Abstract
DJ-1 (or PARK-7) is a multifunctional protein implicated in numerous pathologies including cancer, sterility and Parkinson disease (PD). The popular genetic model Drosophila melanogaster has two orthologs, dj-1: α and β. Dysfunction of dj-1β strongly impairs fly mobility in an age-dependent manner. In this study, we analyze in detail the molecular mechanism underlying the dj-1β mutant phenotype. Mitochondrial hydrogen peroxide production, but not superoxide production, was increased in mutant flies. An increase in peroxide leak from mitochondria causes oxidative damage elsewhere and explains the strong reduction in mobility caused by dj-1β mutation. However, at the same time, increased levels of hydrogen peroxide activated a pro-survival program characterized by (1) an alteration in insulin-like signaling, (2) an increase in mitochondrial biogenesis and (3) an increase in the de-acetylase activity of sirtuins. The activation of this pro-survival program was associated with increased longevity under conditions of moderate oxidative stress. Additionally, the dj-1β mutation unexpectedly accelerated development, a phenotype not previously associated with this mutation. Our results reveal an important role of dj-1β in oxidative stress handling, insulin-like signaling and development in Drosophila melanogaster.
Introduction
DJ-1 (PARK7) is a member of the DJ-1/Pfpl superfamily with homologs in nearly all organisms. DJ-1 has been associated with a variety of human pathologies, including sterility, cancer and Parkinson disease.Citation1 It is abundantly expressed throughout the body with no clear tissue- or organ-specific roles. DJ-1 is found in both the cytosol, nucleus and is also associated with mitochondria.Citation2 Its cellular location is influenced by the cellular redox state and oxidative damage.Citation3 This suggests an important role for DJ-1 in preserving cellular homeostasis.
The association of DJ-1 and mitochondrial dysfunction was inferred from studies in DJ-1-deficient mice and primary neuronal cultures derived from these mice that consistently revealed a striking sensitivity to stress-inducing stimuli, including hydrogen peroxide, rotenone and 1-methyl-4-phenyl 1,2,3,6-tetrahydropyridine (MPTP). All of these oxidative stimuli have the capacity to impair normal mitochondrial function through the inhibition of respiratory complex I.Citation4 DJ-1 has recently been described as a direct regulator of complex I.Citation5 Indeed, knockdown of DJ-1 decreases complex I activity, which would explain why its mutation results in a phenotype similar to rotenone or MPTP administration. The current view is that DJ-1 is a oxidative stress-response protein that defends the cell against reactive oxygen species (ROS) and mitochondrial damage. Despite this, an understanding of DJ-1 biochemical function is still missing.Citation1
Drosophila has two different dj-1 genes: dj-1α, expressed predominantly in testis, and the ubiquitiously expressed dj-1β. Mutations in both of these orthologs,Citation6 or in dj-1β alone,Citation7 cause a Parkinsonian-like syndrome in flies, characterized by a strong decrease in mobility and an increased sensitivity to oxidative stress. We, and others, have shown that mutations in dj-1β (or the analogous gene in mammals) result in increased H2O2 generation in isolated mitochondria.Citation8,Citation9 However, it remains unknown if the increased H2O2 has an impact on oxidative damage in vivo, or if it just affects cellular signaling. Moreover, it is not known if higher levels of H2O2 are caused by an increase in the generation of superoxide or by a decrease in the efficiency of H2O2 detoxification systems.
The alternative oxidase (AOX) is able to bypass blockades in both complex III and IV in fungi and plants.Citation10 When AOX is ectopically expressed in Drosophila melanogaster, it decreases mitochondrial H2O2 and rescues the phenotype caused by mutations in dj-1β.Citation9 However, mutation of dj-1β does not shorten lifespan,Citation11 arguing against a direct role of DJ-1 in the regulation of oxidative stress in vivo. This also raises doubts as to the pathological mechanism implicated in Parkinson disease, associated with DJ-1 mutation.
In this study, we have measured levels of H2O2 and superoxide production in isolated mitochondria. We demonstrate that only H2O2 levels are increased in mutant flies. Using mass spectrometry, we show that protein damage is increased in vivo as a consequence of the increase in mitochondrial H2O2 leak. Moreover, different cellular pathways related to the regulation of longevity are also altered. Strikingly, we found that insulin-like signaling (IIS) is altered, mitochondrial biogenesis is increased, and the de-acetylase activity of sirtuins is increased. We present evidence that deficiency of dj-1β gene function initiates a pro-survival program. This program overcomes the negative longevity effects of oxidative stress, allowing mutant flies to live longer under conditions where oxidative damage is moderately elevated. Unexpectedly, we also found a previously undescribed effect of the dj-1β mutation: a reduction in developmental time. In summary, our data clearly demonstrate a major role for dj-1β in oxidative stress handling, insulin-like signaling and development in Drosophila melanogaster.
Results
dj-1β regulates mitochondrial H2O2 generation
Previously, we have demonstrated that mutation of dj-1β increases production of H2O2 in isolated mitochondria when using homovanillic acid as a fluorescent dye.Citation11 In the present study, we repeated our measurements () using a more sensitive probe (Amplex Red), obtaining the same results. H2O2 production was higher in mitochondria from dj-1β mutant flies when using pyruvate + proline as a substrate (H2O2 produced by complex I + III), but not when using sn-3-P-glycerate + rotenone (H2O2 produced by complex III). Using a new technique,Citation12 we measured mitochondrial superoxide generation using flow cytometry with MitoSOX™. We found that dj-1β mutant mitochondria produced less superoxide than controls with either complex I- and complex III-linked substrates (). The different effects of the dj-1β mutation on superoxide and H2O2 production implicates dj-1β in H2O2, but not superoxide detoxification. This is supported by our previous data showing a reduction in the expression levels of genes involved in H2O2 detoxification, but no change in superoxide-handling enzymes.Citation11
Figure 1. Effects of dj-1β mutation on mitochondrial physiology. (A) Levels of H2O2 production (nmol/min mg prot) shown as mean ± SEM. Between four to six replicate experiments per group. (B) Levels of superoxide production (AUF, arbitrary units of fluorescence) shown as mean ± SEM. Between four to six replicate experiments per group. (C) Mitochondrial oxygen consumption state 3 respiration (nmol O2/s mg prot) shown as mean ± SEM. Between three to six replicate experiments per group. (D) ATP content (µmol/mg protein) in fly homogenates shown as mean ± SEM, 10 replicate experiments per group. (E) Activity of individual flies recorded as average activity per hour (mean ± SEM) over a 48 h period is shown. Between 34–38 individual flies per group. (F) BNE gels of mitochondrial protein extracts from control and mutant flies. In gel, activity of complex I and IV is shown separately. p < 0.05 indicated by *. m, male; f, female.
