Abstract
Normal hematopoiesis is suppressed during the development of leukemia. In the T-ALL leukemia mouse model described in our recent study (Hu X, et al. Blood 2009), the impacts of leukemic environment on normal hematopoietic stem cells (HSCs) and hematopoietic progenitor cells (HPCs) were distinct, in that normal HSCs were preserved in part because of increased mitotic quiescence of HSCs and resulting exhaustion of HPCs proliferation. Stem cell factor (SCF) secreted by leukemic cells in Nalm6 B-ALL model was previously suggested to force normal HSCs/HPCs out of their bone marrow niches and allow leukemic cells to occupy the niches (Colmone A, et al. Science 2008). Here we found that stem cell factor (SCF) expression in PB and BM of T-ALL model was increased, but SCF mRNA and protein levels in normal hematopoietic cells were higher than those in leukemia cells, which suggested that upregulated SCF was mainly contributed by non-leukemic cells in response to the leukemia development. To further elucidate the molecular mechanisms, microarray analysis was conducted on normal HSCs in this model and verified by real-time RT-PCR. The expression of Hes1 and its downstream target p21 were elevated in normal HSCs, whereas their expression showed no significant alteration in HPCs. Interestingly, although overexpression of Hes1 by retroviral infection inhibited the in vitro colony formation of normal hematopoietic cells, in vivo results demonstrated that normal Lin- cells and HSPCs were better preserved when normal Lin- cells with Hes1 overexpression were co-transplanted with T-ALL leukemia cells. Our results suggested that the differential expression of Hes1 between HSCs and HPCs resulted in the distinct responses of these cells to the leukemic condition, and that overexpression of Hes1 could enhance normal HSPCs in the leukemic environment.
Keywords: :
Introduction
Hematopoiesis is strictly regulated by microenvironment factors and intracellular regulators.Citation1-Citation4 Abnormal regulation of hematopoiesis leads to various kinds of hematopoietic disorders.Citation5,Citation6 Leukemia is regarded as a clonal diseaseCitation7 and many intrinsic and extrinsic factors have been verified to play parts in the initiation of leukemia. During leukemogenesis, leukemia cells outcompete their normal counterparts and become dominant by high capacity for self-renewal and low capacity for apoptosis.Citation8 In bone marrow and other hematopoietic tissues where leukemic cells accumulate, normal hematopoiesis is inhibited.
The impact and mechanism of leukemic environment on normal hematopoiesis, especially on normal HSCs and HPCs during leukemogenesis are of great interest. Previous reports demonstrated that leukemia cells that occupied bone marrow niches secreted a great quantity of SCF, which disrupted the behavior of normal HPCs in the human Nalm-6 pre-B acute lymphoblastic leukemia model and therapeutic inhibition of HPC interaction with tumor niches may help maintain normal HPC function in the setting of malignancy.Citation9 We also studied the kinetics of normal HSCs and HPCs in the Notch1-induced murine T-cell leukemia model. Although normal hematopoiesis in the leukemic environment was progressively decreased, the effects of the leukemic environment on normal HSCs and HPCs were different, i.e., the reconstitution potential of normal HSCs from the leukemic environment was preserved, while HPCs were exhausted.Citation10 Cell cycle analysis revealed that normal HSCs in T-ALL leukemia mice were kept in a more quiescent state, while normal HPCs in leukemia mice demonstrated accelerated proliferation. However, the molecular mechanism has not been established.
Cell cycle regulators have been demonstrated to be intrinsically involved in the quiescence of HSCs. Cyclin-dependent kinase inhibitors (CKIs) participate in the sequential activation and inactivation of cyclin-dependent kinases, which are central to the progression of cell cycle. They have been shown to be important in HSCs.Citation11-Citation14 Perturbation of these regulatory circuits may result in changes in stem cell proliferation.Citation15 For instance, p21, a member of CKIs, has been demonstrated to play a role in HSCs in the transition out of the cell cycle and maintenance in G0.Citation16-Citation18 Mammalian homologs of Drosophila hairy and enhancer of split 1 (Hes1) is upstream mediator of p21.Citation19 Hes1 was reported to affect cell differentiation and maintain the cells in an immature state in various tissues,Citation20 including the hematopoietic tissue. It was reported that overexpression of Hes1 inhibited cycling of hematopoietic stem and progenitor cells (HSPCs) in vitro and cell expansion in vivo, associated with upregulation of p21.Citation21
To study the molecular mechanism on how leukemia environment affect HSPCs in Notch1-induced murine T-ALL model, we analyzed SCF expression and underwent microarray analysis on HSCs. Upregulations of both Hes1 and p21 were detected in normal HSCs under leukemic condition. Furthermore, the role of Hes1 on normal Lin- cells, HPCs and HSCs in leukemia mice was studied by transducing Hes1 into mouse Lin- cells and then co-transplanting with leukemia cells to establish the Hes1 leukemia model. Though upregulation of Hes1 in mouse HSPCs inhibited cell proliferation in vitro, it partially preserved normal HSPCs in Notch1-induced leukemia mice.
