Abstract
Energy- and nutrient-sensing proteins such as AMPK, mTOR and S6K1 are now recognized as novel regulators of mitotic completion in proliferating cells. We investigated the cellular distribution of the Ser2481 autophosphorylation of mTOR, which directly monitors mTORC-specific catalytic activity, during mammalian cell mitosis and cytokinesis. Automated immunofluorescence experiments in human carcinoma cell lines revealed that phospho-mTORSer2481 exhibited profound spatial and temporal dynamics during cell division. Phospho-mTORSer2481 was strikingly enriched in mitotic cells, and in prophase, bright phospho-mTORSer2481 staining could be clearly observed among condensed chromosomes. Phospho-mTORSer2481 then redistributes from diffuse cytosolic staining that partially colocalizes with the mitotic spindle during the early phases of mitosis to the furrow at the onset of cytokinesis. Like the bona fide chromosomal passenger proteins (CPPs) INCENP and Aurora B, phospho-mTORSer2481 displayed noteworthy accumulation in the central spindle midzone and the midbody regions, which persisted during the furrowing process. Accordingly, double-staining experiments confirmed that phospho-mTORSer2481 largely colocalized with CCPs in the midbodies. The CPP-like mitotic localization of phospho-mTORSer2481 was fully prevented by the microtubule-depolymerizing drug nocodazole; mitotic traveling of phospho-mTORSer2481 to the midbody during telophase and cytokinesis, where it appears to be integrated into the CPP-driven cytokinetic machinery, may therefore require dynamic microtubules. Although the Ser2448-phosphorylated form of mTOR was also found at high levels during M-phase in human cancer cells, we failed to observe a significant association of phospho-mTORSer2448 with CCP-positive mitotic and cytokinetic structures. Our findings add phospho-mTORSer2481 to the growing list of phospho-active forms of proteins belonging to the AMPK/mTOR/S6K1 signaling axis that reside at the mitotic and cytokinetic apparatus. Future studies should elucidate how the specific ability of phospho-mTORSer2481 to spatially and temporally couple to the cleavage furrow and midbody region as a CPP-like protein can signal to or from adjacent signaling complexes and/or with the basic machinery of cell abscission.
Introduction
The mammalian target of rapamycin (mTOR) is a master integrator of cell growth and division in response to cell energy state, nutrient status and growth factor stimulation.Citation1-Citation6 This serine/threonine kinase is the catalytic component of two distinct signaling complexes, the rapamycin-sensitive mTOR signaling complex 1 (mTORC1) and the rapamycin-insensitive mTOR signaling complex 2 (mTORC2), each of which signals via a different set of effector pathways. mTORC1 regulates cell growth and the translation process in part by phosphorylating S6 kinase (S6K) and the eIF-4E binding protein 1 (eIF-4EBP1). mTORC2 acts as an organizer of the actin cytoskeleton and activates AKT through Ser473 phosphorylation. Although these two TOR complexes constitute an ancestral signaling network conserved throughout eukaryotic evolution to control the fundamental process of cell growth, the challenge now is to definitively understand the central role that mTOR signaling appears to play during development and aging.
An intriguing molecular scenario that may explain how signaling emanating from two TORC complexes—both containing mTOR as the catalytic component—is implicated in disorders such as cancer, cardiovascular disease, obesity and diabetes and relates to the functional significance of mTOR phosphorylation and/or the specificity of mTOR phosphorylation sites for the different mTORCs. Upon activation, mTOR is phosphorylated at several residues, including Ser2448 and Ser2481.Citation7-Citation9 Recent studies have suggested the existence and the identity of mTORC-specific phosphorylation sites on mTOR; for example, mTORC1 contains S6K-regulated phospho-Ser2448 mTOR, which is sensitive to acute rapamycin treatment, whereas mTORC2 contains phospho-Ser2481 mTOR.Citation10 Although the phosphorylation of mTOR at Ser2481 was originally reported to be rapamycin-insensitive,Citation7 prolonged rapamycin treatment can inhibit the assembly and function of the mTORC2 complex.Citation11 Accordingly, Copp et al.Citation10 observed that phosphorylation of mTOR-Ser2481 is dependent on intact mTORC2, and that this site is sensitive to prolonged rapamycin treatment. Soliman et al.,Citation12 however, demonstrated that growth factors such as insulin stimulate the phosphorylation of Ser2481 of mTOR associated with both mTORC1 and mTORC2 in a phosphatidylinositol 3-kinase-dependent manner, whereas acute rapamycin can inhibit insulin-stimulated phospho-mTOR-Ser2481 in mTORC1 but not in mTORC2. In this scenario, Ser2481 autophosphorylation of mTOR therefore appears to directly monitor mTORC-specific catalytic activity.Citation12
The Ser2448 and Ser2481 phosphorylation sites of mTOR are absent in invertebrates but are highly conserved across vertebrate species, suggesting their recent evolution and distinctive regulation of mTOR signaling in vertebrates. However, it cannot be excluded that phospho-Ser2448 mTOR and phospho-Ser2481 mTOR did share a common function once acquired during early vertebrate evolution. In this regard, mTOR phosphorylation at Ser2448 and/or Ser2481 may behave in a similar manner as mitotic survival checkpoints. Yaba et al.Citation13 were pioneers in revealing that phospho-Ser2448 mTOR is present at high levels during M-phase in ovarian granulosa cells. They further reported that phospho-Ser2448 mTOR, but not total mTOR, is enriched on or near the mitotic spindle and also near the contractile ring during cytokinesis. Using spontaneously immortalized rat granulosa cells and co-staining experiments with α-tubulin, they confirmed that Ser2448-phosphorylated mTOR is expressed at higher levels during mitosis relative to neighboring interphase cells and is highly enriched in the region of the mitotic spindle.Citation14 Using childhood adrenocortical cells, Doghman et al.Citation15 similarly reported that the active Ser2448-phosphorylated form of mTOR is present only in mitotic cells in association with the mitotic spindle and midbody in the G2/M phase of the cell cycle. Preliminary findings in our own laboratory suggest that, starting from anaphase, the Ser2481-autophosphorylated form of mTOR appears to move to the midzone and progressively concentrate at the midbody in the cleavage furrow during telophase and cytokinesis.Citation16,Citation17
In the current study, we sought to definitively establish whether rapamycin-sensitive mTOR autophosphorylation at Ser2481 could be added to the growing list of phospho-active forms of proteins that functionally belong to the energy- and nutrient-sensing AMPK/mTOR/S6K1 signaling axis and reside at the mitotic and cytokinetic apparatus. Our current findings reveal for the first time that phospho-mTORSer2481 largely colocalizes with bonafide chromosomal passenger proteins (CPPs) in the midbodies, and that its integration into the CPP-driven cytokinetic machinery during mammalian cell cytokinesis requires dynamic microtubules.