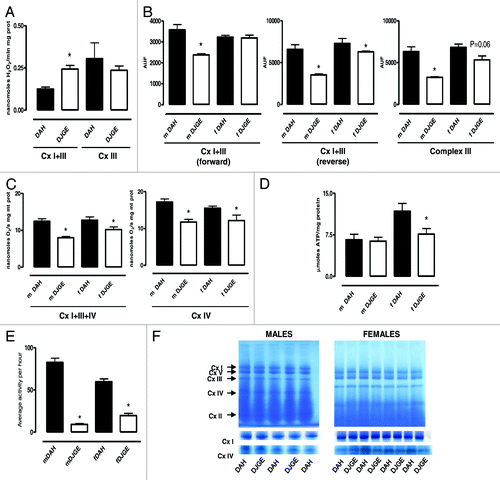
A decrease in the levels of superoxide (with high levels of H2O2) can be caused by partial inhibition of the electron transport chain (ETC). In order to test this possibility, we measured mitochondrial respiration using high-resolution respirometry. We detected a 30% decrease in mitochondrial respiration in the mutant flies either with complex I- or with complex IV-linked substrates (). This inhibition of respiration was accompanied by decreased ATP levels, although only in females (). The unchanged ATP levels in males might be explained by the fact that their motor activity is more greatly decreased by the mutation (): moving less implies a decreased energy expenditure. It has been previously shown that DJ-1 binds directly to complex I in human cells, and that its knockdown decreases complex I activity.Citation5 We therefore investigated if the same phenomenon occurs in Drosophila. We separated the respiratory complexes using Blue Native Electrophoresis, and then we measured the activity of complex I and IV. We did not observe major differences either in the organization or in the activity of the respiratory complexes (). This clearly suggests that the inhibition of respiration is caused by an indirect mechanism, e.g., resulting from oxidative stress, rather than by a decrease level of complex I.
dj-1β regulates oxidative stress in vivo
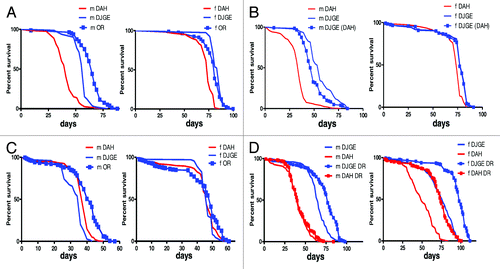
Because of the different effects of dj-1β on different types of ROS, we studied levels of oxidative damage in vivo. We measured five different markers of protein damage, representing three different types of modification: (1) glutamic semialdehyde (GSA) and (2) aminoadipic semialdehyde (AASA) as protein carbonyl markers; (3) carboxyethyl-lysine (CEL) and (4) carboxymethyl-lysine (CML) as glycoxidative markers and (5) malondialdehyde-lysine as a marker of lipoxidative damage (MDA-lys). These markers show a good correlation, both with mitochondrial generation of H2O2 and with the sensitivity to lipoxidation of biological membranes.Citation13 In addition, the levels of these markers are markedly increased in several neurodegenerative diseases.Citation14 The five markers were more abundant (independent of their chemical provenance) in mutant flies (). In order to confirm that mitochondria are both the source and target of damage, we repeated our measurements using isolated mitochondria. Again, we observed higher levels of oxidative damage in mitochondria from mutant flies (). It is noteworthy that levels of oxidative damage in dj-1β mutant flies were higher than in any other wild-type strain studied in our laboratory (Pamplona and Sanz unpublished results). The fact that all markers of damage were increased indicates that the defect in H2O2 detoxification disturbs normal metabolism, increasing oxidative damage in vivo. This has an important physiological correlate in the serious deterioration in fly mobility (). A loss of climbing capacity is a characteristic feature of older flies. Flies deficient in dj-1β thus manifest at least one feature of aging, even though neither mean nor maximum lifespan was decreased. In fact, dj-1β mutant flies are long-lived compared with controls (). Differences in genetic background are not responsible for this, since the effects were still present after 11 generations of backcrossing ().
Figure 2.dj-1β mutation increases oxidative damage in and outside mitochondria. (A) Levels of protein damage in fly homogenates mean ± SEM is shown. Between three to seven replicate experiments per group. (B) Levels of protein damage in fly mitochondria mean ± SEM is shown. Between three to six replicate experiments per group. GSA, glutamic semialdehyde; AASA, aminoadipic semialdehyde; CEL, carboxyethyl-lysine; CML, carboxymethyl-lysine; MDA-lys, malondialdehyde-lysine. Data are average ± SEM of at least three independent samples per group. p < 0.05 indicated by *. m, male; f, female.
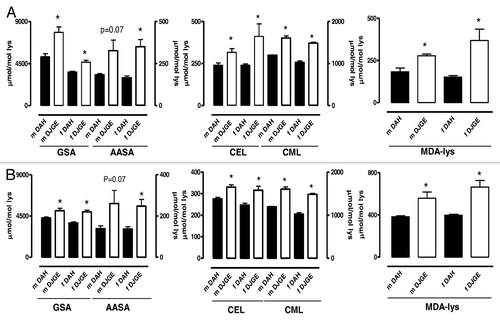
Figure 3. Effect of dj-1β mutation on lifespan. (A) Survival of wild-type strains (DAH and OR) and dj-1β mutant flies (DJGE) at 25°C. n = 80 flies per experiment. Median (maximum) lifespan in days, males DAH 41 (51), males OR 65 (72), males DJGE 58 (60); females DAH 74 (79), females OR 81 (86), females DJGE 86 (86); DAH flies live significantly shorter than DJGE or OR (Log-Rank Test p < 0.001). (B) Survival of wild-type (DAH), dj-1β mutant flies (DJGE) and dj-1β mutant flies backcrossed for 11 generations into DAH (DJGE (DAH)) at 25°C. n = 80 flies. Median (maximum) lifespan in days, males DAH 35 (39), males DJGE 56 (74), males DJGE (DAH) 48 (63); females DAH 74 (79), females DJGE 79 (81), females DJGE(DAH) 77 (81); DAH flies live significantly shorter than DJGE or DJGE (DAH) (Log-Rank Test p < 0.001). (C) Survival of wild-type strains (DAH and OR) and mutant flies (DJGE) at 29°C. n = 200 flies per experiment. Median (maximum) lifespan in days, males DAH 38 (40), males OR 42 (47), males DJGE 36 (38); females DAH 46 (49), females OR 49 (56), females DJGE 46 (49); DJGE male flies live significantly shorter than DJGE or OR (Long Rank Test p < 0.001). No differences in lifespan between DJGE and DAH females. (D) Survival of wild-type (DAH) and dj-1β mutant flies (DJGE) at 25°C in high (HC) and low caloric- (LC) content diets. Male DAH 40 (54), male DJGE 65 (77), male DAH DR 40 (61), male DJGE DR 77 (89); female DAH 49 (68), female DJGE 77 (93), female DAH DR 74 (89), female DJGE DR 98 (108). DJGE flies live significantly longer than DAH in both diets (Log-Rank Test p < 0.001). m, male; f, female.
dj-1β regulates lifespan by activating of pro-survival pathways
If oxidative stress is so highly increased in dj-1β mutant flies as to cause a serious deterioration in motor function at early ages, why is lifespan not decreased? One possibility is that mitochondrial oxidative stress is not a key factor in lifespan determination, as previously proposed.Citation15 Another alternative is that an increase in H2O2 triggers an adaptive response that extends lifespan. That has been described both in worms and in flies related to glucose restrictionCitation16 or superoxide dismutase overxpression.Citation17 In relation to that, oxidative stress activates Jun-N-terminal kinase (JNK), which represses the expression of the insulin-like peptides (Ilp) Ilp2 and Ilp5.Citation18 Consistent with this, decreased expression of Ilp2, -3 and -5 is a characteristic of long-lived flies under dietary restriction (DR).Citation19 We measured the expression levels of Ilp2 and Ilp5 and observed a decrease in the dj-1β mutant background expression levels (). Conversely, a large increase was seen in Ilp3, which was previously observed to be unresponsive to externally applied oxidative stress.Citation18
Figure 4. The dj-1β mutation activates pro-survival pathways in Drosophila melanogaster. (A) Q-PCR of Ilp’s 2, 3 and 5 from dj-1β mutant and control flies, normalized to Actin 88F. Data are presented as mean ± SEM (six replicate experiments). (B) Mitochondrial copy number assayed by Q-PCR of 16S rRNA gene from dj-1β mutant flies and control flies, normalized to levels of Rpl32. Data are presented as mean ± SEM (four to six replicate experiments). (C) Western blots analysis of complex I (NDUFS3 subunit) and loading control PDH (E1α subunit). Data are presented as mean ± SEM (four replicate experiments). (D) Sirtuin activity in homogenates of dj-1β mutant flies and control flies (five replicate experiments). p < 0.05 indicated by *. m, male; f, female. M, dj-1β mutant; C, control.