Results
SCF was secreted by normal hematopoietic cells in T-ALL mice
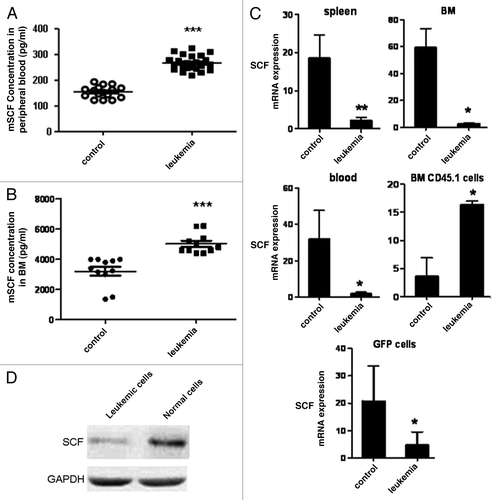
SCF was reported to play important roles on the localization and maintenance of HSCs. Furthermore, it was reported that SCF secreted by Nalm-6 cells constituted the major source of SCF in the malignant microenvironment and therapeutic targeting of SCF may increase the hematopoietic reserve and improve outcomes in BM transplantation and autologous stem cell harvest in the setting of hematologic malignancy.Citation9 To assess whether SCF expression was upregulated in the leukemic environment in our model, we performed ELISA to determine SCF concentration in peripheral blood (PB) serum and bone marrow (BM) samples from leukemia and control mice 10 d after transplantation. The serum SCF level in PB was about (154 ± 2.1) pg/ml in control mice while it was around (268 ± 4.3) pg/ml in leukemia mice (). Furthermore, the BM SCF level was estimated to be (3,200 ± 18.2) pg/ml in control mice while it was around (5,054 ± 22.1) pg/ml in leukemia mice (). These results demonstrated that SCF was upregulated in the Notch1-induced leukemia model. However, SCF mRNA expression was decreased in total bone marrow, peripheral blood and spleen samples, where leukemia cells accounted for the most, and sorted BM leukemia cells were detected. In contrast, a 4.5-fold increase was observed in normal hematopoietic cells when compared with CD45.1+ counterpart cells from control mice (). The level of SCF protein was higher in normal cells compared with the leukemia cells from leukemia mice model (), which suggested that high levels of SCF was contributed by normal cells rather than the malignant cells.
Figure 1. Analyses of SCF expression in T-ALL mice. To assess whether SCF expression was upregulated in the leukemic environment in our model, we performed ELISA to determine SCF concentration in PB serum and BM samples from leukemia and control mice 10 d after transplantation (A and B). The expression of SCF in BM, spleen and blood cells as well as CD45.1+ BM cells and GFP+ BM cells was analyzed by real-time PCR (C). The level of SCF protein in leukemia and normal cells was determined by western blot (D).
Gene expression analyses of normal HSCs in leukemic hosts
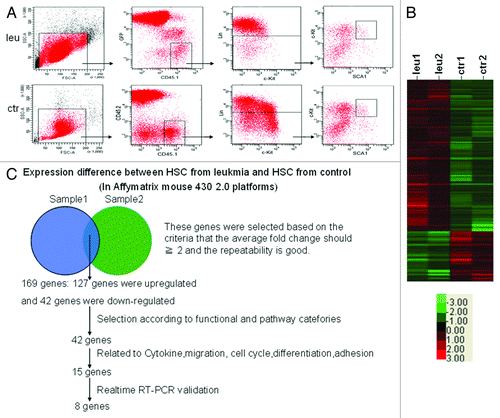
To further study the molecular mechanism how normal HSCs was affected under the Notch1-induced murine T-cell leukemic environment, global gene expression profiling was performed to compare the gene expression differences between normal HSCs from leukemia and control mice. Normal HSCs (CD45.1+CD45.2-GFP-LKS+) from leukemia or control mice were sorted (). The differentially expressed genes in normal HSCs were identified by global expression pattern analyses with two independent microarrays (). The strategy we used to integrate the gene expression data was summarized in . One hundred and sixty-nine genes were expressed differently in normal HSCs in leukemic mice from that in control mice (fold change ≥ 2, score ≥ 2), including 127 genes upregulated and 42 genes downregulated. Among them, 42 genes were selected according to their function and pathway categories. Eight of 15 genes related to cytokine, migration, cell cycle, differentiation and adhesion were verified by real-time PCR. The relative level of each gene expression in normal HSCs from control mice was normalized to 1.
Figure 2. Gene profiling analyses of normal HSCs in leukemic environment. Normal HSCs were obtained from control and leukemia mice on day 10 after transplantation (A). The clustering map showed the genes differently expressed in normal HSCs between leukemia and control group. Red color meant the genes were upregulated while green color meant the genes were downregulated (B). Schematic representation of the analyses strategies integrating expression profiles obtained from two independent experiments (C).
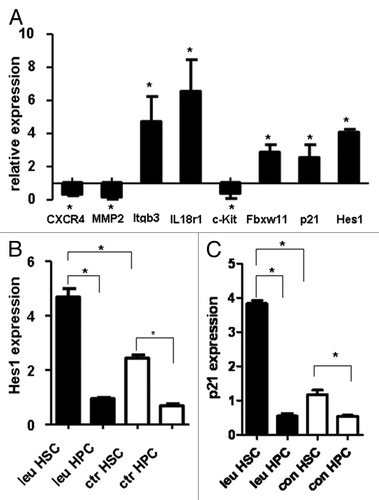
Our results showed that CXCR4, Mmp2 and c-Kit were downregulated, while Itgb3, IL18r1, Fbxw11, p21 and Hes1 were upregulated, which was consistent with the results from our microarray analysis (CXCR4, p = 0.003; Mmp2, p = 0.005; Itgb3, p = 0.018; IL18r1, p = 0.017; c-Kit, p = 0.02; Fbxw11, p = 0.002; p21, p = 0.03; Hes1, p = 0.001, ).
Figure 3. Validation of the results of microarray by real-time PCR. Real-time RT-PCR was used to validate the expression pattern of eight genes using specific murine primers detailed in (A). Quantitative analysis of Hes1 and p21 expression in normal HSCs and HPCs in leukemia and control mice was shown in (B and C). Data are shown as means ± SD *, p < 0.05, statistical analysis was performed using Student’s t-test.
