025.79100.7D
- Turnbull IR, Clark AT, Stromberg PE, Dixon DJ, Woolsey CA, Davis CG, et al. Effects of aging on the immunopathologic response to sepsis. Crit Care Med 2009; 37:1018 - 1023; PMID: 19237912; http://dx.doi.org/10.1097/CCM.0b013e3181968f3a
- McConnell KW, Fox AC, Clark AT, Chang NY, Dominguez JA, Farris AB, et al. The role of heat shock protein 70 in mediating age-dependent mortality in sepsis. J Immunol 2011; 186:3718 - 3725; PMID: 21296977; http://dx.doi.org/10.4049/jimmunol.1003652
- Moran MM, McAlexander MA, Biro T, Szallasi A. Transient receptor potential channels as therapeutic targets. Nat Rev Drug Discov 2011; 10:601 - 620; PMID: 21804597; http://dx.doi.org/10.1038/nrd3456
- Norman DC. Fever in the elderly. Clin Infect Dis 2000; 31:148 - 151; PMID: 10913413; http://dx.doi.org/10.1086/313896
- High KP, Bradley SF, Gravenstein S, Mehr DR, Quagliarello VJ, Richards C, et al. Clinical practice guideline for the evaluation of fever and infection in older adult residents of long-term care facilities: 2008 update by the Infectious Diseases Society of America. J Am Geriatr Soc 2009; 57:375 - 394; PMID: 19278394; http://dx.doi.org/10.1111/j.1532-5415.2009.02175.x
- Rudaya AY, Steiner AA, Robbins JR, Dragic AS, Romanovsky AA. Thermoregulatory responses to lipopolysaccharide in the mouse: dependence on the dose and ambient temperature. Am J Physiol Regul Integr Comp Physiol 2005; 289:1244 - 1252; PMID: 16081879; http://dx.doi.org/10.1152/ajpregu.00370.2005
- Bland JM, Altman DG. The logrank test. BMJ 2004; 328:1073; PMID: 15117797; http://dx.doi.org/10.1136/bmj.328.7447.1073
- Lawless JF. Statistical Models and Methods for Lifetime Data 1982; New York John Wiley & Sons