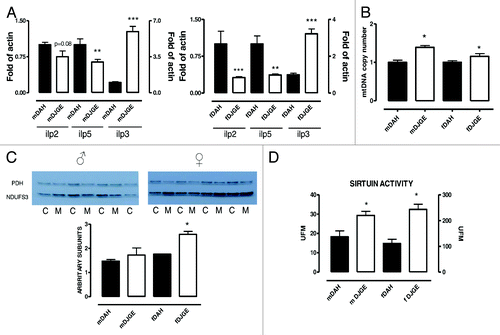
DR or interruption in IIS signaling are characterized by simultaneous activation of several pro-survival pathways in parallel.Citation20 As we have shown, IIS signaling is altered in dj-1β mutant flies. For this reason, we decided to investigate other pathways associated with longevity. Mitochondrial biogenesis is increased during DR,Citation21 and overexpression of the Drosophila PGC-1 homolog Spargel extends lifespan.Citation22We measured mitochondrial DNA (mtDNA) levels and found them to be increased in dj-1β mutants (). Additionally, we investigated the expression of complex I, measuring the concentration of one of its subunits (NDUFS3) by western blotting. Complex I expression was higher in both males and female flies in dj-1β mutant flies (), although the differences were statistically significant only in females. Mitochondrial biogenesis is stimulated by a decrease in ATP production. This finding is therefore consistent with the inhibition of respiration in the mutant flies. Strikingly, proportionate decrease in respiration was similar to the increase in mtDNA copy number (approximately 20%). Moreover, when mitochondrial respiration was analyzed in homogenates, no differences were observed between control and mutant flies (data not shown). Under these experimental conditions, oxygen consumption is measured as the combination of the respiratory efficiency and the mitochondrial mass (mitochondrial number). The increase in the amount of mitochondria in the mutants partially compensates for the loss of respiratory3 activity.
An increase in activity of the sirtuin SIR2 is considered to be enough for lifespan extension both in wormsCitation23 and fliesCitation24 and essential for longevity extension mediated by DR,Citation25 although these findings have recently been questioned.Citation24 We found that sirtuin activity was increased in dj-1β mutant flies (), consistent with other reports of increased activity after oxidative damage.Citation26 Interestingly, sirtuin activity in dj-1β mutant flies was higher than in any wild-type strain in our laboratory (data not shown).
The upregulation of pro-survival pathways may explain the surprising longevity of dj-1β mutant flies. The effects of increased oxidation on lifespan would thus be compensated by downstream activation of global regulators of longevity such as sirtuins. If this is true, a further increase in oxidative stress beyond a critical threshold should be able to overcome these pathways and reduce longevity in the dj-1β mutant flies. To test this hypothesis, we cultured controls and mutant flies at 29°C. Under such conditions, dj-1β mutant flies were observed to have shorter lifespans than controls (), demonstrating that the activation of pro-survival pathways can be overcome by increased levels of ROS.
Alterations to mitochondrial biogenesis and sirtuin activity in dj-1β mutant flies are as predicted, if regulated by IIS in a similar manner to DR. We therefore investigated whether dj-1β mutant flies are still responsive to DR. We cultured mutant and control flies in diets with high or low caloric content. In both diets, mutant flies lived longer than control flies, demonstrating that the mutants remain responsive to DR (). This indicates that functional deficiency of dj-1β does not induce a condition equivalent to DR, and also supports the notion that oxidative stress is not essential for lifespan extension during DR.
dj-1β regulates the rate of development
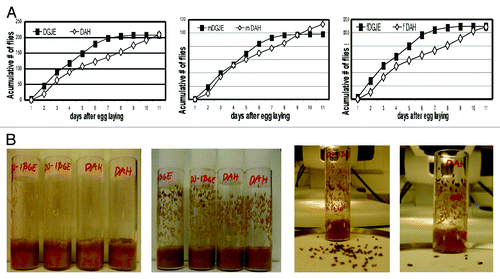
We observed that the phenotype associated with the dj-1β mutation appears at a younger age in each new generation, indicating that this mutation could be conferring some type of adaptive advantage. We also observed that mutant flies developed and eclosed faster than controls. We quantified this phenomenon by counting the number of flies that eclosed after 5 d of mating. No difference in the total number of male and female flies was observed (); however, there were slightly fewer males. More than 50% of mutant flies had emerged by day 4, while wild-type flies did not reach the same levels until day 5. The phenotype is quite interesting, since dj-1β mutant flies develop faster than any other wild-type strain available in our laboratory (data not shown). To the best of our knowledge, this is the first time that a mutation has been described that accelerates so significantly the rate of development in Drosophila. Alterations in development can, in principle, be caused by a dysregulation of IIS. In dj-1β mutant flies, we found a strong increase, approximately 6- and 3-fold in males and females, respectively, in the expression of Ilp3, whereas the expression of other Ilps was lower (). Developmental differences appear when larvae stop feeding and crawl to become pupae (). Interestingly, the transition between larvae to pupae is characterized by an increase in Ilp3 expression.Citation27 Our observations () suggest a tight correlation between these molecular and physiological phenomena and support a causal relationship.
Discussion
Our results reveal a specific role for dj-1β in the regulation of mitochondrially generated ROS. dj-1β influences the level of hydrogen peroxide, but not that of superoxide levels. This difference indicates that dj-1β is not directly involved in the generation of ROS at the level of the ETC, but rather at the level of ROS detoxification. Consistent with this, we previously reported that antioxidant genes responsible for peroxide detoxification are downregulated in mutant flies, but genes related to superoxide detoxification are expressed at similar levels as in wild-types flies.Citation11 This supports a role for dj-1β as a regulator of genes involved in peroxide, but not superoxide detoxification, and would explain our ROS findings in isolated mitochondria. Moreover, dj-1β mutant flies are extremely sensitive to drugs that target glutathione metabolism,Citation28 which is a central component of the defense against peroxides. All these observations support a central role for dj-1β in the detoxification of mitochondrial peroxides. At the moment, we cannot say if the protein acts as a peroxiredoxin or as a regulator of antioxidant genes. In the literature, both possibilities have been suggested.Citation8,Citation11,Citation28 However, whatever the mechanism of action, it is important to highlight that dj-1β is not involved in superoxide detoxification. In fact, a lack of superoxide detoxification creates a different phenotype with regard to mitochondrial physiology. Sod2 knockdown specifically affects proteins with iron-sulfur clusters, e.g., complexes I and II and aconitase located in the mitochondrial matrix.Citation29 Such a phenotype was not observed dj-1β mutants flies.