The HSCs-enriched subset in leukemia mice expressed higher levels of Hes1 and p21
Our previous work showed that normal HSCs from leukemic mice were in a more quiescent state while HPCs were exhausted. Hes1 and p21, both cell cycle inhibitors,Citation21 were upregulated in HSC under leukemic environment. It’s interesting to compare the expression of them between HSCs and HPCs under leukemic and normal environment. Using real-time PCR, we examined expression of Hes1 and p21 in FACS-sorted HSCs and HPCs populations from leukemia and control mice. The results showed that Hes1 expression in the HSCs-enriched population was higher than that in HPCs-enriched population from both leukemia (p = 0.003) and control mice (p = 0.002). More importantly, its expression in HSCs-enriched population from leukemia mice was 2-fold higher than that from control mice (p = 0.0024), while no significant difference could be found in HPCs-enriched population between leukemia and control mice (). Similarly, the expression of p21, which is the downstream target of Hes1, showed the same pattern (). These results suggested that the different responses of Hes1 in HSCs and HPCs in leukemic environment might contribute to the different fates of these two populations in leukemic environment.
Increased expression of Hes1 inhibited colony formation in vitro
To test the roles of Hes1 on mice HSPCs, retrovector-expressing Hes1 with GFP as reporter was constructed. After DNA sequencing to verify the construct, the plasmid was transiently transfected into 293T cells and Hes1 expression in GFP+ cells was analyzed by real-time PCR. The blank retrovector was used as a control. As expected, the FACS-sorted Hes1-transduced (GFP+) cells expressed approximately 5-fold higher levels of Hes1, as compared with the endogenous level of Hes1 measured in blank-transduced cells (p < 0.0001, ). The expression of p21 was also upregulated. However, no significant difference in the expression of CXCR4, Mmp2, Itgb3, Il18r1, c-Kit and Fbxw11 could be detected between Hes1+ and control cells ().
Figure 4. In vitro CFC study on Hes1-transduced HSPCs and HSCs. Infection rates were assessed using transduction of 293T cells. After infection, the cells were analyzed with fluorescence microscope (A) and GFP+ cells were sorted for the expression of Hes1 and other related genes (B). Lin- cells (C) and LSK cells (D) of B6.SJL mice were transduced with Hes1 or control retrovector. Successfully transduced (GFP+) cells were enriched by FACS sorting and plated in standard methylcellulose-containing CFC assays. Data are shown as means ± SD ***, p < 0.0001, statistical analysis was performed using Student’s t-test.
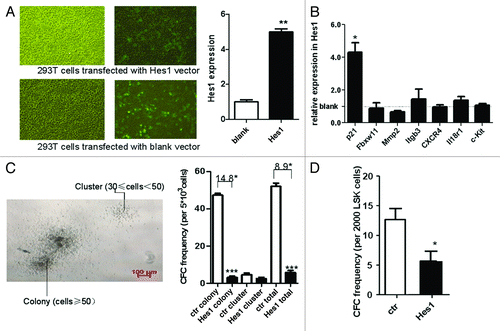
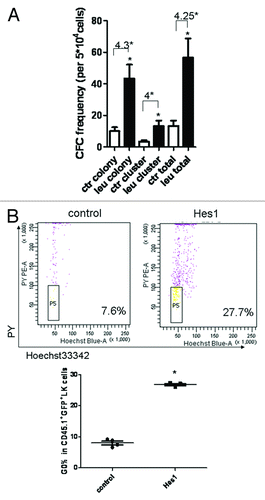
To determine the effects of enforced Hes1 expression on the proliferation of mice HSPCs, and HSCs, Hes1 or blank MSCV transduced Lin- cells and LSK cells were sorted before the CFC assay. We defined cluster (30 ≤ cell number < 50) and colony (cell number ≥ 50). The colony number was 52 ± 1.732 in blank retrovector transduced Lin- cells, while it was 5.8 ± 1.128 in Hes1 transduced Lin- cells (p < 0.0001, ). Also, the total number (colony plus cluster) by Hes1-transduced Lin- cells was lower. Furthermore, the colony number was 12.9 ± 2.548 in blank retrovector transduced HSCs, while it was 6.4 ± 2.274 in Hes1 transduced HSCs (p < 0.0001, ). These results demonstrated that overexpression of Hes1 could inhibit the growth of HPCs and HSCs in vitro.
Normal HSPC was protected in Hes1 mice model
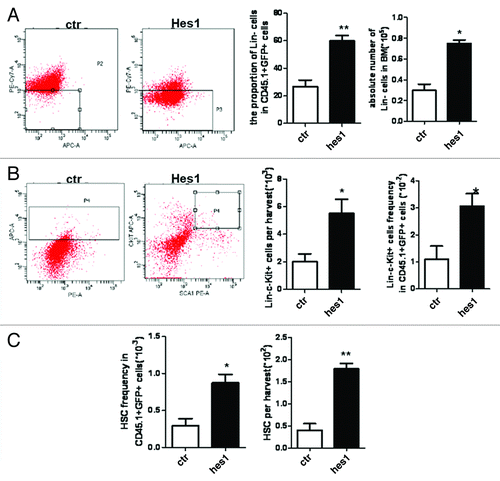
Though the in vitro study suggested that the growth of HSPCs was downregulated, it was interesting to know the in vivo effects of overexpressed Hes1 on HSPCs under the leukemic environment. The Hes1-transduced Lin- cells were co-transplanted with Notch1-induced leukemia cells and the mice were sacrificed on day 10 after transplantation to analyze the proportion and absolute number of Lin- cells and HSPCs in BM. The absolute number and the proportion of Lin-Hes1-GFP+ cells in CD45.1+GFP+ cells in Hes1 mice was higher compared with control mice (). Moreover, the absolute number and the frequency of Lin-c-Kit+ cells (HSPCs) in CD45.1+GFP+ cells in Hes1 mice was higher than that in control mice (p = 0.02, ). Similar results could be observed in HSC population (). We then studied the function of normal HSPCs in different groups. CD45.1+GFP+ BM cells from Hes1 and control groups 10 d after transplantation were isolated for the assessment of CFCs. The frequency of CFCs was dramatically higher in Hes1 group than that in control group (p < 0.0001, ). These results suggest that overexpression of Hes1 in normal HSPCs during leukemia development could protect normal HSPCs against leukemic environment.