Results
Ser2481 autophosphorylation of mTOR is sensitive to the mTOR inhibitor rapamycin
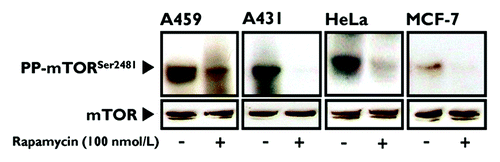
We first wanted to confirm whether prolonged treatment of human cancer cells with rapamycin efficiently inhibits mTOR phosphorylation at Ser2481.Citation10 When actively growing MCF-7 breast cancer cells, A431 epidermoid carcinoma cells, A459 lung carcinoma cells and HeLa cervical carcinoma cells were cultured in the absence or presence of 100 nmol/L rapamycin for 24 h, immunoblotting showed that rapamycin treatment markedly reduced or completely inhibited Ser2481 autophosphorylation of mTOR (). This rapamycin-sensitive blockade of phospho-Ser2481 mTOR was most likely due to prolonged rapamycin treatment inhibiting the assembly of mTORC2, because Ser2481 phosphorylation showed no discernible decreases following acute treatment (i.e., 1 h) with an equivalent dose of rapamycin (data not shown).
Figure 1. Prolonged treatment of human cancer-derived carcinoma cells with rapamycin inhibits mTOR phosphorylation at Ser2481. Actively growing HeLa, MCF-7, A431 and A459 human epithelial carcinoma cells were cultured in the absence or presence of 100 nmol/L rapamycin for 24 h. Whole-cell lysates were analyzed by immunoblotting with antibodies specific for phospho-mTORSer2481 or total mTOR (control), as specified. The figure shows a representative immunoblotting analysis. Equivalent results were obtained in three independent experiments.
Spatiotemporal dynamics of phospho-mTORSer2481 during mitosis and cytokinesis in human cancer cells
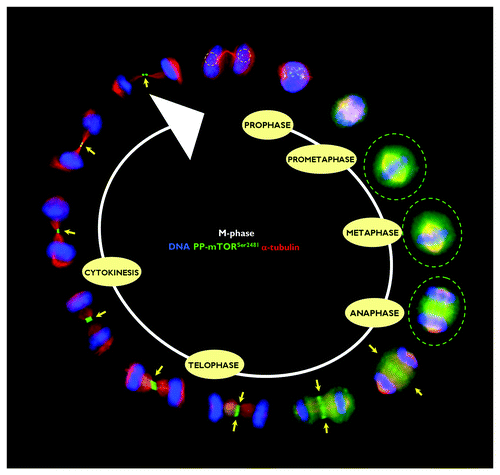
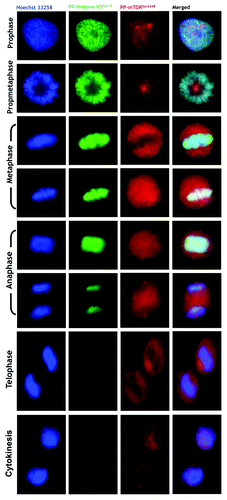
Using an automated confocal imaging system for high-resolution images and 3-D reconstructions, we first explored the spatiotemporal dynamics of phospho-mTORSer2481 during the G2/M stage of the cell cycle in HeLa cancer cells. Similar to that observed when analyzing the spatiotemporal dynamics of phospho-AMPKThr172 during mitosis and cytokinesis,Citation18-Citation23 we observed a previously unrecognized pattern of transient associations between phospho-mTORSer2481 and specific mitotic structures during mitotic chromosome condensation/segregation and cytokinesis, including the central spindle midzone and the midbody (). In prophase, bright phospho-mTORSer2481 staining appeared among condensed chromosomes. Although the faint punctuate staining of prophasic phospho-mTORSer2481 seemed to be distributed along the arms of the chromosomes, we previously reported that there was no evident co-localization between phospho-mTORSer2481 and the Ser10- and Ser28-phosphorylated forms of histone H3.Citation17 In some cases, the staining detected by the rabbit polyclonal phospho-mTOR (Ser2481) antibody (#2974, Cell Signaling Technology®) appeared to accumulate to some extent as discrete centrosomal-like foci at the spindle poles (data not shown). Phospho-mTORSer2481-immunopositive signals in mitotic cells continued to exhibit a diffuse pattern that partially colocalized with the mitotic spindle from prophase to telophase, but phospho-mTORSer2481 was most prominent as a broad cytoplasmic signal until the chromatids were pulled apart and began to migrate toward the poles. Thus, a completely different picture emerged when analyzing the mitotic dynamics of phospho-mTORSer2481 from late anaphase. “PP-mTORSer2481-somes” notably and specifically accumulated at the midzone and midbody until the end of the furrowing process at the completion of telophase and cytokinesis (). Interestingly, the detection of phospho-Ser2481 mTOR in the intercellular bridge reached its maximum during early telophase, and at the completion of telophase, prominent staining of phospho-Ser2481 mTOR as a doublet was apparent on either side of the midbody within the intercellular cytokinetic bridge. Indeed, in the late stages of mitosis and cytokinesis, phosphor-mTORSer2481 persisted close to the midbody in the postmitotic bridges connecting the dividing daughter cells. With time, the phospho-Ser2481 mTOR intensity was barely detectable in the cleavage furrow. Concurrent with this progressive loss of detectable phospho-Ser2481 mTOR at the intercellular bridge was a transient but conspicuous increase in the reactivity of phospho-mTORSer2481 within the reforming nuclei ().
