Abstract
The small nematode C. elegans is characterized by developing through a highly coordinated, reproducible cell lineage that serves as the basis of many studies focusing on the development of multi-lineage organisms. Indeed, the reproducible cell lineage enables discovery of developmental defects that occur in even a single cell. Only recently has attention been focused on how these animals modify their genetically programmed cell lineages to adapt to altered environments. Here, we summarize the current understanding of how C. elegans responds to food deprivation by adapting their developmental program in order to conserve energy. In particular, we highlight the AMPK-mediated and insulin-like growth factor signaling pathways that are the principal regulators of induced cell cycle quiescence.
Introduction
The regulation of both metabolism and the cell cycle has been intensely studied for decades due to their central roles in the etiology and progression of human disease. In the 1920s, Otto Warburg observed that cancerous tissues metabolize glucose at a much higher rate than normal tissue due to an increased rate of glycolysis. Since that discovery, numerous genetic and environmental factors associated with cancer progression have been linked to changes in cell metabolism.Citation1 An understanding of how cancerous cells bypass normal growth control mechanisms may be achieved through investigation of the crosstalk between metabolism and cell cycle regulation. Research in model systems such as C. elegans provides a foundation for understanding these crosstalk mechanisms. C. elegans possesses unique characteristics useful for phenotypic and genetic analyses, including a semi-transparent body that allows for direct visualization of cells at all developmental stages, established forward and reverse genetics methods and a streamlined genome that displays low redundancy while maintaining high conservation within specific gene families.Citation2 Perhaps the most useful characteristic for cell cycle analyses is the highly reproducible cell lineage that is subject to change only in response to genetic alteration or environmental distress.
C. elegans develops through a highly reproducible somatic cell lineage,Citation2,Citation3 which suggests a high degree of spatiotemporal coordination between cell division, apoptosis, cell fate specification and differentiation.Citation4-Citation7 In contrast to the embryo that enjoys the protection of the eggshell and is generally considered self-sufficient, the developing larvae must find a source of food if it is to survive and develop into a fertile adult. At two stages, C. elegans larvae are able to sense environmental cues, such as temperature, crowding and nutrition, and adapt their developmental programs to better suit ambient conditions.Citation8 Immediately after hatching, the larvae make a developmental decision to proceed with post-embryonic development based on the availability of food.Citation9-Citation11 Indeed, the ability to arrest post-embryonic development immediately after hatching in response to food deprivation, a state termed L1 diapause, and to resume larval development in response to food availability is experimentally exploited to generate large populations of synchronously developing larvae.Citation12 Similarly, upon exposure to unfavorable growth conditions during early larval development, the animals can adopt an alternate developmental program called the dauer stage.Citation8,Citation13 Both of these developmental states are characterized by arrested cell cycles; however, the key distinguishing feature is that during dauer arrest, the larvae enter an alternative developmental program suited to withstand stressful conditions, while L1 diapause is simply a temporary arrest of larval development.Citation13 Studies to delineate the developmental mechanisms used to regulate the cell cycle are uncovering an elaborate system of checks and balances that, together, ensure cell cycle progression occurs under the appropriate conditions and at the appropriate time.Citation5,Citation14 Since the components and mechanisms involved in the cell cycle and its regulation are generally conserved with higher organisms, including humans, these studies are invaluable for understanding the molecular basis of and developing treatments for a wide variety of diseases.
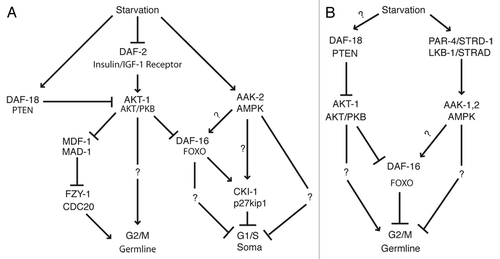
Completion of a cell cycle carries a high energetic cost, thus the ability of a cell to sense its energy status and influence cell cycle progression is important for survival. Two important energy-sensing pathways, AMP-activated protein kinase (AMPK) and insulin/IGF-1 signaling have been well characterized in C. elegans in the context of aging, dauer formation and, more recently, cell cycle progression (). Specifically, these pathways have been implicated in controlling two separate cell cycle processes: G1/S progression in somatic cells and G2/M progression in the germ line. In this review, we discuss how these signaling pathways sense energy availability and override the genetically scheduled cell cycle to arrest cell divisions until conditions are favorable for further growth and development. In addition, pathways involved in oxygen sensing have been shown to regulate cell cycles in C. elegans; this suspended animation is discussed elsewhere in this issue by Padilla and Ladage.