Figure 6. HSCs/HPCs with Hes1 overexpression were preserved under leukemic environment. The Hes1-transduced mouse model is described in detail in Materials and Methods. On day 10 after transplantation, the absolute number and proportion of normal CD45.1+GFP+Lin- cells (A), CD45.1+ GFP+ HSPCs (B), CD45.1+ GFP+ HSCs (C) were analyzed with BD FACS Aria II.
Increased quiescence of Hes1+ HSPCs in leukemic hosts
Since both of in vivo and in vitro assay revealed that Hes1 affected the proliferation of HSPCs, the quiescent fraction of HSPCs in cell cycle under leukemic environment was examined using the DNA dye Hoechst and the RNA dye PY, which has been used for the measurement of stem cell quiescence (G0 phase in cell cyele). CD45.1+GFP+LK+ cells from Hes1 leukemia-bearing hosts in the early stage of leukemia demonstrated a larger fraction of cells in G0 phase than that in control mice (), thereby suggesting that Hes1 is able to maintain HSPCs to be quiescent in the leukemic environment.
Discussion
During leukemogenesis, normal hematopoiesis attenuates while leukemia cells become dominant. However, the impact of the leukemic environment on normal HSCs and HPCs, which is an issue of high significance, is not well-understood. Although we recently reported that the reactions of HSCs and HPCs to the leukemic environment were distinct in T-ALL leukemia, the molecular mechanisms underlying the distinction were not clear.Citation10
SCF, also called mast cells growth factor (MGF), Kit ligand (KL) and Steel factor (SLF), is an acidic glycoprotein secreted by the bone marrow stromal cells. SCF is produced by a wide variety of solid tumors. It’s reported that AML cell lines and primary AML cells have been shown to produce SCF RNA transcripts. Colmone et al. found leukemic cells constituted the major source of SCF in the malignant microenvironment in Nalm-6 pre-B acute lymphoblastic leukemia mouse model and therapeutic targeting of SCF may increase the hematopoietic reserve.Citation9 In contrast to their report, we found that although SCF was higher in Notch1-induced leukemia mice, it was secreted by normal cells in response to the leukemia environment. Considering the differences between the two models, our result suggested that different mechanisms might contribute to the malignant leukemia microenvironment, even though SCF overexpression could be observed in both cases. A recent study demonstrated that SCF can be secreted by perivascular and endothelial cell subsets and these cells served as critical niches for HSCs in homeostatic conditions.Citation22 Further investigation regarding the role of SCF secreted by microenviromental cells is needed.
Then we changed our focus on the gene profile of HSC. After microarray analysis, several genes were selected for the validation by real-time RT-PCR. These genes have been classified into different functions and pathways. CXCR4, Mmp2, c-Kit and Itgb3 are mainly related to HSCs homing, while the function of IL18r1 on HSCs is still unknown.Citation23-Citation28 Hes1 and p21 belong to the same signaling pathway, which is critical in the regulation of tissue renewal and maintenance, especially in the cell cycle regulation of HSCs. Hes1 functions upstream of p21. Loss of p21 may initially expand the stem cell population, since p21 plays a crucial role in maintaining stem cell quiescence.Citation29 Both Hes1 and p21 were upregulated in normal HSCs in our model analyzed by microarray and validated by real-time RT-PCR, suggesting that this pathway is important for the maintenance of normal hematopoiesis in leukemic environment.
Hes1 is a member of basic helix-loop-helix transcription factors, which belongs to the Hes family.Citation30 Its roles in embryogenesis,Citation31,Citation32 chronic myelogenous leukemia,Citation33 development of perinatal T cellsCitation34 and normal hematopoiesis were reported. HES proteins generally act as repressors of transcription.Citation35 It has been reported that Hes1 was involved in cell cycle, and maintained multi-potent precursor cells in an undifferentiated state in several tissues during development and adulthood.Citation36 Hes1 was expressed at high levels in HSC-enriched subpopulation, while at low levels in more mature progenitor cell populations. Hes1 overexpression inhibited cell cycling in vitro and cell expansion in vivo, and long-term HSC reconstitution function was preserved.Citation21 However, these data were obtained in normal hematopoiesis, and its roles in disease conditions, especially in leukemic conditions, have not been studied. Previously, we demonstrated that normal HPCs exhausted after undergoing an accelerated proliferation process, whereas, the function of HSCs is better preserved partially because of increased quiescence in cell cycle.Citation10 Correspondingly, the expression of Hes1 and its downstream factor p21 of normal HSCs under leukemic environment was not only much higher than that of normal HPCs from leukemic environment, but also higher than that of HSCs from normal environment. Our data suggested that in our leukemia model, increased expression of Hes1 might preserve normal HSC function by maintaining them in G0/G1 phase, whereas unchanged expression of Hes1 can’t stop normal HPCs going into rapid proliferation. As insufficient HSCs differentiated into HPCs, HPCs exhausted at the late stage of leukemia.