Figure 2. Spatiotemporal dynamics of phospho-mTORSer2481 during mitosis and cytokinesis. After fixation and permeabilization of asynchronously growing HeLa cells in 96-well clear-bottom imaging tissue culture plates optimized for automated imaging applications, cells were double-stained with antibodies against phospho-mTORSer2481 and α-tubulin and with Hoechst 33258 for nuclear counterstaining. The figure shows representative portions of images containing dividing cells captured with a 40x objective in the channels corresponding to phospho-mTORSer2481 (green), α-tubulin (red) and Hoechst 33258 (blue), and the images were merged with a BD Pathway™ 855 Bioimager System using BD Attovision™ software.
Mitotic traveling of phospho-mTORSer2481 requires dynamic microtubules
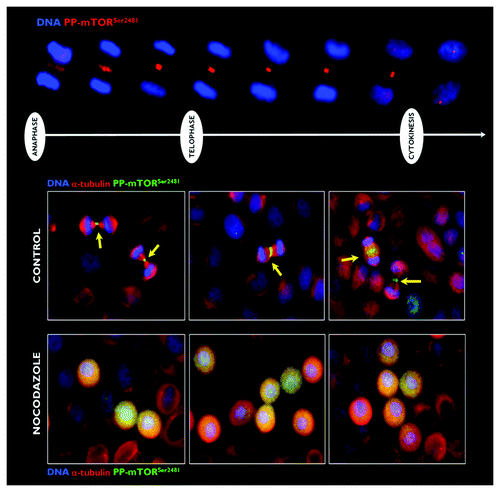
The key feature of the mitotic behavior of phospho-mTORSer2481 was the compaction of the phospho-mTORSer2481 fluorescence to the midzone of the central/nascent midbody during the transition from late anaphase to early telophase. This localization of phospho-mTORSer2481 persisted during the furrowing process, and at the completion of telophase, a striking signal of phospho-mTORSer2481 as a doublet-like structure was clearly observed on either side of the midbody within the intracellular cytokinetic bridge (, top). We aimed to preliminarily assess the molecular associations dictating the mitotic and cytokinetic traveling of phospho-mTORSer2481. When microtubules were eliminated upon treatment with nocodazole, phospho-mTORSer2481 completely lost its ability to target the spindle midzone and the cleavage furrow during telophase and cytokinesis, and it was randomly dispersed across the entire mitotic cytoplasm (, bottom).
Figure 3. Effect of microtubule depolymerization on the central spindle/midzone localization of phospho-mTORSer2481. Top: Spatiotemporal dynamics of phospho-mTORSer2481 during mitosis and cytokinesis in A431 epidermoid cancer cells, which were double-stained with antibodies against phospho-mTORSer2481 (red) and Hoechst 33258 (blue) for nuclear counterstaining. Bottom: Representative images of asynchronous A431 cell cultures propagated under normal conditions (leftpanels) or exposed to 0.2 μg/mL nocodazole for 6-h G2 arrest (right panels). Images were captured with a 20x objective in the channels corresponding to phospho-mTORSer2481 (green), α-tubulin (red) and Hoechst 33258 (blue), and the images were merged with a BD Pathway™ 855 Bioimager System using BD Attovision™ software.
Co-localization analysis of phospho-mTORSer2481 with the chromosomal passenger proteins INCENP and Aurora B
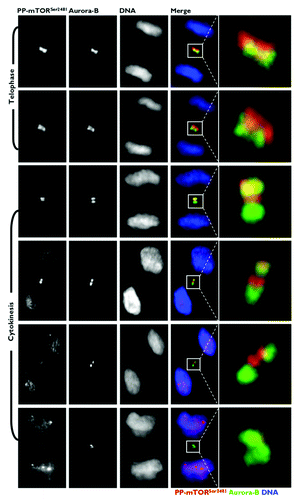
To carefully evaluate the subcellular re-localization of mitotic phospho-mTORSer2481 to areas occupied by bona fide CPPs at central spindle microtubules, we explored the spatiotemporal association between phospho-mTORSer2481 and CPPs that localize to the same subcellular region during mitosis and cytokinesis. Toward the end of anaphase and during telophase, phospho-mTORSer2481 and the CPP INCENP showed similar localizations at the central spindle and midbody (). At late anaphase, INCENP localization was mostly restricted to the central spindle. Late-anaphase phospho-mTORSer2481 began to accumulate in the spindle midzone, although some was not apparently bound to any mitotic structure. More revealing was that there was a prominent overlap of INCENP and phospho-mTORSer2481 in the two bands on either side of the midbody at the cleavage furrow area. The localization of INCENP and phospho-mTORSer2481 continued to overlap in cells at cytokinesis, when both proteins appeared as a doublet facing each other at the apical ends connecting two daughter cells. A similar co-localization pattern occurred when we evaluated the immunofluorescence co-staining of phospho-mTORSer2481 and Aurora B from late anaphase to the completion of cytokinesis (). At this late stage of the mitotic process, however, phospho-mTORSer2481 appeared to position internally, relative to Aurora B. On exit from mitosis, unlike the bona fide CPPs INCENP and Aurora B, there was a noteworthy reappearance of phospho-mTORSer2481 following the onset of chromatin decondensation within the reforming nuclei of both daughter cells.
Figure 4. Co-localization analysis of phospho-mTORSer2481 and the CPP INCENP during mitosis and cytokinesis. Asynchronously growing A431 cells were fixed and stained as described in the Materials and Methods. The figure shows representative portions of images containing dividing cells captured with a 40x objective in the channels corresponding to phospho-mTORSer2481 (red), INCENP (green) and Hoechst 33258 (blue), and the images were merged with a BD PathwayTM 855 Bioimager System using BD AttovisionTM software. The rectangular regions (white lines) are enlarged and shown as high-magnification insets in the right panels.
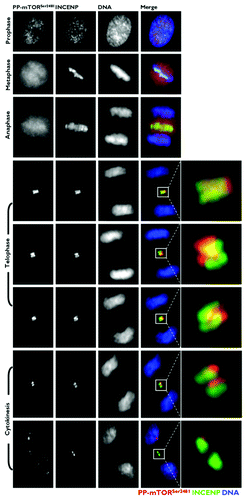
Figure 5. Co-localization analysis of phospho-mTORSer2481 and the CPP Aurora B during mitosis and cytokinesis. Asynchronously growing A431 cells were fixed and stained as described in the Materials and Methods. The figure shows representative portions of images containing dividing cells captured with a 40x objective in the channels corresponding to phospho-mTORSer2481 (red), Aurora B (green) and Hoechst 33258 (blue), and the images were merged with a BD PathwayTM 855 Bioimager System using BD AttovisionTM software. The rectangular regions (white lines) are enlarged and shown as high-magnification insets in the right panels.