The Core Insulin/IGF-1 Signaling Pathway is Conserved
Intensive investigation of the insulin signal transduction cascade in C. elegans has yielded a detailed mechanistic understanding of a process that is highly conserved among higher organisms.Citation15-Citation18 These studies have revealed a core-signaling pathway that is involved in several nutrition-dependent developmental decisions described below. Several recent reviews have focused specifically on the mechanism of insulin/IGF-1 signal transduction;Citation15,Citation19-Citation22 thus, only those genes directly relevant to the discussion on cell cycle control are presented here. While approximately 40 genes have been identified as encoding putative insulin ligands,Citation23-Citation26 a single gene called daf-2 encodes the insulin receptor.Citation27 The signaling cascade is initiated upon ligand engagement by DAF-2, which activates the AGE-1 PI3-kinase.Citation28 AGE-1 converts phosphoinositide-3,4-P2(PIP2) to phosphoinositide-3,4,5-P3 (PIP3). The increase in PIP3 concentration results in the activation of two AKT/PKB kinase homologs, AKT-1 and AKT-2.Citation29,Citation30 The AGE-1 activity is counteracted by DAF-18, the C. elegans homolog of the phosphatase PTEN, which dephosphorylates PIP3.Citation31 AKT-1 and AKT-2 regulate the nucleocytoplasmic localization of the DAF-16/FOXO transcription factor.Citation30,Citation32-Citation35 Phosphorylated DAF-16 is retained within the cytoplasm, such that active insulin signaling prevents DAF-16-mediated transcriptional activation.Citation32,Citation33 This canonical pathway functions in the context of multiple processes, including response to UV exposure,Citation36 heatCitation35 or oxidative stress,Citation35 dauer formationCitation27 and aging.Citation27,Citation37 As described below, the insulin/IGF-1 signaling pathway also plays a central role in regulating the cell cycle at both the G1/SCitation38 and G2/M transitionsCitation10,Citation39,Citation40 of somatic and germ cells, respectively.
Starvation-Induced Cell Cycle Quiescence Requires Components of the Insulin/IGF-1 Signaling Pathway
Insulin/IGF-1 signaling plays a vital role in initiating post-embryonic development of the newly hatched larvae. Specifically, in response to starvation, components of the insulin/IGF-1 pathway mediate a G1-phase arrest of somatic cells during L1 diapause.Citation38 Based on the examination of several cell lineages, wild-type animals arrest cell divisions during L1 diapause, while animals deficient in daf-16/FOXO activity fail to arrest the cell cycle.Citation38 Accordingly, animals lacking the opposing daf-2 activity display a heightened sensitivity to food deprivation.Citation38 These findings establish that insulin signaling is an important contributor to nutrient-dependent G1 cell cycle arrest in C. elegans. Further, the failure of daf-16/FOXO mutant animals to arrest cell divisions correlates with a failure to accumulate CKI-1/p27kip1, suggesting that insulin/IGF-1 signaling controls L1 diapause arrest through the promotion of cyclin-dependent kinase inhibitory activity.Citation38 These results are supported by data obtained in other experimental systems demonstrating that FOXO transcription factors can regulate cell cycle progression through control of p27kip1.Citation41-Citation43 Although inactivation of daf-16/FOXO results in failure to accumulate CKI-1/p27kip1 in C. elegans, further studies are needed to establish the precise mechanism of daf-16-mediated cell cycle arrest.
In addition to a role in starvation-induced G1 arrest of somatic cells, insulin/IGF-1 signaling also mediates an arrest at the G2/M transition in germ cells.Citation10,Citation39,Citation44 Analyses of animals lacking the p27kip1-related G1/S inhibitor encoded by cki-1 revealed over proliferation of a wide variety of somatic cell types,Citation11 but obvious hyperproliferation was not observed within the germ line.Citation44 This observation suggests that regulation of germ cell divisions does not occur at the G1/S transition as in the somatic tissues.Citation10 While primordial germ cells do, in fact, arrest cell cycles during L1 diapause, the cells arrest with 4N DNA content and duplicated centrosomes, indicating that the arrest occurs after completion of S phase.Citation10 Similar to somatic cells, the germline cell cycle arrest in L1 diapause is dependent on components of the insulin/IGF-1 signaling pathway. Loss-of-function (lf) mutation within daf-18/PTEN allows germ cells to inappropriately proliferate during L1 diapause.Citation10 The failure of germ cells to arrest in daf-18/PTEN(lf) mutant animals is due to overactive insulin/IGF-1 signaling, since further loss of the genetically opposing insulin/IGF-1 signaling components, such as age-1 or akt-1, suppress the hyperproliferation defect.Citation10 Interestingly, daf-16 function is not required for germ cell arrest during L1 diapause.Citation10 Since the proposed cell cycle quiescence function of DAF-16/FOXO is to promote expression of the G1/S inhibitor CKI-1,Citation38 daf-16 function may be dispensable in organizing the G2/M arrest of the germ cells. This suggests that the germline L1 diapause arrest may involve a non-canonical insulin/IGF-1 pathway that diverges prior to the activity of the canonical downstream effector, DAF-16/FOXO.
The requirement for insulin/IGF-1 signaling in germ cell arrest is not limited to L1 diapause, as insulin signaling components are also necessary for germ cell arrest during the dauer stage. At this stage, gain-of-function (gf) mutation of akt-1 or daf-18(lf) disrupts the ability of germline cells to arrest proliferation during the dauer stage.Citation39 In contrast to L1 diapause, daf‑16/FOXO activity appears to be required in the germ line for dauer stage proliferation arrest.Citation39 The differential requirement may reflect inherent differences between the mechanisms used to arrest germ cells during L1 and dauer stages. Despite these differences, it is clear that components of the insulin/IGF-1 signaling pathway play an indispensible role in sensing the low nutrient conditions and temporarily halting cell divisions within the germ line.