In order to further evaluate the effect of overexpression of Hes1 on HSPCs under leukemic environment, we infected mouse BM Lin- cells with Hes1-overexpressing retrovirus. Overexpression of Hes1 reduced the proliferation of HSPCs by in vitro CFC experiment, which was consistent with previous reports that enforced Hes1 expression inhibited cell cycling with G1 arrest.Citation21,Citation33,Citation37 Moreover, the functionality of Hes1 in the context of leukemia development was further assessed. Enforced expression of Hes1 in normal Lin- cells in leukemia mice resulted in an increased number of Lin- cells and HSPCs. To study the function of HSPCs in Hes1 mice model, CFC assay was performed, demonstrating that the frequency of CFCs was higher in the Hes1 group than that in control group. It’s worth noting that the two CFC experiments were designed to explain two different things. In the in vitro CFC assay, the same amount of HSPCs, either transduced with Hes1 or blank vector, were seeded to see the difference between colony formations, which explained the inhibition effect of overexpressed Hes1 on HSPCs. In contrast, in the co-transplant mice CFC assay, the same amount of CD45.1+Hes1+ cells (please note that the absolute numbers of CD45.1+Hes1+ HSPCs, especially non-G0/G1 phase HSPCs were different) were seeded to see functional HSPCs could be preserved, which explained that overexpression of Hes1 could preserve HSPCs under leukemia condition. Since the proportion and absolute number of Hes1-GFP+ HSPC in BM were higher than that of blank-GFP+ HSPC in control mice BM, the observation that CFC number of Hes1 mice was higher than that of control mice is reasonable, although Hes1 maintained HSPCs in quiescence under Nothc1-induced leukemic environment. The results demonstrated that in T-ALL leukemia mice, overexpression of Hes1 partially protected the function of HSPCs.
In summary, our data indicated that overexpression of Hes1 in normal HSPCs in T-ALL leukemia mice could partially maintain the number and function of normal HSPCs through the p21 signaling pathway. Our findings also suggested one of the molecular mechanisms governing the different responses of HSCs and HPCs under T-ALL leukemic condition. Further studies aiming to define defending mechanism(s) of normal HSCPs in different types of leukemia are under way.
Materials and Methods
Animals, cell lines and antibodies
Wild-type C57BL/6J (CD45.2) mice were obtained from the Experimental Animal Center of Chinese Academy of Medical Sciences, and B6.SJL-PtprcaPepcb/BoyJ mice (B6.SJL, CD45.1) were purchased from the Jackson Laboratory. All mice were maintained in the certified animal facility of the Institute of Hematology, Chinese Academy of Medical Science. All mice used in the experiments were female. The procedures involved in the animal work were approved by the Animal Care and Use Committee at the institutions involved in this study. 293T cell line was purchased from Cell Center, Chinese Academy of Medical Sciences. All the antibodies were purchased from eBioscience unless otherwise noted.
Notch1-induced murine T-ALL leukemia model
The Notch1-induced murine T-ALL leukemia model was established as previously reported.Citation10 Briefly, bone marrow nucleated cells (BMNCs; 107/host) from B6.SJL mice at the age of 6–8 wk were transplanted into lethally irradiated (9.5 Gy) female C57BL/6J recipients (6–8 wk old) with 106 intracellular domain of Notch1 (ICN1) plasmid (MSCV-ICN1-IRES-GFP)-transduced Lin-Sca-1+ cells from C57BL/6J mice. In control group, recipients (C57BL/6J mice) were transplanted with 108 BMNCs from C57BL/6J mice and 107 BMNCs from B6.SJL mice. On day 7 after transplantation, GFP+ immature T cells (CD4+CD8+) accumulated in peripheral blood (PB), spleen and bone marrow (BM) of leukemia group. On day 10 after transplantation, GFP+ leukemia cells in BM accounted for 75–85%.
Enzyme-linked immunosorbent assay (ELISA)
On day 10 after transplantation, peripheral blood were obtained from the eye vein of mice of each group and then clotted at room temperature for 2 h before centrifuging for 20 min at 2,000 g to collect serum samples. For BM samples, each femur was flushed with 500 ul phosphate-buffered saline (PBS), and the suspension was centrifuged at 1,300 rpm to remove hematopoietic cells. Peripheral serum and BM samples were assayed immediately using Quantikine mouse SCF Kit (R&D Systems).
Western blot
Total proteins of 106 normal and leukemia cells sorted from leukemia mice model were obtained using PARIS Protein and RNA isolation kit (Ambion) according to the manufacturer’s instructions. Aliquots of protein extracts were loaded onto 4–20% Criterion Precast Gel (BioRad). After electrophoresis and transfer onto Hybond-P membrane (Amersham Biosciences), SCF were blotted, followed by GAPDH to confirm equal protein loading. The antibodies were diluted at 1:200 for rabbit IgG against SCF (Abcam) and 1:5,000 for GAPDH (CST). The blots were visualized using ECL Western Blotting Detection Reagents (Amersham Biosciences).
Flow cytometric analysis and cell sorting
On day10 after transplantation, mice were sacrificed and BM cells were obtained by flushing ilias, femurs and tibias. HSCs and HPCs were defined by the immunophenotypes as Lin-c-Kit+Sca1+ (LKS+) and Lin-c-Kit+Sca1- (LKS-), respectively. To isolate HSCs and HPCs, BM cells were first enriched using the Lin-conjugated Immunomagnetic Negative Selection Kit (CD3, CD4, CD8, B220, Gr-1, Mac-1, Ter-119; Miltenyi Biotec) according to the manufacturer’s instructions. Then the negatively selected cells were stained with PE-Cy5.5-conjugated CD45.1, FITC-conjugated CD45.2, PE-Cy7-conjugated lineage (CD3, CD4, CD8, B220, Gr-1, Mac-1, Ter-119), PE-conjugated Sca1, APC-conjugated c-Kit antibodies. CD45.1+GFP-CD45.2- LKS+ (normal HSCs) were sorted with FACS Aria II sorter (BD Biosciences) for gene microarray analyses (Affymatrix mouse 430 2.0). CD45.1+GFP-CD45.2-LKS+ (normal HSCs) and CD45.1+ GFP-CD45.2-LKS- (normal HPCs) cells were sorted directly to the tubes and lysed for real-time polymerase chain reaction (PCR). During the sorting procedure, DAPI was used to exclude the dead cells ().