Specificity analysis of the CPP-like behavior of phospho-mTORSer2481
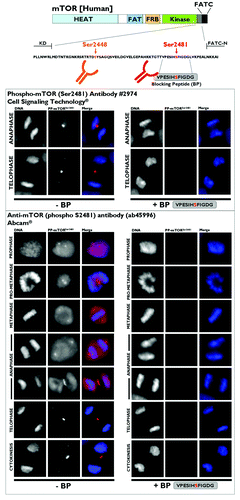
As controls for the specificity of the staining pattern observed for mitotic/cytokinetic phospho-mTORSer2481, we performed several additional experiments. First, immunohistochemical analysis of various mammalian cell types differing in tissue origin revealed that the presence of phospho-mTORSer2481 in midbodies is a general phenomenon in mammalian cells. Thus, the anti-phospho-mTORSer2481 antibody stained the furrowing region in MCF10A, MCF-7, MDA-MB-231, A431, HT29, A459, PC9, HepG2 and other cell types, all of which are representative models of epithelial and mesenchymal cancer cell types. The anti-phospho-mTORSer2481 antibody also labeled midbodies in human skin fibroblastic cells and 3T3-L1 mouse pre-adipocyte fibroblasts (data not shown). Second, the use of a series of alternative fixation and permeabilization regimens did not alter the CPP-like staining pattern of phospho-mTORSer2481, supporting the idea that this localization does not reflect artifactual precipitation of the antibody onto the furrowing area in dividing cells (data not shown). Third, we compared the staining patterns obtained with the phospho-mTOR(Ser2481) antibody (#2974, Cell Signaling Technology®), which was the antibody used in the above experiments, with three other commercially available anti-phospho-mTORSer2481 antibodies (ab45996 from Abcam®, 09–343 from Merck Millipore and sc-293132 from Santa Cruz Biotechnology). Used strictly in parallel and under identical conditions, all commercially available antibodies we tested yielded an equivalent localization of phospho-mTORSer2481 at the midbodies and furrowing areas of dividing cells ( shows representative panels of the staining patterns obtained with ab45996). However, it should be noted that, as mentioned above, when using the phospho-mTOR(Ser2481) antibody #2974 from Cell Signaling Technology® (which was produced by immunizing animals with a synthetic phosphopeptide corresponding to residues surrounding Ser2481 of human mTOR), a weak but significant centrosomal-like localization of phospho-mTORSer2481 was observed with other anti-phospho-mTORSer2481 antibodies, such as ab45996 (which was raised against a KLH-conjugated synthetic peptide derived from the region between residue 2450 and the C terminus of human mTOR phosphorylated at Ser2481). Fourth, to unambiguously demonstrate the specificity of the mitotic and cytokinetic staining of the Ser-2481-autophosphorylated form of mTOR, we performed absorption studies of the two antibodies with the blocking peptide VPESIHSFIGDG, which encompassed and included the amino acid residues surrounding mTOR Ser2481. Absorption of either antibody (#2974 or ab45996) by inclusion of the VPESIHSFIGDG peptide completely eliminated phospho-mTORSer2481-reactive species in all staining reactions. Moreover, cross-absorption of the primary antibodies against Aurora A, Aurora B and INCENP with a similar concentration of the phospho-mTORSer2481-blocking peptide failed to affect staining reactivity in any site (data not shown).
Figure 6. Investigation of the specificity of phospho-mTORSer2481 antibodies. Top: Schematic of mTOR protein. The region between the kinase domain (KD) and an NH2-terminal extension of the FATC domain (FATC-N) is conserved among vertebrates,Citation10 including the marked phosphorylation sites S2448 and S2481. (HEAT, Huntington, elongation factor 3, PR65/A, TOR; FAT, FRAP, ATM, TRRAP; FRB, FKBP12 rapamycin-binding; KD, kinase domain; RD, regulatory domain; FATC, FRAP ATM TRRAP carboxy-terminus.) Bottom: Characterization of the specificity of anti-phospho-mTORSer2481 antibodies. The VPESIH(S)FIGDG-blocking peptide was obtained from Abcam®. We added VPESIH(S)FIGDG to each antibody at a 2:1 (v/v) ratio in a total volume of 100 μL and then incubated this combination for a minimum of 30 min prior to adding the required volumes to the imaging tissue culture plates. Immunofluorescence analyses showed that the mitotic/cytokinetic geography of phospho-mTORSer2481 was fully blocked by peptide competition.
Phospho-mTORSer2448 does not mimic the CPP-like midbody localization of phospo-mTORSer2481
Finally, we explored the similarities and differences between the mitotic and cytokinetic geography of phospho-mTORSer2481 and phospho-mTORSer2448. We performed double-immunofluorescence staining of the two phospho-isoforms of mTOR with one of the mitosis-specific phosphorylated forms of histone H3 (). Similar to that observed with phospho-mTORSer2481, phospho-mTORSer2448 showed enriched expression that correlated strongly and specifically with the mitotic status of cultured cancer cells. On the basis of the double-immunofluorescence staining, we concluded that all mitotic figures positively marked with the Ser10-phosphorylated G2/M marker also co-expressed high levels of phospho-mTORSer2448. Whereas phospho-histone H3Ser10 likewise indicated chromosome condensation at the onset of mitosis (prophase, metaphase and the beginning of anaphase), but not during late anaphase, telophase or cytokinesis, phospho-mTORSer2448 expression was observed in all stages of mitosis. Although phospho-mTORSer2448 showed an adjacent pattern to condensed chromatin in the early stages of mitosis that was particularly visible when the chromosomes arranged at the metaphase plate and was comparable to that observed with phospho-mTORSer2481, we failed to observe any tight localization of phospho-mTORSer2448 to the midbody region within the intracellular bridge during late stages of mitosis and cytokinesis ().