One important difference between hydrogen peroxide and superoxide is reactivity. H2O2 can diffuse far away from its origin, whereas superoxide cannot go through lipid membranes and has a much more limited area of action. This suggests that a decreased detoxification of mitochondrial H2O2 will increase oxidative stress both inside and outside of mitochondria, and this is exactly what we observed when we measured protein damage. The damage elicited is nonspecific, as shown by high levels of all markers of damage, independent of their chemical origin. The fact that oxidative damage was higher in dj-1β mutants than in any other wild-type strain in our laboratory [including Canton-S, Oregon-R and Dahomey (DAH)] suggests a central role of this protein in the regulation of oxidative stress. The increase in hydrogen peroxide is associated with decreased ETC activity, reducing its capacity for ATP production. The ultimate effect is to limit the available energy, consistent with our finding of decreased locomotor activity in young flies. Decreased activity and high levels of mitochondrial ROS are typical traits of older flies, which are also expressed in young dj-1β mutant flies.Citation11 Alterations in different types of ROS (superoxide vs. H2O2) have distinct effects on physiology and lifespan in different organisms. In worms, an increase in superoxide levels does not affect lifespanCitation30 or even extends it,Citation12 whereas, in flies, an increase in superoxides is associated with a shorter lifespanCitation31 and muscle degeneration.Citation32 However, in flies, H2O2 can exert a positive effect on longevity through direct administration,Citation33 mutation of dj-1β (this study) or overexpression of sod1Citation34 or sod2.Citation35 Extension of lifespan as a consequence of increase in mitochondrial oxidative stress has been called mitohormesis,Citation36 and the extension is normally explained by a long-term increase in antioxidant levels.Citation36 However, increased administration or overexpression of the same upregulated antioxidants do not clearly extend lifespan either in worms or mammals (reviewed in ref. Citation37). Our results indicate that the activation of pro-survival mechanism(s) related to IIS signaling and growth is a more feasible explanation for mitohormesis.Citation38 Surprisingly, decreasing H2O2 in the mitochondrial matrix through overexpression of catalase shortens Drosophila lifespan.Citation39 Data related to the effect of dj-1β on longevity are contradictory. In flies, mutation of dj-1β has been reported both to decrease and to have no effect on longevity;Citation6,Citation40 whereas, no change in lifespan was seen in mice, in spite of higher levels of mitochondrial H2O2.Citation8 Differences in longevity are sometimes confounded by genetic background effects that have not been properly controlled. In our case, differences between controls and mutants cannot be attributed to background effects, since mutant flies are long-lived before and after backcrossing into the Dahomey reference strain over 11 generations. In our laboratory, the longest-lived wild-type strain is Oregon-R (OR). OR is also the strain with the lowest mitochondrial ROS production both in vitroCitation11 and in vivo (Pamplona and Sanz, unpublished results). Differences in longevity between the OR and DAH strains are independent of temperature. However, the lifespan increase associated with the dj-1β mutation was not observed when temperature was increased. On the other hand, mutant flies liver longer when grown on diets of both high and low caloric content. The effect of temperature, but not DR, is related to oxidative stress in Drosophila lifespan extension.Citation15 The fact that the survival advantage of dj-1β mutants was lost at high temperature, but not when caloric content was altered, is consistent with the inference that dj-1β is a regulator of oxidative stress in vivo. Moreover, when dj-1β mutant flies were cultured at high temperatures, they became immobilized at the bottom of the vial, whereas wild-type flies move faster. This supports the idea that age-related loss of mobility is caused by a ROS-mediated mechanism.
High levels of H2O2 from mitochondria can activate an adaptive response (mitohormesis) extending lifespan through the preservation of vital tissues.Citation41 Consistent with this, dj-1β mutant flies exhibited decreased expression of Ilp2 and Ilp5 (IIS signaling), increased levels of mtDNA and complex I (mitochondrial biogenesis) and more activity of sirtuins in fly homogenate. Independently, it has been shown that JNK is activated by mitochondrial H2O2.Citation42 Once active, JNK specifically reduces expression levels of Ilp2,Citation18 as also happens in dj-1β mutant flies. Strikingly, the knockout of Ilp2 extends Drosophila lifespan.Citation43 Downstream in the IIS pathway, it was found that knockdown models for dj-1 (α or β) exhibit a decrease in AKT phosphorylation,Citation44 which is an essential step in the activation of a pro-survival program mediated by FOXO translocation to the nucleus.Citation45 Our results, and those of others, indicate that a reduction in AKT phosphorylation is a common, non-cell-autonomous response when oxidative damage is increased by internal or external causes.Citation18,Citation46 The activation of such pro-survival pathways related with IIS signaling and growth can explain the positive effects of mitohormesis on lifespan independently of an upregulation in expression of antioxidants.Citation41 We must remember that antioxidants by themselves do not extend lifespan, whereas the activation of these other pathways do without a consistent changing in antioxidant defenses.
It is reasonable that an increase in oxidative stress can be interpreted by the cell as a signal to activate pro-survival pathways. For example, an increase in mitochondrial biogenesis is essential for lifespan extension during DR, both in DrosophilaCitation21 and in mouse.Citation47 Either ROS or a partial inhibition of mitochondrial respiration can promote mitochondrial biogenesis by activating PGC-1αCitation48 or the AMPK signaling pathway,Citation49 respectively. It is important to note that higher mitochondria density can confer benefits without changing the total level of oxidative damage,Citation15 and the overexpression of spargel (homolog of PGC-1) extends Drosophila lifespan. On the other hand, decreased respiration activates a survival-promoting strategy that is present in yeast,Citation10 worms,Citation50 fliesCitation51 and even mice.Citation52 An interruption of normal electron flow can increase or decrease longevity depending upon downstream consequences. For example, in worms, mutations in complex I, III or IV increase lifespan,Citation12 whereas disruption of complex II shortens lifespan.Citation53 Something similar occurs in Drosophila; when specific subunits of complex I, III, IV and V, but not II, are knocked down, lifespan can be extended.Citation54 From this point of view, a mild inhibition of respiration caused by more oxidative stress (dj-1β mutants) would be equivalent to a mild reduction in the levels of respiratory complexes I, III or IV (RNAi lines).Citation54
High levels of oxidative stress damage DNA, promoting the mobilization of sirtuins,Citation26 which can protect against genetic and epigenetic modifications of the genome.Citation55 dj-1β mutant flies have the highest levels both of oxidative damage and sirtuin activity compared with all wild-type strains in our laboratory. This should protect the mutant flies against genetic and epigenetic modifications, contributing to their extended lifespan. In the past, Sir2 was considered a major regulator of longevity and an instrumental gene for the prolongation of lifespan mediated by DR.Citation25 However, these results have recently been called into question.Citation24 It is possible that Sir2 does not directly regulate longevity, but that the protective effects of Sir2 or other sirtuins can nevertheless help overcome oxidative damage caused by the dj-1β mutation. In fact, knockdown of Sir2 in Drosophila fat body reduces resistance to starvation,Citation56 stress that initially is associated with an increase in oxidative stress.Citation57
It is generally accepted that the activation of a single pathway is insufficient to extend lifespan. For example, DR and other genetic strategies that increase lifespan are characterized by simultaneous changes in the expression of several different genes.Citation58 In the future, it would be interesting to determine whether the relevant pathways are regulated by the same master mechanism or by different signals. Different tissues are more sensitive to varying kinds of damage. For example, muscle is particularly sensitive to ROS, and sarcopenia is very sensitive to oxidative stress.Citation59 Alternatively, damage to muscle can activate a signal that leads to the preservation of other tissues. The dj-1β mutant fly is a clear example of how high levels of oxidative damage can be compatible with a long lifespan, a rare phenotype in Drosophila.Citation37
During our experiments, we observed that the mutant phenotype appeared earlier in each successive generation. Additionally, mutant flies eclosed more quickly than controls. This suggests that the mutation confers some adaptative advantage under our laboratory conditions. Strikingly, more rapid development was accompanied by a several-fold higher expression level of Ilp3 in mutant flies. Fly development is strictly regulated by the expression of specific llps.Citation60 For example, the expression of Ilp3 is increased prior to the pupariation when the non-feeding state starts.Citation27 Coincidentally, this is the moment when differences in the developmental rate between mutant and wild-type flies became evident. Increased Ilp3 expression can result from a compensatory mechanism induced by the decreased expression of other Ilps.Citation18,Citation61 ROS have been proposed as regulators of development,Citation62 and H2O2 promotes cellular proliferation,Citation63 differentiationCitation64 or senescence.Citation65 The effects of H2O2 depend on cell type, cell cycle stage and also concentration. The fact that dj-1β mutant flies develop faster than controls suggests that ROS may indeed be an important regulator of development. In fact, there is an increase in H2O2 levels during the transition from feeding to non-feeding states in Drosophila larvae.Citation66 Moreover, the administration of acetyl cysteine in C. elegansCitation12 reduces lifespan in long-lived mutants with overproduction of ROS and also slows development. Antagonist pleiotropy suggests that aging results from the accumulation of mutations that are not subject to natural selection, because the accumulation is only detrimental at an advanced age. These mutations may have a beneficial effect at earlier stages. The dj-1β mutation may be a good example of this phenomenon. It promotes more rapid development, but as a trade-off, it impairs mobility at an earlier age. In an environment where resources are abundant (our laboratory conditions), this is advantageous and can be favorably selected. This would explain why the phenotype appears at an earlier age in each successive generation.