Microarray analyses
Total RNA was extracted from normal HSCs from leukemia and control mice and evaluated in duplicate with Mouse 430 2.0 Genechips (Affymetrix). Microarray data was analyzed with the Bioconductor package. The Rank Prod program was used to select differentially expressed genes with a cutoff P value of 0.01 and an estimated false-positive rate of 0.05. Gene annotation was obtained from Ingenuity Pathway Analysis and Gene Ontology Annotation databases.
Real-time RT-PCR analyses
Total RNA was extracted with the Rneasy mini kit (Qiagen) or Trizol (Invitrogen) according to the manufacturer’s instructions. BM, spleen and blood cells, as well as CD45.1+ BM cells and GFP+ BM cells after sorted, were used for SCF analyses. Approximately 3,000 HSCs and 7 × 104 HPCs were sorted before RNA extraction for gene expression analyses. Reverse transcription was achieved using QuantiTect Reverse Transcription Kit (Qiagen). Real-time PCR was performed using an ABI-Prism 7500 Sequence Detector (Applied Biosystems). Typically, 5 ng of reverse-transcribed cDNA per sample was used in triplicate. The parameters for the thermal cycling of PCR were as follows: 15 sec at 95°C and 60 sec at 60°C, 45 cycles. The sequences of all the primers were listed in .
Table 1. The sequences of primers
Construction of MSCV-Hes1-IRES-GFP vector
Wild-type Hes1 cDNA was obtained by RT-PCR from BM samples of female B6.SJL mice. The MSCV-IRES-GFP vector (provided by Dr. David Scadden, Harvard University) was used to construct Hes1. Total RNA of CD45.1 mice BM cells was extracted with Trizol (Invitrogen) and then reverse transcripted using Superscript III (Invitrogen). The following primers were used for amplifying Hes1 (GenBank NM008235) fragments: forward primer 5′-GGACTAGT ATG CCA GCT GAT ATA ATG GAG-3′, reverse primer 5′-GAAGATCT AGG TGG GCT AGG GAC TTT AC-3′. The MSCV-IRES-GFP vector was ligased with Hes1 cDNA for 12 h. The identities of the Hes1 constructs were confirmed by DNA sequencing. Retrovector production and multiplicity of infection were assessed using transient transduction of 293T cells. After transduction, cells were analyzed with fluorescence microscope and GFP+ cells were sorted for expression of Hes1 and other related genes.
Transduction of murine primary BM cells
The plasmid (MSCV-Hes1-IRES-GFP or MSCV-IRES-GFP used as blank vector) was co-transfected into package cell line 293T with pCMV-VSV-G and pKAT, using lipofectamine 2000 (Invitrogen). Virus supernatant was harvested 48 h and 72 h after transfection. BM cells from B6.SJL mice were enriched with biotin-conjugated lineage Immunomagnetic Beads Kit. Lin- cells were cultured in IMDM with 50 ng/ml mSCF, 25 ng/ml Flt-3 and 10 ng/ml TPO for 24 h, and then transduced with virus supernatant. Forty-eight hours after transduction, cells were sorted by FACS (BD Aria II), based on GFP fluorescence.
Establishment of Hes1-transduced mice models and in vivo assay
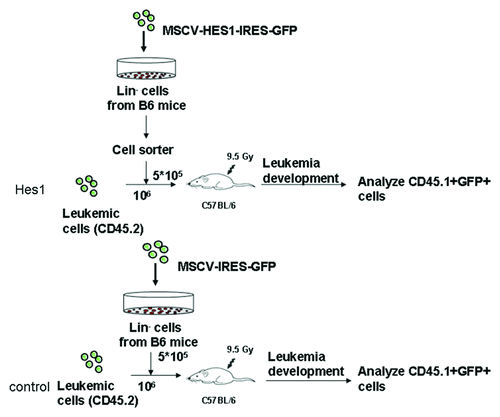
To test the function of Hes1 on normal HSPCs in T-ALL leukemic mice, Hes1-transduced mice models were established. Lin- cells from B6.SJL mice transduced with either MSCV-Hes1-IRES-GFP or blank-GFP plasmid were sorted for Hes1-GFP+ or blank-GFP+ cells (CD45.1) and then injected into lethally irradiated C57BL/6J (CD45.2) by tail vein at a quantity of 2 × 105 cells per mouse with 106 cells of Notch1-induced leukemia cells (CD45.2) (). On day 10 after transplantation, mice were sacrificed and BM cells were stained with PerCP-Cy5.5 CD45.1, FITC CD45.2, PE-Cy7 Lin, PE Sca1, APC c-Kit antibodies. The proportion and absolute number of CD45.1+GFP+Lin- cells and CD45.1+GFP+ HSPCs were analyzed with BD FACS Aria II. GFP+CD45.1+ cells were then sorted for Colony-forming cell (CFC) assay.
Figure 5. Establishment of Hes1-transduced mouse model. Lin- cells from B6.SJL mice at the age of 6–8 wk were transduced with either MSCV-Hes1-IRES-GFP or blank plasmid for 72 h. After transduction, Hes1-GFP+ or blank-GFP+ cells (CD45.1) were sorted and then injected into lethally irradiated C57BL/6J (CD45.2) by tail vein at a quantity of 2 × 105 cells per mouse with 106 cells of Notch1-induced leukemia cells (CD45.2).
Colony-forming cell assay
To study the in vitro effects of Hes1 on mouse HPCs and HSCs, Lin- cells and LSK cells of B6.SJL mice were transduced with Hes1 or blank retrovector. Successfully transduced (GFP+) cells were enriched by FACS sorting and plated in standard methylcellulose-containing CFC assays. The cells were placed into 24-well plate in 0.5 ml methylcellulose medium M3434 (StemCell Technologies) at a density of 104 Lin- cells or 2,000 LSK cells/ml in tetraplicate. On day 10, the CFC colonies were counted under a reverse microscope.