Figure 7. Mitotic dynamics of phospho-mTORSer2481 and phospho-mTORSer2448 during mitosis and cytokinesis. Asynchronously growing A431 cells were fixed and stained as described in the Materials and Methods. The figure shows representative portions of images containing dividing cells captured with a 20x objective in the channels corresponding to phospho-mTORSer2481 (red), phospho-mTORSer2448 (red), phospho-Histone H3Ser10 (green) and Hoechst 33258 (blue), and the images were merged with a BD PathwayTM 855 Bioimager System using BD AttovisionTM software. (P, prophase; PM, prometaphase; A, anaphase; M, metaphase; T, telophase; Cy, cytokinesis.)
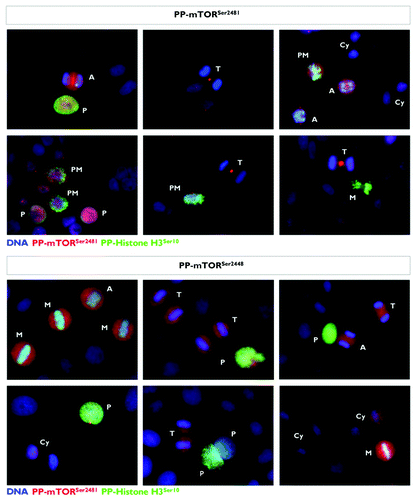
Figure 8. Spatiotemporal relationship between phospho-mTORSer2448 and phospho-histone H3Ser10 during mitosis and cytokinesis. After fixation and permeabilization of asynchronously growing A431 cells in 96-well clear-bottom imaging tissue culture plates optimized for automated imaging applications, cells were double-stained with antibodies against phospho-mTORSer2448 and α-tubulin and with Hoechst 33258 for nuclear counterstaining. The figure shows representative portions of images containing dividing cells captured with a 40x objective in the channels corresponding to phospho-mTORSer2448 (red), phospho-histone H3Ser10 (green) and Hoechst 33258 (blue), and the images were merged with a BD Pathway™ 855 Bioimager System using BD Attovision™ software.
Discussion
Our current findings strongly support a recently discovered scenario, in which some kinase-active forms of the AMPK/mTOR/S6K1 axis, a multifaceted integrator of cell growth and division in response to cell energy state, nutrient status and growth factor stimulation, unpredictably appear to act as novel components of mitosis and cytokinesis machinery. Using tissue sections of human carcinomas and cell cultures of mammalian cells, we previously revealed that the active form of the α-catalytic subunit of AMPK (i.e., phospho-AMPKThr172), a pivotal upstream regulator of mTOR activity, is specifically increased in mitotic cells and transiently associates with several mitosis-specific structures, including centrosomes, spindle poles, the central spindle midzone and the midbody, throughout all of the mitotic stages and during the furrowing process in cytokinesis.Citation18-Citation23 Indeed, a trimeric complex of AMPK containing the γ2 regulatory subunit appears to become selectively activated in the cytokinetic apparatus and to be directly linked to mitotic processes.Citation22,Citation24,Citation25 Other groups have confirmed these observations,Citation26-Citation28 and we now know that mitotic AMPK regulates mitotic spindle orientation through phosphorylation of the myosin regulatory light chain, and that it ensures the normal recruitment of myosin molecules into the contractile ring structure to allow the proper transition from metaphase to anaphase and the completion of cytokinesis.Citation26-Citation29 Phosphorylation at Thr421/Ser424 of p70S6K1, a downstream effector of mTOR, is also strongly upregulated during mitosis, and the S6 kinase p54S6K2 has been identified as a centrosome-located kinase throughout the cell cycle.Citation30-Citation32 Our current findings confirm that mTOR Ser2481 autophosphorylation, a recently described pharmacodynamic biomarker that directly monitors the effects of rapamycin on the assembly and function of mTORC complexes, should be added to the growing list of phospho-active forms of proteins of the AMPK/mTOR/S6K1 signaling axis that reside at the mitotic and cytokinetic apparatus.
Expression of the Ser2481-autophosphorylated form of mTOR is detectable in all stages of mitosis, with distinct subpopulations accumulating at the spindle equator, the midbody as well as the cleavage furrow at the junction between dividing daughter cells.Citation16,Citation17 Here, we focused on the molecular dynamics of the intense staining of phospho-mTORSer2481 in an equatorial plane corresponding to the spindle microtubule overlap zone as well as its persistence in a region corresponding to the cleavage plane throughout telophase and into cytokinesis. Our findings suggest that phospho-mTORSer2481 may behave as a CPP-like protein at the spindle midzone during anaphase and at the cleavage furrow and midbody during cytokinesis. Our current data also strongly suggest that phospho-mTORSer2481 functions as a microtubule-binding protein that translocates to the equatorial plane prior to cleavage furrow formation through molecular interactions requiring dynamic microtubules. Because the formation of the contractile rings is a rather late phenomenon during mitosis, and phospho-mTORSer2481 relocates to the equatorial plate at the metaphase-anaphase transition, it may be reasonable to suggest that phospho-mTORSer2481 is a member of the class of CPPs. Indeed, the staining pattern obtained with anti-phospho-mTORSer2481 antibodies was notably superimposable to that obtained with antibodies against the bona fide CPPs INCENP and Aurora B, which were located in the midbody at the position of maximal furrow constriction. However, it should be noted that members of the CPP class of proteins share some traits that we failed to observe when assessing the mitotic/cytokinetic geography of phospho-mTORSer2481 in dividing cells. All CPPs associate with chromosomes during metaphase and colocalize with the microtubules of the overlap zone of the central spindle during anaphase. In contrast, phospho-mTORSer2481 does not appear to use the chromosomes to correctly position itself at the metaphase plate and at the midbodies. At the metaphase-anaphase transition, phospho-mTORSer2481 is also released from the cytoplasm and/or centrosomal-like areas and then localizes to the central spindle region in a microtubule-dependent manner. Although our demonstration that phospho-mTORSer2481 is associated with midbody regions during the cell cycle suggests a functional cooperation between this phospho-active form of mTOR and CPPs to achieve the completion of cytokinesis, forthcoming studies should elucidate how the specific ability of phospho-mTORSer2481 to spatially and temporally couple to the cleavage furrow and midbody region as a CPP-like protein can signal to or from adjacent signaling complexes at the basic machinery of cell abscission.