How does insulin/IGF-1 signaling exert control over the core cell cycle machinery to inhibit G2/M progression in the germ cells? Interestingly, the cell cycle arrest requires the spindle assembly checkpoint (SAC), which delays the metaphase-to-anaphase transition until the sister chromatids have completed attachments to the mitotic spindle.Citation45 Animals that are hemizygous (i.e., Δ/+) for the SAC component encoded by mdf-1/MAD1 exhibit a germ cell hyperproliferation phenotype similar to daf-18(lf) mutant animals.Citation40 This hyperproliferation defect is not enhanced in combination with daf-18(lf), suggesting that daf-18 and mdf-1 may act within a linear pathway.Citation40 DAF-18 and MDF-1 appear to control the cell cycle through the activity of fzy-1, the C. elegans anaphase-promoting complex (APC) component, CDC20.Citation40 These genetic interactions support a model wherein the DAF-18/PTEN-mediated signaling pathway promotes germline cell cycle arrest through activation of the SAC, which, in turn, inhibits APC activity to result in cell cycle arrest.Citation40,Citation46 A mechanism directly connecting insulin/IGF-1 signaling and the SAC is indicated by a bioinformatics analysis of AKT-1 that identified several potential phosphorylation sites on MDF-1, several of which could be phosphorylated by AKT-1 in vitro.Citation40 Moreover, expression of a phosphorylation site-defective MDF-1 weakly suppressed the daf-18(lf) hyperproliferation phenotype, suggesting that DAF-18 may mediate germ cell arrest during nutrient stress by preventing AKT-1 phosphorylation and inhibtion of MDF-1.Citation40 Together, these data suggest a mechanism to explain how the non-canonical insulin/IGF-1 signaling pathway can mediate a daf-16-independent G2/M arrest through control of the SAC.
Insulin/IGF-1 signaling also plays an important role in the maintenance of germline proliferation under normal, non-starvation growth conditions. Animals carrying a temperature sensitive (ts) daf-2 allele that are grown at a semi-permissive temperature do not undergo dauer formation but display decreased germline proliferation, illustrating a constitutive role for insulin signaling in promoting germline proliferation.Citation47 The ability to separate germline arrest from dauer formation is notable, since it suggests that the cell cycle arrest does not depend on entry into the dauer arrest state.Citation47 The arrested germ cells of these daf-2(ts) mutant animals exhibited a 4N DNA content, consistent with a G2/M delay.Citation47 The daf-2(ts) hypoproliferation defect also requires the activity of both daf-18/PTEN and daf-16/FOXO, indicating vital functions of the downstream targets of DAF-2 activation.Citation47 Based on RNAi analyses of putative insulin ligands, ins-3 and ins-33 may encode the insulin ligands that function to control germline proliferation.Citation47 A genetic screen for mutations that suppress germline tumor formation also found a vital role for daf-2 in germline proliferation. This model uses a gain-of-function allele of glp-1, one of two C. elegans Notch receptor homologs, to promote the production of a germline tumor.Citation48 The screen identified a daf-2(lf) mutation that suppresses germline tumor formation through daf-16/FOXO-dependent decrease of proliferation and increase in apoptosis.Citation49 Putative DAF-16/FOXO target genes that mediate the arrest were subsequently identified and included both positive and negative regulators of germline tumor formation.Citation50 Further analyses of these DAF-16 target genes may reveal the mechanism used by insulin/IGF-1 signaling to control germline proliferation.
AMPK Monitors Conditions for Growth
The AMP-activated protein kinase (AMPK) signaling pathway is an ancient energy-sensing pathway that is conserved throughout eukaryotic evolution.Citation51 A wide variety of metabolism-associated signals are integrated by AMPK through mechanisms that are poorly understood.Citation51 Similarly, the mechanisms by which activated AMPK effects changes in the cell are not fully characterized,Citation51 although its phosphorylation site specificity is well-documented.Citation52,Citation53 Characterization of the AMPK heterotrimeric complex is complicated by the fact that higher organisms encode several isoforms of each subunit, which allows for the formation of multiple AMPK complexes.Citation51 The C. elegans genome encodes two catalytic α subunits, aak-1 and aak-2; two β regulatory subunits, aakb-1 and aakb-2; and five γ regulatory subunits, aakg-1 through aakg-5.Citation54-Citation56 Despite the potential of only 20 unique AMPK complexes in C. elegans, the assignment of a specific function to an individual complex has not yet been achieved. Through the study of other model systems, numerous targets of AMPK have been identified that implicate AMPK-mediated signaling in the positive regulation of catabolic processes and the negative regulation of anabolic processes through a variety of mechanisms.Citation51 We will focus on the roles of AMPK signaling in the inhibition of cell cycle progression during low-nutrition development.