In summary, DJ-1β regulates oxidative stress, insulin signaling and development in Drosophila melanogaster. Since signaling pathways are highly conserved across different phyla, these data should prove useful to scientists studying the role of DJ-1 in human diseases such as Parkinson disease or cancer.
Materials and Methods
Fly stocks and lifespan studies
Wild-type strains used were our own stocks of Dahomey (DAH) and Oregon-R (OR). The loss-of-function dj-1β mutant fly or dj-1βGE23381 (DJGE in all figures) has been previously described.Citation7 Lifespan experiments were repeated in DJGE flies after 11 generations of backcrossing into DAH; both mitochondrial and nuclear DNA were in DAH background in DJGE (DAH) flies. Flies were maintained on standard media (1% agar, 1.5% sucrose, 3% glucose, 3.5% dried yeast, 1.5% maize, 1% wheat, 1% soya, 3% treacle, 0.5% propionic acid, 0.1% Nipagin). For experiments evaluating the effect of caloric content on lifespan, we used the diets described by reference Citation67 (high caloric-content diet: 2% agar, 15% dried yeast, 15% sucrose, 3% nipagin, 0.3% propionic acid; low caloric-content diet: 2% agar, 6.5% dried yeast, 6.5% sucrose, 3% nipagin, 0.3% propionic acid). Flies were collected using CO2 anesthesia within 24 h of eclosion and then kept at a density of 20 flies (males and females separately) per vial at 25°C or 29°C in a controlled 12 h light:dark cycle. Flies were transferred to new vials every 2–3 d. Lifespan studies were performed with at least 80 flies per genotype. The number of dead flies was recorded every 2–3 d, and from this, the mean and maximum lifespan (the last 10% of surviving flies) were calculated. For the remainder of the experiments, 10-day-old flies were used.
Developmental assay
Five virgin females were crossed for five days with three males. Pictures of the vials were taken every day. After eclosion, the number of flies eclosing per day was counted for 10 d.
Mitochondrial O2 consumption and measurement of mitochondrial H2O2 and superoxide production
Mitochondria were isolated as describedCitation68 and used to measure ROS production and oxygen consumption. Mitochondrial oxygen consumption was measured by high-resolution respirometry using an oxygraph 2-K (Oroboros). Mitochondria were incubated in assay buffer (120 mM KCl, 5 mM KH2PO4, 3 mM Hepes, 1 mM EGTA, 1 mM MgCl2, 0.2% bovine serum albumin, pH 7.2 at 25°C) and a combination of complex I- (5 mM pyruvate + 5 mM proline) or complex IV- (50 μM TMPD + 1 mM ascorbate + 10 μM Antimycin + 10 μM rotenone to inhibit complex III and complex I, respectively) linked substrates. State 3 respiration was initiated by adding 500 µM of ADP to the sample. In parallel, we assayed H2O2 production as previously described.Citation69 In order to measure superoxide, we adapted the method described by reference Citation12 in C. elegans to Drosophila. Briefly, isolated mitochondria were incubated with assay buffer containing complex I- (5 mM pyruvate + 5 mM proline) or complex III-linked [20 mM sn-3-glycerol-phosphate with and without rotenone (10 µM)] substrates and 5 μM MitoSox™ at 25°C for 10 min. Mitochondria were then sorted using an Accuri C6 Flow cytometer (BD Accuri Cytometers), and fluorescence was measured using the appropriate channel and software provided by the manufacturer.
Western blot analysis
Protein extraction, SDS-PAGE and western blotting were as described in reference Citation69. Antibodies used together with the appropriate secondary antibodies, were as follows: against the complex I NDUFS3 subunit (MitoSciences) used at 1:20,000; against the pyruvate dehydrogenase (PDH) E1α subunit (MitoSciences) used at 1:10,000; the secondary antibody was HRP-conjugated horse anti-mouse IgG (H+L) (Vector Laboratories), used at 1:10,000.
Blue native-PAGE (BNE) and in-gel histochemistry
BNE was performed using NativePAGETM Novex® 3–12% Bis-Tris Gels according to reference Citation69. For in-gel assay of complex I, gels were incubated in complex I activity buffer (2 mM Tris, 0.1 mg/ml NADH, 2.5 mg/ml Nitroblue tetrazolium chloride, pH 7.4) for 20 min, after which they were fixed in destain solution for 20 min. For in-gel assay of complex IV, gels were incubated in complex IV activity buffer [5 mg 3.3′-diamidobenzidine tetrahydrochloride (DAB) dissolved in 9 ml phosphate buffer (0.05 M, pH 7.4), 1 nM catalase (20 μg/ml), 10 mg cytochrome c and 750 mg sucrose] for 2 h, after which they were fixed in destain solution for 20 min. All activity staining was performed at room temperature.
ATP measurement
Total ATP content was measured using the luciferin-luciferase-based ATP determination kit (Molecular Probes). One to five flies were homogenized in 100 µl 6M guanidinium chloride. Homogenates were centrifuged at 16,000 gmax for 5 min. Supernatants were dissolved 1:750 in TE buffer. Ten microliters of diluted sample were mixed with reaction solution containing luciferin-luciferase, and triplicates were measured by luminometry. A standard curve of different ATP dilutions was run in parallel, and results were normalized by protein content (mg) after application of the standard curve.
Fly activity
The locomotor activity of individual flies was measured in a Digitherm CircKinetics monitoring incubator (Tritech Research) at 25°C in a controlled 12:12 h dark:light cycle. Individual flies were put in capillaries with standard fly food, and their activity was monitored over 72 h using TriKinetics Activity Monitors (Trikinetics Inc.). Flies were acclimated for the first 24 h; hand activity was monitored during the next 48 h; the number of times that flies crossed the center of the vial per hour was counted and integrated using TriKinetics software.
RNA/DNA quantification
Isolation of mRNA, cDNA and q-RT-PCR synthesis was as described in full detail in reference Citation69. For mtDNA copy number, total DNA was isolated according to reference Citation69 and analyzed by q-PCR using the same conditions as used for mRNA analysis. Data were extracted and analyzed using Applied Biosystems StepOne software version 2.1. Primer sequences were 16S (forward: ttcgtccaaccattcattcc, reverse: tctaacctgcccactgaa), Act88F (forward: agggtgtgatggtgggtatg, reverse: cttctccatgtcgtcccagt) Ilp2 (forward: agcaagcctttgtccttcatctc, reverse: acaccatactcagcacctcgttg), Ilp3 (forward: tgtgtgtatggcttcaacgcaatg, reverse: cactcaacagtctttccagcaggg), Ilp 5 (forward: gaggcaccttgggcctattc, reverse:catgtggtgagattcggagc), Rpl32 (forward: gttcgatccgtaaccgatgtt, reverse: caccagtcggatcgatatgc).