Altogether, our findings are in agreement with the notion that mTOR complexes are hyperactive during mitosis and suggest that all or at least part of their components (e.g., raptor)Citation33-Citation35 may physically and functionally interact at the mitotic and cytokinetic apparatus during cell division. It remains to be demonstrated whether the Ser2481 autophosphorylation may allow mTOR to adapt to target other mitotic proteins, and the precise mechanism of regulation of mTOR autophosphorylation at Ser2481 during mitosis and cytokinesis remains unclear. However, the fact that M phase-associated upregulation of phospho-mTORSer2481 occurs in human cancers in vivo,Citation17 together with the confirmatory finding that prolonged but not acute treatment with rapamycin drastically reduces the amount of mTOR-associated Ser2481 autophosphorylation, may open a new avenue to better understand the role of mTOR phosphorylation and mTOR signaling complexes in relation to their pharmacological targeting with rapalogs to prevent and/or treat cell division-related diseases, including cancer and aging.Citation36-Citation47 These findings should also be particularly valuable, given that adult stem cell divisions during an organism’s lifespan can greatly impact longevity and aging.
Materials and Methods
Cell lines and culture conditions
LKB1+/+ A431 human epidermoid cancer cells and LKB1−/− HeLa human cervical cancer cells were obtained from the American Type Culture Collection (ATCC). Cells were routinely grown in Dulbecco's modified Eagle's medium (Gibco) containing 10% heat-inactivated fetal bovine serum (FBS, Bio-Whittaker), 1% L-glutamine, 1% sodium pyruvate, 50 U/mL penicillin and 50 μg/mL streptomycin. Cells were maintained at 37°C in a humidified atmosphere of 95% air and 5% CO2. Cells were screened periodically for Mycoplasma contamination.
Reagents
Rapamycin and nocodazole were purchased from Sigma Chemical Co. The following antibodies were used in this study: rabbit polyclonal anti-total mTOR (#2972; Cell Signaling Technology, Inc.); rabbit polyclonal anti-phospho-mTOR (S2448) (49F9) (#2976; Cell Signaling Technology, Inc.); rabbit polyclonal anti-phospho-mTOR (S2481) (#2974; Cell Signaling Technology); rabbit polyclonal anti-mTOR (phospho-S2481) (ab45996; Abcam®); rabbit polyclonal anti-phospho-mTOR (Ser2481) (Cat. No. 09–343; Merck Millipore); mouse monoclonal anti-p-FRAP (296.Ser2481) (Cat. No. sc-293132; Santa Cruz Biotechnology); mouse monoclonal anti-Aurora B (Cat. No. ab3635; Abcam plc); mouse monoclonal anti-INCENP (Cat. No. 39–2800; clone 58–217; Zymed Laboratories Inc.) and mouse monoclonal anti-phospho-histone H3 (Ser10) (Cat. No. 97065; clone 6G3; Cell Signaling Technology). The mTOR peptide-phospho S2481 (ab46195) was purchased from Abcam®.
Immunoblot analysis
Western blots were performed using an SDS-PAGE Electrophoresis System as described previously,Citation18 employing anti-total mTOR or anti-phospho-mTORSer2481, as specified. Immunoblotting experiments were repeated at least three times, and the blots were re-probed for β-actin (Santa Cruz Biotechnology, Cat. #sc-47778) to control for protein loading and transfer. Densitometric values of protein bands were quantified using Scion Image software (Scion Corporation).
Immunofluorescence staining and high-content confocal imaging
Cells were seeded at approximately 3,000 cells/well in 96-well clear-bottom imaging tissue culture plates (Becton Dickinson Biosciences) optimized for automated imaging applications. Triton® X-100 permeabilization and blocking, primary antibody staining, secondary antibody staining using Alexa Fluor® 488/594 goat anti-rabbit/mouse IgGs (Invitrogen, Probes) and counterstaining (using Hoechst 33258; Invitrogen) were performed according to BD Biosciences protocols. Images were captured in different channels for Alexa Fluor® 488 (pseudo-colored green), Alexa Fluor® 594 (pseudo-colored red) and Hoechst 33258 (pseudo-colored blue) using a BD PathwayTM 855 Bioimager System (Becton Dickinson Biosciences) with a 20x or 40x objective (NA 075 Olympus). Merged images were obtained according to the Recommended Assay Procedure using BD AttovisionTM software.
Acknowledgments
This work was financially supported through funding from the Instituto de Salud Carlos III [Ministerio de Sanidad y Consumo, Fondo de Investigación Sanitaria (FIS), Spain, Grants CP05–00090 and PI06–0778 and RD06–0020–0028); the Fundación Científica de la Asociación Española Contra el Cáncer (AECC, Spain) and the Ministerio de Ciencia e Innovación (SAF2009–11579, Plan Nacional de I+D+ I, MICINN, Spain]. A.V.-M. received the Sara Borrell post-doctoral contract (CD08/00283, Ministerio de Sanidad y Consumo, FIS, Spain). S.C. received a Research Fellowship (Formación de Personal Investigador, FPI) from the Ministerio de Ciencia e Innovación (MICINN, Spain).