Using cultured mammalian cells, the critical role of AMPK signaling in nutrient-dependent G1 arrest has been established. Cells expressing a constitutively active AMPK or treated with an activator of AMPK activity, AICAR, increase expression of p21cip1, p27kip1 and p53 and arrest divisions.Citation42,Citation57,Citation58 This arrest appears to be p53-dependent, since cells lacking p53 do not arrest following AMPK activation, and p53 phosphorylation coincides with activation of AMPK and glucose-dependent G1 arrest.Citation58 Moreover, several tumor cell lines show low or no expression of the primary upstream kinase that activates AMPK, LKB1.Citation59 Consistent with its proposed role as a tumor suppressor, expression of LKB-1 in these cells results in elevated expression of the p21cip1 cyclin-dependent kinase inhibitor and G1 arrest.Citation60
C. elegans AMPK Regulates Cell Cycle Quiescence in Low Food Conditions
In C. elegans, the crucial functions of AMPK in cell cycle control have only recently been revealed. Animals deficient for the AMPK catalytic subunit encoded by aak-2 failed to arrest somatic cell divisions during L1 diapause.Citation38 Although the molecular mechanism for the defect remains unknown, previously described results from mammalian studies suggest several possibilities. aak-2 may regulate cell cycle arrest by promoting the activity of the p53 homolog cep-1 or the cyclin-dependent kinase inhibitor orthologs cki-1 and cki-2. Since cep-1 has no known role in regulation of G1/S progression in somatic cells,Citation61 cki-1 and cki-2 are more likely candidates. Ultimately, unbiased identification of additional AMPK targets will clarify how AMPK controls cell cycle quiescence.
The AMPK requirement during induced cell cycle quiescence is better characterized in the germline than in somatic cells. Using a genetic screen to identify genes required to arrest germline proliferation in a temperature-sensitive dauer constitutive daf-2(ts) strain, a dominant-negative (Dn) allele of aak-2 was identified.Citation39 Here, despite the presence of food, the impaired daf-2(ts) activity results in dauer development and arrest of germ cell divisions.Citation27,Citation39,Citation62 The aak-2(Dn) allele disrupts the arrest and allows proliferation to proceed.Citation39 Using RNAi to inhibit the activity of the aak-1 ortholog, a weak germline hyperproliferation defect is also observed in the dauer stage.Citation39 However, the phenotype is significantly enhanced when aak-1(RNAi) is combined with the aak-2 mutation, suggesting that the two genes act in a redundant manner.Citation39 It is notable that a par-4/LKB1 mutation results in a phenotype similar to the aak-1;aak-2 double loss,Citation39 consistent with LKB1 as an activator of AMPK.Citation51 Interestingly, the phenotype caused by this par-4 mutant allele is enhanced by additional loss of aak-2 function, suggesting that either (1) other upstream factors act redundantly on par-4 to regulate aak-2 or (2) the par-4 mutant allele used in this study still retains some ability to activate aak-2.Citation39 Furthermore, strd-1, the C. elegans homolog of the STRAD accessory protein that associates with LKB-1 to activate AMPK, is also required for germline arrest.Citation63 strd-1(lf) and par-4(lf) mutant animals display a similar hyperproliferation phenotype.Citation63 Consistent with in vivo cooperation between PAR-4 and STRD-1 to regulate AAK-2, phosphorylation of AAK-2 is not detected in either par-4(lf) or strd-1(lf) mutant animals.Citation63 Moreover, PAR-4 and STRD-1 appear to co-localize in early embryonic cells.Citation63 Taken together, these results delineate the AMPK-mediated mechanism that promotes germline cell cycle quiescence during dauer arrest.
Crosstalk between AMPK and Insulin/IGF-1 Signaling
Several studies in C. elegans suggest cooperation between insulin/IGF-1 and AMPK signaling. The extension of lifespan by reduced insulin/IGF-1 signaling requires aak-2 function,Citation54,Citation55,Citation64 indicating that AMPK acts downstream of insulin/IGF-1 signaling.Citation55,Citation64 Subsequently, it was determined that lifespan extension by constitutive AMPK activation requires daf-16 function.Citation64 Since DAF-16 and FOXO3 are the targets of transcription activating phosphorylation by AMPK, a function for AMPK in lifespan extension may be to activate DAF-16.Citation64,Citation65 Both aak-2(lf) and daf-16(lf) mutant animals exhibit the germ cell arrest failure phenotype in dauer,Citation39 consistent with AMPK activation of DAF-16 to promote germ cell arrest. However, genetic analyses of mutations disrupting other insulin/IGF-1 signaling components in combinations with the aak-2(lf) mutation suggest that AMPK and insulin/IGF-1 pathways may act within partially redundant mechanisms.Citation39 Further studies are clearly necessary to confirm the significance of DAF-16 co-regulation by AMPK and insulin/IGF-1 pathways to control cell cycle quiescence.
Conclusion
Studies of C. elegans demonstrate the presence of a nutrient-sensing system that is able to alter the highly ordered developmental cell lineage. We described two distinct stages when developing worms can modify their cell lineage programs upon encountering a low-food environment. First, the newly hatched larvae can delay post-embryonic development in a state referred to as L1 diapause until suitable nutrition is encountered. Second, in response to sub-optimal growth conditions encountered during the early larval stages, development can be directed toward an alternate L3 stage termed the dauer stage. While the transition into the dauer state also involves alterations to the cell fate program, both of these survival conditions necessitate changes to the normal timing of the energy intensive cell division cycles. Thus, while the genetically programmed cell lineage is highly reproducible under standard laboratory culture conditions, in less hospitable environments the developmental program exhibits a level of plasticity to facilitate survival of the animal.