Assay of sirtuin activity
Sirtuin activity was assayed in fly homogenates, based on the deacetylation of the substrate Fluor de Lys-SIRT1 (Enzo Life Sciences) according to reference Citation69.
Analysis of protein oxidative damage markers by mass spectrometry
The levels of markers, aminoadipic semialdehyde (AASA, oxidation), glutamic semialdehyde (GSA, oxidation), carboxymethyl-lysine (CML, glycoxidation), carboxyethyl-lysine (CEL, glycoxidation) and malondialdehydelysine (MDAL, lipoxidation) were determined by gas chromatography/mass spectrometry (GC/MS) according to reference Citation69.
Statistical analysis
Data were analyzed using GraphPad Prism 4 and the unpaired t-test was used for statistical testing of mutant and wild-type flies of the same sex. Lifespan data were analyzed using the Kaplan Meier Log-Rank Test. The level of statistical significance was established as p < 0.05.
| Abbreviations: | ||
| MPTP | = | 1-methyl-4-phenyl 1,2,3,6-tetrahydropyridine |
| AOX | = | alternative oxidase |
| AASA | = | aminoadipic semialdehyde |
| CEL | = | carboxyethyl-lysine |
| BNE | = | blue native-polyacrylamide gel electrophoresis |
| CML | = | carboxymethyl-lysine |
| DAH | = | Dahomey |
| DR | = | dietary restriction |
| DJGE | = | dj-1βGE23381 |
| ETC | = | electron transport chain |
| GSA | = | glutamic semialdehyde |
| JNK | = | Jun-N-terminal kinase |
| Ilp | = | insulin-like peptides |
| IIS | = | insulin-like signalling |
| MDA-lys | = | malondialdehyde-lysine |
| mtDNA | = | mitochondrial DNA |
| PDH | = | pyruvate dehydrogenase |
| OR | = | Oregon-R |
| PD | = | Parkinson disease |
| ROS | = | reactive oxygen species |
Acknowledgments
This work was supported by the Academy of Finland, the Tampere University Hospital Medical Research Fund, the European Research Council, the Spanish Ministry of Science and Innovation and the Autonomous Government of Catalonia.
References
- Wilson MA. The role of cysteine oxidation in DJ-1 function and dysfunction. Antioxid Redox Signal 2011; 15:111 - 22; http://dx.doi.org/10.1089/ars.2010.3481; PMID: 20812780
- Zhang L, Shimoji M, Thomas B, Moore DJ, Yu SW, Marupudi NI, et al. Mitochondrial localization of the Parkinson’s disease related protein DJ-1: implications for pathogenesis. Hum Mol Genet 2005; 14:2063 - 73; http://dx.doi.org/10.1093/hmg/ddi211; PMID: 15944198
- Lev N, Ickowicz D, Melamed E, Offen D. Oxidative insults induce DJ-1 upregulation and redistribution: implications for neuroprotection. Neurotoxicology 2008; 29:397 - 405; http://dx.doi.org/10.1016/j.neuro.2008.01.007; PMID: 18377993
- Bezard E, Przedborski S. A tale on animal models of Parkinson’s disease. Mov Disord 2011; 26:993 - 1002; http://dx.doi.org/10.1002/mds.23696; PMID: 21626544
- Hayashi T, Ishimori C, Takahashi-Niki K, Taira T, Kim YC, Maita H, et al. DJ-1 binds to mitochondrial complex I and maintains its activity. Biochem Biophys Res Commun 2009; 390:667 - 72; http://dx.doi.org/10.1016/j.bbrc.2009.10.025; PMID: 19822128
- Meulener M, Whitworth AJ, Armstrong-Gold CE, Rizzu P, Heutink P, Wes PD, et al. Drosophila DJ-1 mutants are selectively sensitive to environmental toxins associated with Parkinson’s disease. Curr Biol 2005; 15:1572 - 7; http://dx.doi.org/10.1016/j.cub.2005.07.064; PMID: 16139213
- Park J, Kim SY, Cha GH, Lee SB, Kim S, Chung J. Drosophila DJ-1 mutants show oxidative stress-sensitive locomotive dysfunction. Gene 2005; 361:133 - 9; http://dx.doi.org/10.1016/j.gene.2005.06.040; PMID: 16203113
- Andres-Mateos E, Perier C, Zhang L, Blanchard-Fillion B, Greco TM, Thomas B, et al. DJ-1 gene deletion reveals that DJ-1 is an atypical peroxiredoxin-like peroxidase. Proc Natl Acad Sci USA 2007; 104:14807 - 12; http://dx.doi.org/10.1073/pnas.0703219104; PMID: 17766438
- Fernandez-Ayala DJ, Sanz A, Vartiainen S, Kemppainen KK, Babusiak M, Mustalahti E, et al. Expression of the Ciona intestinalis alternative oxidase (AOX) in Drosophila complements defects in mitochondrial oxidative phosphorylation. Cell Metab 2009; 9:449 - 60; http://dx.doi.org/10.1016/j.cmet.2009.03.004; PMID: 19416715
- Dufour E, Boulay J, Rincheval V, Sainsard-Chanet A. A causal link between respiration and senescence in Podospora anserina. Proc Natl Acad Sci USA 2000; 97:4138 - 43; http://dx.doi.org/10.1073/pnas.070501997; PMID: 10759557
- Sanz A, Fernández-Ayala DJ, Stefanatos RK, Jacobs HT. Mitochondrial ROS production correlates with, but does not directly regulate lifespan in Drosophila. Aging (Albany NY) 2010; 2:200 - 23; PMID: 20453260
- Yang W, Hekimi S. A mitochondrial superoxide signal triggers increased longevity in Caenorhabditis elegans. PLoS Biol 2010; 8:e1000556; http://dx.doi.org/10.1371/journal.pbio.1000556; PMID: 21151885
- Sanz A, Caro P, Ayala V, Portero-Otin M, Pamplona R, Barja G. Methionine restriction decreases mitochondrial oxygen radical generation and leak as well as oxidative damage to mitochondrial DNA and proteins. FASEB J 2006; 20:1064 - 73; http://dx.doi.org/10.1096/fj.05-5568com; PMID: 16770005
- Martínez A, Portero-Otin M, Pamplona R, Ferrer I. Protein targets of oxidative damage in human neurodegenerative diseases with abnormal protein aggregates. Brain Pathol 2010; 20:281 - 97; http://dx.doi.org/10.1111/j.1750-3639.2009.00326.x; PMID: 19725834
- Jacobson J, Lambert AJ, Portero-Otín M, Pamplona R, Magwere T, Miwa S, et al. Biomarkers of aging in Drosophila. Aging Cell 2010; 9:466 - 77; http://dx.doi.org/10.1111/j.1474-9726.2010.00573.x; PMID: 20367621
- Zarse K, Schmeisser S, Groth M, Priebe S, Beuster G, Kuhlow D, et al. Impaired insulin/IGF1 signaling extends life span by promoting mitochondrial L-proline catabolism to induce a transient ROS signal. Cell Metab 2012; 15:451 - 65; http://dx.doi.org/10.1016/j.cmet.2012.02.013; PMID: 22482728
- Cabreiro F, Ackerman D, Doonan R, Araiz C, Back P, Papp D, et al. Increased life span from overexpression of superoxide dismutase in Caenorhabditis elegans is not caused by decreased oxidative damage. Free Radic Biol Med 2011; 51:1575 - 82; http://dx.doi.org/10.1016/j.freeradbiomed.2011.07.020; PMID: 21839827