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- Bjornsti MA, Houghton PJ. The TOR pathway: a target for cancer therapy. Nat Rev Cancer 2004; 4:335 - 48; http://dx.doi.org/10.1038/nrc1362; PMID: 15122205
- Wullschleger S, Loewith R, Hall MN. TOR signaling in growth and metabolism. Cell 2006; 124:471 - 84; http://dx.doi.org/10.1016/j.cell.2006.01.016; PMID: 16469695
- Sabatini DM. mTOR and cancer: insights into a complex relationship. Nat Rev Cancer 2006; 6:729 - 34; http://dx.doi.org/10.1038/nrc1974; PMID: 16915295
- Guertin DA, Sabatini DM. Defining the role of mTOR in cancer. Cancer Cell 2007; 12:9 - 22; http://dx.doi.org/10.1016/j.ccr.2007.05.008; PMID: 17613433
- Bhaskar PT, Hay N. The two TORCs and Akt. Dev Cell 2007; 12:487 - 502; http://dx.doi.org/10.1016/j.devcel.2007.03.020; PMID: 17419990
- Hall MN. mTOR-what does it do?. Transplant Proc 2008; 40:Suppl S5 - 8; http://dx.doi.org/10.1016/j.transproceed.2008.10.009; PMID: 19100909
- Peterson RT, Beal PA, Comb MJ, Schreiber SL. FKBP12-rapamycin-associated protein (FRAP) autophosphorylates at serine 2481 under translationally repressive conditions. J Biol Chem 2000; 275:7416 - 23; http://dx.doi.org/10.1074/jbc.275.10.7416; PMID: 10702316
- Chiang GG, Abraham RT. Phosphorylation of mammalian target of rapamycin (mTOR) at Ser-2448 is mediated by p70S6 kinase. J Biol Chem 2005; 280:25485 - 90; http://dx.doi.org/10.1074/jbc.M501707200; PMID: 15899889
- Holz MK, Blenis J. Identification of S6 kinase 1 as a novel mammalian target of rapamycin (mTOR)-phosphorylating kinase. J Biol Chem 2005; 280:26089 - 93; http://dx.doi.org/10.1074/jbc.M504045200; PMID: 15905173
- Copp J, Manning G, Hunter T. TORC-specific phosphorylation of mammalian target of rapamycin (mTOR): phospho-Ser2481 is a marker for intact mTOR signaling complex 2. Cancer Res 2009; 69:1821 - 7; http://dx.doi.org/10.1158/0008-5472.CAN-08-3014; PMID: 19244117
- Sarbassov DD, Ali SM, Sengupta S, Sheen JH, Hsu PP, Bagley AF, et al. Prolonged rapamycin treatment inhibits mTORC2 assembly and Akt/PKB. Mol Cell 2006; 22:159 - 68; http://dx.doi.org/10.1016/j.molcel.2006.03.029; PMID: 16603397
- Soliman GA, Acosta-Jaquez HA, Dunlop EA, Ekim B, Maj NE, Tee AR, et al. mTOR Ser-2481 autophosphorylation monitors mTORC-specific catalytic activity and clarifies rapamycin mechanism of action. J Biol Chem 2010; 285:7866 - 79; http://dx.doi.org/10.1074/jbc.M109.096222; PMID: 20022946
- Yaba A, Bianchi V, Borini A, Johnson J. A putative mitotic checkpoint dependent on mTOR function controls cell proliferation and survival in ovarian granulosa cells. Reprod Sci 2008; 15:128 - 38; http://dx.doi.org/10.1177/1933719107312037; PMID: 18276949
- Yu J, Yaba A, Kasiman C, Thomson T, Johnson J. mTOR controls ovarian follicle growth by regulating granulosa cell proliferation. PLoS One 2011; 6:e21415; http://dx.doi.org/10.1371/journal.pone.0021415; PMID: 21750711
- Doghman M, El Wakil A, Cardinaud B, Thomas E, Wang J, Zhao W, et al. Regulation of insulin-like growth factor-mammalian target of rapamycin signaling by microRNA in childhood adrenocortical tumors. Cancer Res 2010; 70:4666 - 75; http://dx.doi.org/10.1158/0008-5472.CAN-09-3970; PMID: 20484036
- Vazquez-Martin A, Oliveras-Ferraros C, Bernadó L, López-Bonet E, Menendez JA. The serine 2481-autophosphorylated form of mammalian Target Of Rapamycin (mTOR) is localized to midzone and midbody in dividing cancer cells. Biochem Biophys Res Commun 2009; 380:638 - 43; http://dx.doi.org/10.1016/j.bbrc.2009.01.153; PMID: 19285014
- Lopez-Bonet E, Vazquez-Martin A, Pérez-Martínez MC, Oliveras-Ferraros C, Pérez-Bueno F, Bernadó L, et al. Serine 2481-autophosphorylation of mammalian target of rapamycin (mTOR) couples with chromosome condensation and segregation during mitosis: confocal microscopy characterization and immunohistochemical validation of PP-mTOR(Ser2481) as a novel high-contrast mitosis marker in breast cancer core biopsies. Int J Oncol 2010; 36:107 - 15; PMID: 19956839
- Vazquez-Martin A, López-Bonet E, Oliveras-Ferraros C, Pérez-Martínez MC, Bernadó L, Menendez JA. Mitotic kinase dynamics of the active form of AMPK (phospho-AMPKalphaThr172) in human cancer cells. Cell Cycle 2009; 8:788 - 91; http://dx.doi.org/10.4161/cc.8.5.7787; PMID: 19221486
- Vazquez-Martin A, Oliveras-Ferraros C, Menendez JA. The active form of the metabolic sensor: AMP-activated protein kinase (AMPK) directly binds the mitotic apparatus and travels from centrosomes to the spindle midzone during mitosis and cytokinesis. Cell Cycle 2009; 8:2385 - 98; http://dx.doi.org/10.4161/cc.8.15.9082; PMID: 19556893
- Vazquez-Martin A, Oliveras-Ferraros C, Lopez-Bonet E, Menendez JA. AMPK: Evidence for an energy-sensing cytokinetic tumor suppressor. Cell Cycle 2009; 8:3679 - 83; http://dx.doi.org/10.4161/cc.8.22.9905; PMID: 19844168
- Vazquez-Martin A, Oliveras-Ferraros C, Cufí S, Menendez JA. Polo-like kinase 1 regulates activation of AMP-activated protein kinase (AMPK) at the mitotic apparatus. Cell Cycle 2011; 10:1295 - 302; http://dx.doi.org/10.4161/cc.10.8.15342; PMID: 21474997
- Menendez JA, Vazquez-Martin A. AMPK: A bona fide resident of the mitotic spindle midzone. Cell Cycle 2012; 11:841 - 2; http://dx.doi.org/10.4161/cc.11.5.19461; PMID: 22336917