C. elegans appear to mainly rely on the AMPK and insulin signaling pathways to induce cell cycle quiescence during conditions of low nutrient availability. While significant progress has been made, several outstanding questions remain regarding the functions of AMPK and insulin signaling pathways. Although clearly related to nutrient availability, the specific environmental cue or metabolite that initiates the insulin cascade to promote cell cycle quiescence is not defined. Nor is the mechanism used by AMPK to control cell cycles understood. Since several genes appear to act in a cell-autonomous manner, how the spatially and functionally unrelated cells coordinate initiation and maintenance of cell cycle arrest throughout the diverse cell types of the entire animal is not understood. Lastly, LKB1 was recently shown to play an AMPK-independent role in maintaining the quiescence of hematopoietic stem cells in mice.Citation66-Citation68 Indeed, a function for AMPK signaling to control cell cycle progression during nutrient-replete C. elegans growth conditions has not been described. We suggest that the combined knowledge gained by studies of the intersection between metabolism and cell cycle using model systems such as C. elegans, Drosophila and mice can be developed into useful chemotherapeutic agents for treatment of a range of diseases from diabetes mellitus to cancer.
Acknowledgments
We are grateful to David Fay and Patricia Ernst for critical comments. We apologize to our colleagues whose original articles were not cited because of space constraints. The authors’ lab is supported by the NIH (GM077031).
References
- Hsu PP, Sabatini DM. Cancer cell metabolism: Warburg and beyond. Cell 2008; 134:703 - 7; http://dx.doi.org/10.1016/j.cell.2008.08.021; PMID: 18775299
- Sulston JE, Horvitz HR. Post-embryonic cell lineages of the nematode, Caenorhabditis elegans.. Dev Biol 1977; 56:110 - 56; http://dx.doi.org/10.1016/0012-1606(77)90158-0; PMID: 838129
- Sulston JE, Schierenberg E, White JG, Thomson JN. The embryonic cell lineage of the nematode Caenorhabditis elegans.. Dev Biol 1983; 100:64 - 119; http://dx.doi.org/10.1016/0012-1606(83)90201-4; PMID: 6684600
- Conradt B, Xue D. Programmed cell death. WormBook 2005; 1 - 13; PMID: 18061982
- van den Heuvel S. Cell-cycle regulation. WormBook 2005; 1 - 16; PMID: 18050422
- Sternberg PW, Félix MA. Evolution of cell lineage. Curr Opin Genet Dev 1997; 7:543 - 50; http://dx.doi.org/10.1016/S0959-437X(97)80084-6; PMID: 9309188
- Bertrand V, Hobert O. Lineage programming: navigating through transient regulatory states via binary decisions. Curr Opin Genet Dev 2010; 20:362 - 8; http://dx.doi.org/10.1016/j.gde.2010.04.010; PMID: 20537527
- Golden JW, Riddle DL. The Caenorhabditis elegans dauer larva: developmental effects of pheromone, food, and temperature. Dev Biol 1984; 102:368 - 78; http://dx.doi.org/10.1016/0012-1606(84)90201-X; PMID: 6706004
- Muñoz MJ, Riddle DL. Positive selection of Caenorhabditis elegans mutants with increased stress resistance and longevity. Genetics 2003; 163:171 - 80; PMID: 12586705
- Fukuyama M, Rougvie AE, Rothman JH. C. elegans DAF-18/PTEN mediates nutrient-dependent arrest of cell cycle and growth in the germline. Curr Biol 2006; 16:773 - 9; http://dx.doi.org/10.1016/j.cub.2006.02.073; PMID: 16631584
- Hong Y, Roy R, Ambros V. Developmental regulation of a cyclin-dependent kinase inhibitor controls postembryonic cell cycle progression in Caenorhabditis elegans.. Development 1998; 125:3585 - 97; PMID: 9716524
- Stiernagle T. Maintenance of C. elegans.. WormBook 2006; 1 - 11; PMID: 18050451
- Hu PJ. Dauer. WormBook 2007; 1 - 19; PMID: 17988074
- Kirienko NV, Mani K, Fay DS. Cancer models in Caenorhabditis elegans.. Dev Dyn 2010; 239:1413 - 48; PMID: 20175192
- Landis JN, Murphy CT. Integration of diverse inputs in the regulation of Caenorhabditis elegans DAF-16/FOXO. Dev Dyn 2010; 239:1405 - 12; PMID: 20140911