- Karpac J, Hull-Thompson J, Falleur M, Jasper H. JNK signaling in insulin-producing cells is required for adaptive responses to stress in Drosophila. Aging Cell 2009; 8:288 - 95; http://dx.doi.org/10.1111/j.1474-9726.2009.00476.x; PMID: 19627268
- Wang PY, Neretti N, Whitaker R, Hosier S, Chang C, Lu D, et al. Long-lived Indy and calorie restriction interact to extend life span. Proc Natl Acad Sci USA 2009; 106:9262 - 7; http://dx.doi.org/10.1073/pnas.0904115106; PMID: 19470468
- Stefanatos R, Sanz A. Mitochondrial complex I: a central regulator of the aging process. Cell Cycle 2011; 10:1528 - 32; http://dx.doi.org/10.4161/cc.10.10.15496; PMID: 21471732
- Zid BM, Rogers AN, Katewa SD, Vargas MA, Kolipinski MC, Lu TA, et al. 4E-BP extends lifespan upon dietary restriction by enhancing mitochondrial activity in Drosophila. Cell 2009; 139:149 - 60; http://dx.doi.org/10.1016/j.cell.2009.07.034; PMID: 19804760
- Rera M, Bahadorani S, Cho J, Koehler CL, Ulgherait M, Hur JH, et al. Modulation of longevity and tissue homeostasis by the Drosophila PGC-1 homolog. Cell Metab 2011; 14:623 - 34; http://dx.doi.org/10.1016/j.cmet.2011.09.013; PMID: 22055505
- Tissenbaum HA, Guarente L. Increased dosage of a sir-2 gene extends lifespan in Caenorhabditis elegans. Nature 2001; 410:227 - 30; http://dx.doi.org/10.1038/35065638; PMID: 11242085
- Burnett C, Valentini S, Cabreiro F, Goss M, Somogyvári M, Piper MD, et al. Absence of effects of Sir2 overexpression on lifespan in C. elegans and Drosophila. Nature 2011; 477:482 - 5; http://dx.doi.org/10.1038/nature10296; PMID: 21938067
- Rogina B, Helfand SL. Sir2 mediates longevity in the fly through a pathway related to calorie restriction. Proc Natl Acad Sci USA 2004; 101:15998 - 6003; http://dx.doi.org/10.1073/pnas.0404184101; PMID: 15520384
- Oberdoerffer P, Michan S, McVay M, Mostoslavsky R, Vann J, Park SK, et al. SIRT1 redistribution on chromatin promotes genomic stability but alters gene expression during aging. Cell 2008; 135:907 - 18; http://dx.doi.org/10.1016/j.cell.2008.10.025; PMID: 19041753
- Slaidina M, Delanoue R, Gronke S, Partridge L, Léopold P. A Drosophila insulin-like peptide promotes growth during nonfeeding states. Dev Cell 2009; 17:874 - 84; http://dx.doi.org/10.1016/j.devcel.2009.10.009; PMID: 20059956
- van der Brug MP, Blackinton J, Chandran J, Hao LY, Lal A, Mazan-Mamczarz K, et al. RNA binding activity of the recessive parkinsonism protein DJ-1 supports involvement in multiple cellular pathways. Proc Natl Acad Sci USA 2008; 105:10244 - 9; http://dx.doi.org/10.1073/pnas.0708518105; PMID: 18626009
- Missirlis F, Hu J, Kirby K, Hilliker AJ, Rouault TA, Phillips JP. Compartment-specific protection of iron-sulfur proteins by superoxide dismutase. J Biol Chem 2003; 278:47365 - 9; http://dx.doi.org/10.1074/jbc.M307700200; PMID: 12972424
- Van Raamsdonk JM, Hekimi S. Superoxide dismutase is dispensable for normal animal lifespan. Proc Natl Acad Sci USA 2012; 109:5785 - 90; http://dx.doi.org/10.1073/pnas.1116158109; PMID: 22451939
- Mukherjee S, Forde R, Belton A, Duttaroy A. SOD2, the principal scavenger of mitochondrial superoxide, is dispensable for embryogenesis and imaginal tissue development but essential for adult survival. Fly (Austin) 2011; 5:39 - 46; PMID: 21212740
- Godenschwege T, Forde R, Davis CP, Paul A, Beckwith K, Duttaroy A. Mitochondrial superoxide radicals differentially affect muscle activity and neural function. Genetics 2009; 183:175 - 84; http://dx.doi.org/10.1534/genetics.109.103515; PMID: 19546321
- Sohal RS. Effect of hydrogen peroxide administration on life span, superoxide dismutase, catalase, and glutathione in the adult housefly, Musca domestica. Exp Gerontol 1988; 23:211 - 6; http://dx.doi.org/10.1016/0531-5565(88)90008-3; PMID: 2849553
- Martin I, Jones MA, Grotewiel M. Manipulation of Sod1 expression ubiquitously, but not in the nervous system or muscle, impacts age-related parameters in Drosophila. FEBS Lett 2009; 583:2308 - 14; http://dx.doi.org/10.1016/j.febslet.2009.06.023; PMID: 19540235
- Curtis C, Landis GN, Folk D, Wehr NB, Hoe N, Waskar M, et al. Transcriptional profiling of MnSOD-mediated lifespan extension in Drosophila reveals a species-general network of aging and metabolic genes. Genome Biol 2007; 8:R262; http://dx.doi.org/10.1186/gb-2007-8-12-r262; PMID: 18067683
- Ristow M, Zarse K. How increased oxidative stress promotes longevity and metabolic health: The concept of mitochondrial hormesis (mitohormesis). Exp Gerontol 2010; 45:410 - 8; http://dx.doi.org/10.1016/j.exger.2010.03.014; PMID: 20350594
- Muller FL, Lustgarten MS, Jang Y, Richardson A, Van Remmen H. Trends in oxidative aging theories. Free Radic Biol Med 2007; 43:477 - 503; http://dx.doi.org/10.1016/j.freeradbiomed.2007.03.034; PMID: 17640558
- Kirby K, Hu J, Hilliker AJ, Phillips JP. RNA interference-mediated silencing of Sod2 in Drosophila leads to early adult-onset mortality and elevated endogenous oxidative stress. Proc Natl Acad Sci USA 2002; 99:16162 - 7; http://dx.doi.org/10.1073/pnas.252342899; PMID: 12456885
- Mockett RJ, Sohal BH, Sohal RS. Expression of multiple copies of mitochondrially targeted catalase or genomic Mn superoxide dismutase transgenes does not extend the life span of Drosophila melanogaster. Free Radic Biol Med 2010; 49:2028 - 31; http://dx.doi.org/10.1016/j.freeradbiomed.2010.09.029; PMID: 20923705
- Hao LY, Giasson BI, Bonini NM. DJ-1 is critical for mitochondrial function and rescues PINK1 loss of function. Proc Natl Acad Sci USA 2010; 107:9747 - 52; http://dx.doi.org/10.1073/pnas.0911175107; PMID: 20457924
- Ristow M, Schmeisser S. Extending life span by increasing oxidative stress. Free Radic Biol Med 2011; 51:327 - 36; http://dx.doi.org/10.1016/j.freeradbiomed.2011.05.010; PMID: 21619928
- Owusu-Ansah E, Yavari A, Mandal S, Banerjee U. Distinct mitochondrial retrograde signals control the G1-S cell cycle checkpoint. Nat Genet 2008; 40:356 - 61; http://dx.doi.org/10.1038/ng.2007.50; PMID: 18246068
- Grönke S, Clarke DF, Broughton S, Andrews TD, Partridge L. Molecular evolution and functional characterization of Drosophila insulin-like peptides. PLoS Genet 2010; 6:e1000857; http://dx.doi.org/10.1371/journal.pgen.1000857; PMID: 20195512