- Vazquez-Martin A, Cufí S, Oliveras-Ferraros C, Menendez JA. Polo-like kinase 1 directs the AMPK-mediated activation of myosin regulatory light chain at the cytokinetic cleavage furrow independently of energy balance. Cell Cycle 2012; 11:2422 - 6; http://dx.doi.org/10.4161/cc.20438; PMID: 22672936
- Pinter K, Jefferson A, Czibik G, Watkins H, Redwood C. Subunit composition of AMPK trimers present in the cytokinetic apparatus: Implications for drug target identification. Cell Cycle 2012; 11:917 - 21; http://dx.doi.org/10.4161/cc.11.5.19412; PMID: 22333580
- Carmena M. A mitosis-specific AMPK trimer?. Cell Cycle 2012; 11:1269; http://dx.doi.org/10.4161/cc.19902; PMID: 22426423
- Banko MR, Allen JJ, Schaffer BE, Wilker EW, Tsou P, White JL, et al. Chemical genetic screen for AMPKα2 substrates uncovers a network of proteins involved in mitosis. Mol Cell 2011; 44:878 - 92; http://dx.doi.org/10.1016/j.molcel.2011.11.005; PMID: 22137581
- Robitaille AM, Hall MN. Ramping up mitosis: an AMPKα2-regulated signaling network promotes mitotic progression. Mol Cell 2012; 45:8 - 9; http://dx.doi.org/10.1016/j.molcel.2011.12.018; PMID: 22244327
- Wrighton KH. AMPK moonlights in mitosis. Nat Rev Mol Cell Biol 2012; 13:64; PMID: 22251904
- Thaiparambil JT, Eggers CM, Marcus AI. AMPK regulates mitotic spindle orientation through phosphorylation of myosin regulatory light chain. Mol Cell Biol 2012; 32:3203 - 17; http://dx.doi.org/10.1128/MCB.00418-12; PMID: 22688514
- Schmidt T, Wahl P, Wüthrich RP, Vogetseder A, Picard N, Kaissling B, et al. Immunolocalization of phospho-S6 kinases: a new way to detect mitosis in tissue sections and in cell culture. Histochem Cell Biol 2007; 127:123 - 9; http://dx.doi.org/10.1007/s00418-006-0255-5; PMID: 17136413
- Egervári G, Márk A, Hajdu M, Barna G, Sápi Z, Krenács T, et al. Mitotic lymphoma cells are characterized by high expression of phosphorylated ribosomal S6 protein. Histochem Cell Biol 2011; 135:409 - 17; http://dx.doi.org/10.1007/s00418-011-0803-5; PMID: 21424608
- Rossi R, Pester JM, McDowell M, Soza S, Montecucco A, Lee-Fruman KK. Identification of S6K2 as a centrosome-located kinase. FEBS Lett 2007; 581:4058 - 64; http://dx.doi.org/10.1016/j.febslet.2007.07.047; PMID: 17678899
- Gwinn DM, Asara JM, Shaw RJ. Raptor is phosphorylated by cdc2 during mitosis. PLoS One 2010; 5:e9197; http://dx.doi.org/10.1371/journal.pone.0009197; PMID: 20169205
- Ramírez-Valle F, Badura ML, Braunstein S, Narasimhan M, Schneider RJ. Mitotic raptor promotes mTORC1 activity, G(2)/M cell cycle progression, and internal ribosome entry site-mediated mRNA translation. Mol Cell Biol 2010; 30:3151 - 64; http://dx.doi.org/10.1128/MCB.00322-09; PMID: 20439490
- Vazquez-Martin A, Cufí S, Oliveras-Ferraros C, Menendez JA. Raptor, a positive regulatory subunit of mTOR complex 1, is a novel phosphoprotein of the rDNA transcription machinery in nucleoli and chromosomal nucleolus organizer regions (NORs). Cell Cycle 2011; 10:3140 - 52; http://dx.doi.org/10.4161/cc.10.18.17376; PMID: 21900751
- Blagosklonny MV. Aging and immortality: quasi-programmed senescence and its pharmacologic inhibition. Cell Cycle 2006; 5:2087 - 102; http://dx.doi.org/10.4161/cc.5.18.3288; PMID: 17012837
- Blagosklonny MV. An anti-aging drug today: from senescence-promoting genes to anti-aging pill. Drug Discov Today 2007; 12:218 - 24; http://dx.doi.org/10.1016/j.drudis.2007.01.004; PMID: 17331886
- Blagosklonny MV. Prevention of cancer by inhibiting aging. Cancer Biol Ther 2008; 7:1520 - 4; http://dx.doi.org/10.4161/cbt.7.10.6663; PMID: 18769112
- de Magalhães JP, Faragher RG. Cell divisions and mammalian aging: integrative biology insights from genes that regulate longevity. Bioessays 2008; 30:567 - 78; http://dx.doi.org/10.1002/bies.20760; PMID: 18478536
- Blagosklonny MV. Validation of anti-aging drugs by treating age-related diseases. Aging (Albany NY) 2009; 1:281 - 8; PMID: 20157517
- Blagosklonny MV. Aging-suppressants: cellular senescence (hyperactivation) and its pharmacologic deceleration. Cell Cycle 2009; 8:1883 - 7; http://dx.doi.org/10.4161/cc.8.12.8815; PMID: 19448395
- Blagosklonny MV. TOR-driven aging: speeding car without brakes. Cell Cycle 2009; 8:4055 - 9; http://dx.doi.org/10.4161/cc.8.24.10310; PMID: 19923900
- Blagosklonny MV. Rapamycin and quasi-programmed aging: four years later. Cell Cycle 2010; 9:1859 - 62; http://dx.doi.org/10.4161/cc.9.10.11872; PMID: 20436272
- Blagosklonny MV. Molecular damage in cancer: an argument for mTOR-driven aging. Aging (Albany NY) 2011; 3:1130 - 41; PMID: 22246147
- Menendez JA, Cufí S, Oliveras-Ferraros C, Vellon L, Joven J, Vazquez-Martin A. Gerosuppressant metformin: less is more. Aging (Albany NY) 2011; 3:348 - 62; PMID: 21483040
- Menendez JA, Vellon L, Oliveras-Ferraros C, Cufí S, Vazquez-Martin A. mTOR-regulated senescence and autophagy during reprogramming of somatic cells to pluripotency: a roadmap from energy metabolism to stem cell renewal and aging. Cell Cycle 2011; 10:3658 - 77; http://dx.doi.org/10.4161/cc.10.21.18128; PMID: 22052357
- Blagosklonny MV. Cell cycle arrest is not yet senescence, which is not just cell cycle arrest: terminology for TOR-driven aging. Aging (Albany NY) 2012; 4:159 - 65; PMID: 22394614