- Mukhopadhyay A, Oh SW, Tissenbaum HA. Worming pathways to and from DAF-16/FOXO. Exp Gerontol 2006; 41:928 - 34; http://dx.doi.org/10.1016/j.exger.2006.05.020; PMID: 16839734
- Gami MS, Wolkow CA. Studies of Caenorhabditis elegans DAF-2/insulin signaling reveal targets for pharmacological manipulation of lifespan. Aging Cell 2006; 5:31 - 7; http://dx.doi.org/10.1111/j.1474-9726.2006.00188.x; PMID: 16441841
- Baumeister R, Schaffitzel E, Hertweck M. Endocrine signaling in Caenorhabditis elegans controls stress response and longevity. J Endocrinol 2006; 190:191 - 202; http://dx.doi.org/10.1677/joe.1.06856; PMID: 16899554
- Murphy CT. The search for DAF-16/FOXO transcriptional targets: approaches and discoveries. Exp Gerontol 2006; 41:910 - 21; http://dx.doi.org/10.1016/j.exger.2006.06.040; PMID: 16934425
- Amrit FR, May RC. Younger for longer: insulin signalling, immunity and ageing. Curr Aging Sci 2010; 3:166 - 76; http://dx.doi.org/10.2174/1874609811003030166; PMID: 20735349
- Kaletsky R, Murphy CT. The role of insulin/IGF-like signaling in C. elegans longevity and aging. Dis Model Mech 2010; 3:415 - 9; http://dx.doi.org/10.1242/dmm.001040; PMID: 20354111
- Kleemann GA, Murphy CT. The endocrine regulation of aging in Caenorhabditis elegans.. Mol Cell Endocrinol 2009; 299:51 - 7; http://dx.doi.org/10.1016/j.mce.2008.10.048; PMID: 19059305
- Pierce SB, Costa M, Wisotzkey R, Devadhar S, Homburger SA, Buchman AR, et al. Regulation of DAF-2 receptor signaling by human insulin and ins-1, a member of the unusually large and diverse C. elegans insulin gene family. Genes Dev 2001; 15:672 - 86; http://dx.doi.org/10.1101/gad.867301; PMID: 11274053
- Li W, Kennedy SG, Ruvkun G. daf-28 encodes a C. elegans insulin superfamily member that is regulated by environmental cues and acts in the DAF-2 signaling pathway. Genes Dev 2003; 17:844 - 58; http://dx.doi.org/10.1101/gad.1066503; PMID: 12654727
- Murphy CT, Lee SJ, Kenyon C. Tissue entrainment by feedback regulation of insulin gene expression in the endoderm of Caenorhabditis elegans.. Proc Natl Acad Sci U S A 2007; 104:19046 - 50; http://dx.doi.org/10.1073/pnas.0709613104; PMID: 18025456
- Kawano T, Ito Y, Ishiguro M, Takuwa K, Nakajima T, Kimura Y. Molecular cloning and characterization of a new insulin/IGF-like peptide of the nematode Caenorhabditis elegans.. Biochem Biophys Res Commun 2000; 273:431 - 6; http://dx.doi.org/10.1006/bbrc.2000.2971; PMID: 10873623
- Kimura KD, Tissenbaum HA, Liu Y, Ruvkun G. daf-2, an insulin receptor-like gene that regulates longevity and diapause in Caenorhabditis elegans.. Science 1997; 277:942 - 6; http://dx.doi.org/10.1126/science.277.5328.942; PMID: 9252323
- Morris JZ, Tissenbaum HA, Ruvkun G. A phosphatidylinositol-3-OH kinase family member regulating longevity and diapause in Caenorhabditis elegans.. Nature 1996; 382:536 - 9; http://dx.doi.org/10.1038/382536a0; PMID: 8700226
- Paradis S, Ailion M, Toker A, Thomas JH, Ruvkun GA. A PDK1 homolog is necessary and sufficient to transduce AGE-1 PI3 kinase signals that regulate diapause in Caenorhabditis elegans. Genes Dev 1999; 13:1438 - 52; http://dx.doi.org/10.1101/gad.13.11.1438; PMID: 10364160
- Hertweck M, Göbel C, Baumeister RC. C. elegans SGK-1 is the critical component in the Akt/PKB kinase complex to control stress response and life span. Dev Cell 2004; 6:577 - 88; http://dx.doi.org/10.1016/S1534-5807(04)00095-4; PMID: 15068796
- Ogg S, Ruvkun G. The C. elegans PTEN homolog, DAF-18, acts in the insulin receptor-like metabolic signaling pathway. Mol Cell 1998; 2:887 - 93; http://dx.doi.org/10.1016/S1097-2765(00)80303-2; PMID: 9885576
- Lin K, Dorman JB, Rodan A, Kenyon C. daf-16: An HNF-3/forkhead family member that can function to double the life-span of Caenorhabditis elegans.. Science 1997; 278:1319 - 22; http://dx.doi.org/10.1126/science.278.5341.1319; PMID: 9360933
- Ogg S, Paradis S, Gottlieb S, Patterson GI, Lee L, Tissenbaum HA, et al. The Fork head transcription factor DAF-16 transduces insulin-like metabolic and longevity signals in C. elegans.. Nature 1997; 389:994 - 9; http://dx.doi.org/10.1038/40194; PMID: 9353126