To study the in vivo effects of Hes1 on the function of mouse HSPCs in leukemic environment, BM CD45.1+GFP+Lin- cells on day 10 after transplantation were sorted from Hes1 or control mice and placed into 24-well plate at a density of 105 cells/ml for CFC assay.
Cell cycle analyses
For cell cycle analyses of the HSPCs with Hes1 overexpression in the leukemia environment, the whole BM cells from Hes1 or control mice model were isolated on day 5 after transplantation. Then the cells were permeabilized and stained with Hoechst33342, followed by 1 ug/ml PY. The proportion of CD45.1+GFP+Lin-c-Kit+ cells (HSPCs) in G0 phase was determined by flow cytometry with quantitation of DNA and RNA.
Statistical analysis
Data were presented as means ± SD. The significant difference was examined using the Student’s t-test. p values of less than 0.05 were considered to be significant. Statistical analyses were performed using GraphPad Prism version 4.0 (GraphPad Software, www.graphpad.com).
Acknowledgments
The work was supported by the Grants 2011CB964801, 2009CB521803, 2010DFB30270 and 2012CB96600 from the Ministry of Science and Technology of China, Grants 81090410, 90913018, 30825017 and 81130074 from the National Natural Science Foundation of China (NSFC), Grants 09ZCZDSF03800 and 11JCZDJC18200 from Tianjin Science and Technology Programs. T.C. was a recipient of the Scholar Award from the Leukemia and Lymphoma Society (2008–2013).
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Contributions
C.T. performed the experiments, analyzed the data and wrote the paper; G.Z. analyzed the data, co-supervised the research work, revised the paper; Z.C., Q.L. contributed to the leukemia model; Z.J. performed the flow cytometry work and analyzed the data; J.W. performed some of the flow cytometry work; W.Y. analyzed the data and co-supervised the research work; T.C. designed the experiments, analyzed the data, revised the paper and oversaw the overall project.
References
- Lapidot T. Mechanism of human stem cell migration and repopulation of NOD/SCID and B2mnull NOD/SCID mice. The role of SDF-1/CXCR4 interactions. Ann N Y Acad Sci 2001; 938:83 - 95; http://dx.doi.org/10.1111/j.1749-6632.2001.tb03577.x; PMID: 11458529
- Christopherson KW 2nd, Hangoc G, Mantel CR, Broxmeyer HE. Modulation of hematopoietic stem cell homing and engraftment by CD26. Science 2004; 305:1000 - 3; http://dx.doi.org/10.1126/science.1097071; PMID: 15310902
- Calvi LM, Adams GB, Weibrecht KW, Weber JM, Olson DP, Knight MC, et al. Osteoblastic cells regulate the haematopoietic stem cell niche. Nature 2003; 425:841 - 6; http://dx.doi.org/10.1038/nature02040; PMID: 14574413
- Iscove NN, Nawa K. Hematopoietic stem cells expand during serial transplantation in vivo without apparent exhaustion. Curr Biol 1997; 7:805 - 8; http://dx.doi.org/10.1016/S0960-9822(06)00341-1; PMID: 9368765
- Rujkijyanont P, Beyene J, Wei K, Khan F, Dror Y. Leukaemia-related gene expression in bone marrow cells from patients with the preleukaemic disorder Shwachman-Diamond syndrome. Br J Haematol 2007; 137:537 - 44; http://dx.doi.org/10.1111/j.1365-2141.2007.06608.x; PMID: 17539775
- Chatterjee S, Dutta RK, Basak P, Das P, Das M, Pereira JA, et al. Alteration in marrow stromal microenvironment and apoptosis mechanisms involved in aplastic anemia: an animal model to study the possible disease pathology. Stem Cells Int 2010; 2010:932354; http://dx.doi.org/10.4061/2010/932354; PMID: 21048856
- Notta F, Mullighan CG, Wang JC, Poeppl A, Doulatov S, Phillips LA, et al. Evolution of human BCR-ABL1 lymphoblastic leukaemia-initiating cells. Nature 2011; 469:362 - 7; http://dx.doi.org/10.1038/nature09733; PMID: 21248843
- Cheng T. Cell cycle inhibitors in normal and tumor stem cells. Oncogene 2004; 23:7256 - 66; http://dx.doi.org/10.1038/sj.onc.1207945; PMID: 15378085
- Colmone A, Amorim M, Pontier AL, Wang S, Jablonski E, Sipkins DA. Leukemic cells create bone marrow niches that disrupt the behavior of normal hematopoietic progenitor cells. Science 2008; 322:1861 - 5; http://dx.doi.org/10.1126/science.1164390; PMID: 19095944
- Hu X, Shen H, Tian C, Yu H, Zheng G, XuFeng R, et al. Kinetics of normal hematopoietic stem and progenitor cells in a Notch1-induced leukemia model. Blood 2009; 114:3783 - 92; http://dx.doi.org/10.1182/blood-2009-06-227843; PMID: 19652197
- Sherr CJ. G1 phase progression: cycling on cue. Cell 1994; 79:551 - 5; http://dx.doi.org/10.1016/0092-8674(94)90540-1; PMID: 7954821
- Sherr CJ, Roberts JM. Inhibitors of mammalian G1 cyclin-dependent kinases. Genes Dev 1995; 9:1149 - 63; http://dx.doi.org/10.1101/gad.9.10.1149; PMID: 7758941
