Eukaryotic cells ensure that DNA is replicated only once per cell cycle. The initiation of DNA replication is dependent on the formation of the pre-replicative complex (pre-RC) on replication origins. The six components of origin recognition complex (Orc1–6p) are bound to the origins, followed by Cdc6p, Cdt1p and the Mcm2–7p complex, to constitute pre-RC. The pre-RC is then activated by the Dbf kinase-Cdc7p complex to initiate DNA replication, where the Mcm complex acts as a helicase.Citation1 After initiation, the pre-RC complex is disassembled and/or inactivated in order to inhibit re-initiation until the next cell cycle. The inhibition of the pre-RC assembly can be achieved by protein degradation through ubiquitination, translocation and/or phosphorylation of the pre-RC components. Regulation of the Orc complex across species is diverse. In yeast, the Orc complex is probably associated on the origin throughout the cell cycle, and the Orc proteins are phosphorylated by cyclin/CDK complex to inhibit the function.Citation2 In humans, Orc1 is ubiquitinated by SCFskp2 complex and degraded by the proteasome.Citation3 Orc2 is associated with heterochromatin, centromere and centrosome depending on the cell cycle stage.Citation3,Citation4
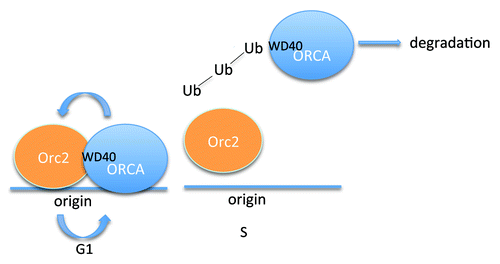
Previously Shen et al. showed that Orc-associated protein (ORCA) utilizes WD40 repeats to associate with DNA replication factors such as Orc, Cdt1 and Geminin in human cells. The WD40 repeats in ORCA are also required for chromatin binding, suggesting that ORCA may anchor other DNA replication factors to chromatin. ORCA stabilizes Orc to chromatin, indicating that ORCA positively regulates the initiation of DNA replication.Citation5 The siRNA treatment against Orc2 showed decreased amount of ORCA, suggesting that the stability of ORCA is dependent on Orc2.Citation6 Therefore the Orc2-ORCA binding facilitates the stability of this complex. It has been shown that ORCA protein levels are peaked at G1 phase. In a previous issue of Cell Cycle, Shen et al. investigated the molecular mechanism by which ORCA is regulated in a cell cycle-dependent manner.Citation7 The authors showed that ORCA is polyubiquitinated at the WD40 repeat region, where it is recognized by Orc2. They observed an elevated ORCA ubiquitination level at G1/S boundary that coincides with ORCA protein degradation. Furthermore, Orc2 is associated only with non-ubiquinated ORCA, indicating that Orc2 prevents ORCA from ubiquitin-mediated degradation. The authors proposed a model that Orc2 releases from chromatin after G1, which may trigger ORCA ubiquitination and degradation (). Therefore, ORCA degradation prevents Orc2 loading on the chromatin after G1 to allow only one S-phase per cell cycle. This cell cycle-dependent ORCA regulation may allow Orc2 to obtain diverse functions as well as timely initiation of DNA replication on the specific origins. The novel mechanism to control Orc components through ORCA may be a key mechanism to control DNA re-replication.
References
- Bell SP, et al. Annu Rev Biochem 2002; 71:333 - 74; http://dx.doi.org/10.1146/annurev.biochem.71.110601.135425; PMID: 12045100
- Nguyen VQ, et al. Nature 2001; 411:1068 - 73; http://dx.doi.org/10.1038/35082600; PMID: 11429609
- Méndez J, et al. Mol Cell 2002; 9:481 - 91; http://dx.doi.org/10.1016/S1097-2765(02)00467-7; PMID: 11931757
- Prasanth SG, et al. EMBO J 2004; 23:2651 - 63; http://dx.doi.org/10.1038/sj.emboj.7600255; PMID: 15215892
- Shen Z, et al. Mol Cell 2010; 40:99 - 111; http://dx.doi.org/10.1016/j.molcel.2010.09.021; PMID: 20932478
- Shen Z, et al. Mol Cell Biol 2012; 32:3107 - 20; http://dx.doi.org/10.1128/MCB.00362-12; PMID: 22645314
- Shen Z, et al. Cell Cycle 2012; 11; http://dx.doi.org/10.4161/cc.21870; PMID: 22935713