- Paradis S, Ruvkun G. Caenorhabditis elegans Akt/PKB transduces insulin receptor-like signals from AGE-1 PI3 kinase to the DAF-16 transcription factor. Genes Dev 1998; 12:2488 - 98; http://dx.doi.org/10.1101/gad.12.16.2488; PMID: 9716402
- Henderson ST, Johnson TE. daf-16 integrates developmental and environmental inputs to mediate aging in the nematode Caenorhabditis elegans.. Curr Biol 2001; 11:1975 - 80; http://dx.doi.org/10.1016/S0960-9822(01)00594-2; PMID: 11747825
- Murakami S, Johnson TE. A genetic pathway conferring life extension and resistance to UV stress in Caenorhabditis elegans.. Genetics 1996; 143:1207 - 18; PMID: 8807294
- Kenyon C, Chang J, Gensch E, Rudner A, Tabtiang RA. A C. elegans mutant that lives twice as long as wild type. Nature 1993; 366:461 - 4; http://dx.doi.org/10.1038/366461a0; PMID: 8247153
- Baugh LR, Sternberg PW. DAF-16/FOXO regulates transcription of cki-1/Cip/Kip and repression of lin-4 during C. elegans L1 arrest. Curr Biol 2006; 16:780 - 5; http://dx.doi.org/10.1016/j.cub.2006.03.021; PMID: 16631585
- Narbonne P, Roy R. Inhibition of germline proliferation during C. elegans dauer development requires PTEN, LKB1 and AMPK signalling. Development 2006; 133:611 - 9; http://dx.doi.org/10.1242/dev.02232; PMID: 16407400
- Watanabe S, Yamamoto TG, Kitagawa R. Spindle assembly checkpoint gene mdf-1 regulates germ cell proliferation in response to nutrition signals in C. elegans.. EMBO J 2008; 27:1085 - 96; http://dx.doi.org/10.1038/emboj.2008.32; PMID: 18309291
- Dijkers PF, Medema RH, Pals C, Banerji L, Thomas NS, Lam EW, et al. Forkhead transcription factor FKHR-L1 modulates cytokine-dependent transcriptional regulation of p27(KIP1). Mol Cell Biol 2000; 20:9138 - 48; http://dx.doi.org/10.1128/MCB.20.24.9138-9148.2000; PMID: 11094066
- Liang J, Shao SH, Xu ZX, Hennessy B, Ding Z, Larrea M, et al. The energy sensing LKB1-AMPK pathway regulates p27(kip1) phosphorylation mediating the decision to enter autophagy or apoptosis. Nat Cell Biol 2007; 9:218 - 24; http://dx.doi.org/10.1038/ncb1537; PMID: 17237771
- Medema RH, Kops GJ, Bos JL, Burgering BM. AFX-like Forkhead transcription factors mediate cell-cycle regulation by Ras and PKB through p27kip1. Nature 2000; 404:782 - 7; http://dx.doi.org/10.1038/35008115; PMID: 10783894
- Fukuyama M, Gendreau SB, Derry WB, Rothman JH. Essential embryonic roles of the CKI-1 cyclin-dependent kinase inhibitor in cell-cycle exit and morphogenesis in C elegans.. Dev Biol 2003; 260:273 - 86; http://dx.doi.org/10.1016/S0012-1606(03)00239-2; PMID: 12885569
- Musacchio A, Salmon ED. The spindle-assembly checkpoint in space and time. Nat Rev Mol Cell Biol 2007; 8:379 - 93; http://dx.doi.org/10.1038/nrm2163; PMID: 17426725
- Kitagawa R, Law E, Tang L, Rose AM. The Cdc20 homolog, FZY-1, and its interacting protein, IFY-1, are required for proper chromosome segregation in Caenorhabditis elegans.. Curr Biol 2002; 12:2118 - 23; http://dx.doi.org/10.1016/S0960-9822(02)01392-1; PMID: 12498686
- Michaelson D, Korta DZ, Capua Y, Hubbard EJ. Insulin signaling promotes germline proliferation in C. elegans.. Development 2010; 137:671 - 80; http://dx.doi.org/10.1242/dev.042523; PMID: 20110332
- Francis R, Barton MK, Kimble J, Schedl T. gld-1, a tumor suppressor gene required for oocyte development in Caenorhabditis elegans.. Genetics 1995; 139:579 - 606; PMID: 7713419
- Pinkston JM, Garigan D, Hansen M, Kenyon C. Mutations that increase the life span of C. elegans inhibit tumor growth. Science 2006; 313:971 - 5; http://dx.doi.org/10.1126/science.1121908; PMID: 16917064
- Pinkston-Gosse J, Kenyon C. DAF-16/FOXO targets genes that regulate tumor growth in Caenorhabditis elegans.. Nat Genet 2007; 39:1403 - 9; http://dx.doi.org/10.1038/ng.2007.1; PMID: 17934462
- Hardie DG. AMP-activated protein kinase: an energy sensor that regulates all aspects of cell function. Genes Dev 2011; 25:1895 - 908; http://dx.doi.org/10.1101/gad.17420111; PMID: 21937710