- Yang Y, Gehrke S, Haque ME, Imai Y, Kosek J, Yang L, et al. Inactivation of Drosophila DJ-1 leads to impairments of oxidative stress response and phosphatidylinositol 3-kinase/Akt signaling. Proc Natl Acad Sci USA 2005; 102:13670 - 5; http://dx.doi.org/10.1073/pnas.0504610102; PMID: 16155123
- Wang MC, Bohmann D, Jasper H. JNK extends life span and limits growth by antagonizing cellular and organism-wide responses to insulin signaling. Cell 2005; 121:115 - 25; http://dx.doi.org/10.1016/j.cell.2005.02.030; PMID: 15820683
- Karpac J, Younger A, Jasper H. Dynamic coordination of innate immune signaling and insulin signaling regulates systemic responses to localized DNA damage. Dev Cell 2011; 20:841 - 54; http://dx.doi.org/10.1016/j.devcel.2011.05.011; PMID: 21664581
- Nisoli E, Tonello C, Cardile A, Cozzi V, Bracale R, Tedesco L, et al. Calorie restriction promotes mitochondrial biogenesis by inducing the expression of eNOS. Science 2005; 310:314 - 7; http://dx.doi.org/10.1126/science.1117728; PMID: 16224023
- Strobel NA, Peake JM, Matsumoto A, Marsh SA, Coombes JS, Wadley GD. Antioxidant supplementation reduces skeletal muscle mitochondrial biogenesis. Med Sci Sports Exerc 2011; 43:1017 - 24; http://dx.doi.org/10.1249/MSS.0b013e318203afa3; PMID: 21085043
- Hardie DG. Sensing of energy and nutrients by AMP-activated protein kinase. Am J Clin Nutr 2011; 93:891S - 6; http://dx.doi.org/10.3945/ajcn.110.001925; PMID: 21325438
- Yang W, Hekimi S. Two modes of mitochondrial dysfunction lead independently to lifespan extension in Caenorhabditis elegans. Aging Cell 2010; 9:433 - 47; http://dx.doi.org/10.1111/j.1474-9726.2010.00571.x; PMID: 20346072
- Hur JH, Cho J, Walker DW. Aging: Dial M for Mitochondria. Aging (Albany NY) 2010; 2:69 - 73; PMID: 20228940
- Dell’agnello C, Leo S, Agostino A, Szabadkai G, Tiveron C, Zulian A, et al. Increased longevity and refractoriness to Ca(2+)-dependent neurodegeneration in Surf1 knockout mice. Hum Mol Genet 2007; 16:431 - 44; http://dx.doi.org/10.1093/hmg/ddl477; PMID: 17210671
- Kuang J, Ebert PR. The failure to extend lifespan via disruption of complex II is linked to preservation of dynamic control of energy metabolism. Mitochondrion 2011; PMID: 22122855
- Copeland JM, Cho J, Lo T Jr., Hur JH, Bahadorani S, Arabyan T, et al. Extension of Drosophila life span by RNAi of the mitochondrial respiratory chain. Curr Biol 2009; 19:1591 - 8; http://dx.doi.org/10.1016/j.cub.2009.08.016; PMID: 19747824
- Bosch-Presegué L, Raurell-Vila H, Marazuela-Duque A, Kane-Goldsmith N, Valle A, Oliver J, et al. Stabilization of Suv39H1 by SirT1 is part of oxidative stress response and ensures genome protection. Mol Cell 2011; 42:210 - 23; http://dx.doi.org/10.1016/j.molcel.2011.02.034; PMID: 21504832
- Banerjee KK, Ayyub C, Sengupta S, Kolthur-Seetharam U. dSir2 deficiency in the fatbody, but not muscles, affects systemic insulin signaling, fat mobilization and starvation survival in flies. Aging (Albany NY) 2012; 4:206 - 23; PMID: 22411915
- Sorensen M, Sanz A, Gómez J, Pamplona R, Portero-Otín M, Gredilla R, et al. Effects of fasting on oxidative stress in rat liver mitochondria. Free Radic Res 2006; 40:339 - 47; http://dx.doi.org/10.1080/10715760500250182; PMID: 16517498
- Bauer J, Antosh M, Chang C, Schorl C, Kolli S, Neretti N, et al. Comparative transcriptional profiling identifies takeout as a gene that regulates life span. Aging (Albany NY) 2010; 2:298 - 310; PMID: 20519778
- Jang YC, Lustgarten MS, Liu Y, Muller FL, Bhattacharya A, Liang H, et al. Increased superoxide in vivo accelerates age-associated muscle atrophy through mitochondrial dysfunction and neuromuscular junction degeneration. FASEB J 2010; 24:1376 - 90; http://dx.doi.org/10.1096/fj.09-146308; PMID: 20040516
- Haselton AT, Fridell YW. Adult Drosophila melanogaster as a model for the study of glucose homeostasis. Aging (Albany NY) 2010; 2:523 - 6; PMID: 20689157
- Broughton S, Alic N, Slack C, Bass T, Ikeya T, Vinti G, et al. Reduction of DILP2 in Drosophila triages a metabolic phenotype from lifespan revealing redundancy and compensation among DILPs. PLoS One 2008; 3:e3721; http://dx.doi.org/10.1371/journal.pone.0003721; PMID: 19005568
- de Magalhães JP, Church GM. Cells discover fire: employing reactive oxygen species in development and consequences for aging. Exp Gerontol 2006; 41:1 - 10; http://dx.doi.org/10.1016/j.exger.2005.09.002; PMID: 16226003
- Burdon RH. Superoxide and hydrogen peroxide in relation to mammalian cell proliferation. Free Radic Biol Med 1995; 18:775 - 94; http://dx.doi.org/10.1016/0891-5849(94)00198-S; PMID: 7750801
- Lee S, Tak E, Lee J, Rashid MA, Murphy MP, Ha J, et al. Mitochondrial H2O2 generated from electron transport chain complex I stimulates muscle differentiation. Cell Res 2011; 21:817 - 34; http://dx.doi.org/10.1038/cr.2011.55; PMID: 21445095
- Furukawa A, Tada-Oikawa S, Kawanishi S, Oikawa S. H2O2 accelerates cellular senescence by accumulation of acetylated p53 via decrease in the function of SIRT1 by NAD+ depletion. Cell Physiol Biochem 2007; 20:45 - 54; PMID: 17595514
- Albrecht SC, Barata AG, Grosshans J, Teleman AA, Dick TP. In vivo mapping of hydrogen peroxide and oxidized glutathione reveals chemical and regional specificity of redox homeostasis. Cell Metab 2011; 14:819 - 29; http://dx.doi.org/10.1016/j.cmet.2011.10.010; PMID: 22100409
- Mair W, Piper MD, Partridge L. Calories do not explain extension of life span by dietary restriction in Drosophila. PLoS Biol 2005; 3:e223; http://dx.doi.org/10.1371/journal.pbio.0030223; PMID: 16000018
- Sanz A, Stefanatos R, McIlroy G. Production of reactive oxygen species by the mitochondrial electron transport chain in Drosophila melanogaster. J Bioenerg Biomembr 2010; 42:135 - 42; http://dx.doi.org/10.1007/s10863-010-9281-z; PMID: 20300811
- Sanz A, Soikkeli M, Portero-Otín M, Wilson A, Kemppainen E, McIlroy G, et al. Expression of the yeast NADH dehydrogenase Ndi1 in Drosophila confers increased lifespan independently of dietary restriction. Proc Natl Acad Sci USA 2010; 107:9105 - 10; http://dx.doi.org/10.1073/pnas.0911539107; PMID: 20435911