- Hawley SA, Davison M, Woods A, Davies SP, Beri RK, Carling D, et al. Characterization of the AMP-activated protein kinase kinase from rat liver and identification of threonine 172 as the major site at which it phosphorylates AMP-activated protein kinase. J Biol Chem 1996; 271:27879 - 87; http://dx.doi.org/10.1074/jbc.271.44.27879; PMID: 8910387
- Stein SC, Woods A, Jones NA, Davison MD, Carling D. The regulation of AMP-activated protein kinase by phosphorylation. Biochem J 2000; 345:437 - 43; http://dx.doi.org/10.1042/0264-6021:3450437; PMID: 10642499
- Apfeld J, O’Connor G, McDonagh T, DiStefano PS, Curtis R. The AMP-activated protein kinase AAK-2 links energy levels and insulin-like signals to lifespan in C. elegans.. Genes Dev 2004; 18:3004 - 9; http://dx.doi.org/10.1101/gad.1255404; PMID: 15574588
- Curtis R, O’Connor G, DiStefano PS. Aging networks in Caenorhabditis elegans: AMP-activated protein kinase (aak-2) links multiple aging and metabolism pathways. Aging Cell 2006; 5:119 - 26; http://dx.doi.org/10.1111/j.1474-9726.2006.00205.x; PMID: 16626391
- Beale EG. 5′-AMP-activated protein kinase signaling in Caenorhabditis elegans.. Exp Biol Med (Maywood) 2008; 233:12 - 20; http://dx.doi.org/10.3181/0705-MR-117; PMID: 18156301
- Imamura K, Ogura T, Kishimoto A, Kaminishi M, Esumi H. Cell cycle regulation via p53 phosphorylation by a 5′-AMP activated protein kinase activator, 5-aminoimidazole- 4-carboxamide-1-beta-D-ribofuranoside, in a human hepatocellular carcinoma cell line. Biochem Biophys Res Commun 2001; 287:562 - 7; http://dx.doi.org/10.1006/bbrc.2001.5627; PMID: 11554766
- Jones RG, Plas DR, Kubek S, Buzzai M, Mu J, Xu Y, et al. AMP-activated protein kinase induces a p53-dependent metabolic checkpoint. Mol Cell 2005; 18:283 - 93; http://dx.doi.org/10.1016/j.molcel.2005.03.027; PMID: 15866171
- Tiainen M, Ylikorkala A, Mäkelä TP. Growth suppression by Lkb1 is mediated by a G(1) cell cycle arrest. Proc Natl Acad Sci U S A 1999; 96:9248 - 51; http://dx.doi.org/10.1073/pnas.96.16.9248; PMID: 10430928
- Tiainen M, Vaahtomeri K, Ylikorkala A, Mäkelä TP. Growth arrest by the LKB1 tumor suppressor: induction of p21(WAF1/CIP1). Hum Mol Genet 2002; 11:1497 - 504; http://dx.doi.org/10.1093/hmg/11.13.1497; PMID: 12045203
- Waters K, Yang AZ, Reinke V. Genome-wide analysis of germ cell proliferation in C.elegans identifies VRK-1 as a key regulator of CEP-1/p53. Dev Biol 2010; 344:1011 - 25; http://dx.doi.org/10.1016/j.ydbio.2010.06.022; PMID: 20599896
- Albert PS, Riddle DL. Mutants of Caenorhabditis elegans that form dauer-like larvae. Dev Biol 1988; 126:270 - 93; http://dx.doi.org/10.1016/0012-1606(88)90138-8; PMID: 3350212
- Narbonne P, Hyenne V, Li S, Labbé JC, Roy R. Differential requirements for STRAD in LKB1-dependent functions in C. elegans.. Development 2010; 137:661 - 70; http://dx.doi.org/10.1242/dev.042044; PMID: 20110331
- Greer EL, Dowlatshahi D, Banko MR, Villen J, Hoang K, Blanchard D, et al. An AMPK-FOXO pathway mediates longevity induced by a novel method of dietary restriction in C. elegans.. Curr Biol 2007; 17:1646 - 56; http://dx.doi.org/10.1016/j.cub.2007.08.047; PMID: 17900900
- Greer EL, Oskoui PR, Banko MR, Maniar JM, Gygi MP, Gygi SP, et al. The energy sensor AMP-activated protein kinase directly regulates the mammalian FOXO3 transcription factor. J Biol Chem 2007; 282:30107 - 19; http://dx.doi.org/10.1074/jbc.M705325200; PMID: 17711846
- Gan B, Hu J, Jiang S, Liu Y, Sahin E, Zhuang L, et al. Lkb1 regulates quiescence and metabolic homeostasis of haematopoietic stem cells. Nature 2010; 468:701 - 4; http://dx.doi.org/10.1038/nature09595; PMID: 21124456
- Nakada D, Saunders TL, Morrison SJ. Lkb1 regulates cell cycle and energy metabolism in haematopoietic stem cells. Nature 2010; 468:653 - 8; http://dx.doi.org/10.1038/nature09571; PMID: 21124450
- Gurumurthy S, Xie SZ, Alagesan B, Kim J, Yusuf RZ, Saez B, et al. The Lkb1 metabolic sensor maintains haematopoietic stem cell survival. Nature 2010; 468:659 - 63; http://dx.doi.org/10.1038/nature09572; PMID: 21124451