- Boyer MJ, Cheng T. The CDK inhibitors: potential targets for therapeutic stem cell manipulations?. Gene Ther 2008; 15:117 - 25; http://dx.doi.org/10.1038/sj.gt.3303064; PMID: 17989702
- Zou P, Yoshihara H, Hosokawa K, Tai I, Shinmyozu K, Tsukahara F, et al. p57(Kip2) and p27(Kip1) cooperate to maintain hematopoietic stem cell quiescence through interactions with Hsc70. Cell Stem Cell 2011; 9:247 - 61; http://dx.doi.org/10.1016/j.stem.2011.07.003; PMID: 21885020
- Li J. Quiescence regulators for hematopoietic stem cell. Exp Hematol 2011; 39:511 - 20; http://dx.doi.org/10.1016/j.exphem.2011.01.008; PMID: 21288477
- Cheng T, Rodrigues N, Shen H, Yang Y, Dombkowski D, Sykes M, et al. Hematopoietic stem cell quiescence maintained by p21cip1/waf1. Science 2000; 287:1804 - 8; http://dx.doi.org/10.1126/science.287.5459.1804; PMID: 10710306
- Yu H, Yuan Y, Shen H, Cheng T. Hematopoietic stem cell exhaustion impacted by p18 INK4C and p21 Cip1/Waf1 in opposite manners. Blood 2006; 107:1200 - 6; http://dx.doi.org/10.1182/blood-2005-02-0685; PMID: 16234365
- Cheng T, Shen H, Rodrigues N, Stier S, Scadden DT. Transforming growth factor beta 1 mediates cell-cycle arrest of primitive hematopoietic cells independent of p21(Cip1/Waf1) or p27(Kip1). Blood 2001; 98:3643 - 9; http://dx.doi.org/10.1182/blood.V98.13.3643; PMID: 11739168
- Sang L, Coller HA, Roberts JM. Control of the reversibility of cellular quiescence by the transcriptional repressor HES1. Science 2008; 321:1095 - 100; http://dx.doi.org/10.1126/science.1155998; PMID: 18719287
- Tomita K, Hattori M, Nakamura E, Nakanishi S, Minato N, Kageyama R. The bHLH gene Hes1 is essential for expansion of early T cell precursors. Genes Dev 1999; 13:1203 - 10; http://dx.doi.org/10.1101/gad.13.9.1203; PMID: 10323870
- Yu X, Alder JK, Chun JH, Friedman AD, Heimfeld S, Cheng L, et al. HES1 inhibits cycling of hematopoietic progenitor cells via DNA binding. Stem Cells 2006; 24:876 - 88; http://dx.doi.org/10.1634/stemcells.2005-0598; PMID: 16513761
- Ding L, Saunders TL, Enikolopov G, Morrison SJ. Endothelial and perivascular cells maintain haematopoietic stem cells. Nature 2012; 481:457 - 62; http://dx.doi.org/10.1038/nature10783; PMID: 22281595
- Nie Y, Han YC, Zou YR. CXCR4 is required for the quiescence of primitive hematopoietic cells. J Exp Med 2008; 205:777 - 83; http://dx.doi.org/10.1084/jem.20072513; PMID: 18378795
- Sugiyama T, Kohara H, Noda M, Nagasawa T. Maintenance of the hematopoietic stem cell pool by CXCL12-CXCR4 chemokine signaling in bone marrow stromal cell niches. Immunity 2006; 25:977 - 88; http://dx.doi.org/10.1016/j.immuni.2006.10.016; PMID: 17174120
- Kipreos ET, Pagano M. The F-box protein family. Genome Biol 2000; 1:S3002; http://dx.doi.org/10.1186/gb-2000-1-5-reviews3002; PMID: 11178263
- Barbash O, Diehl JA. SCF(Fbx4/alphaB-crystallin) E3 ligase: when one is not enough. Cell Cycle 2008; 7:2983 - 6; http://dx.doi.org/10.4161/cc.7.19.6775; PMID: 18818515
- Onoyama I, Nakayama KI. Fbxw7 in cell cycle exit and stem cell maintenance: insight from gene-targeted mice. Cell Cycle 2008; 7:3307 - 13; http://dx.doi.org/10.4161/cc.7.21.6931; PMID: 18948752
- Matsuoka S, Oike Y, Onoyama I, Iwama A, Arai F, Takubo K, et al. Fbxw7 acts as a critical fail-safe against premature loss of hematopoietic stem cells and development of T-ALL. Genes Dev 2008; 22:986 - 91; http://dx.doi.org/10.1101/gad.1621808; PMID: 18367647
- Stier S, Cheng T, Forkert R, Lutz C, Dombkowski DM, Zhang JL, et al. Ex vivo targeting of p21Cip1/Waf1 permits relative expansion of human hematopoietic stem cells. Blood 2003; 102:1260 - 6; http://dx.doi.org/10.1182/blood-2002-10-3053; PMID: 12702511
- Davis RL, Turner DL. Vertebrate hairy and Enhancer of split related proteins: transcriptional repressors regulating cellular differentiation and embryonic patterning. Oncogene 2001; 20:8342 - 57; http://dx.doi.org/10.1038/sj.onc.1205094; PMID: 11840327
- Jarriault S, Brou C, Logeat F, Schroeter EH, Kopan R, Israel A. Signalling downstream of activated mammalian Notch. Nature 1995; 377:355 - 8; http://dx.doi.org/10.1038/377355a0; PMID: 7566092
- Jarriault S, Le Bail O, Hirsinger E, Pourquié O, Logeat F, Strong CF, et al. Delta-1 activation of notch-1 signaling results in HES-1 transactivation. Mol Cell Biol 1998; 18:7423 - 31; PMID: 9819428
- Nakahara F, Sakata-Yanagimoto M, Komeno Y, Kato N, Uchida T, Haraguchi K, et al. Hes1 immortalizes committed progenitors and plays a role in blast crisis transition in chronic myelogenous leukemia. Blood 2010; 115:2872 - 81; http://dx.doi.org/10.1182/blood-2009-05-222836; PMID: 19861684
- Kaneta M, Osawa M, Sudo K, Nakauchi H, Farr AG, Takahama Y. A role for pref-1 and HES-1 in thymocyte development. J Immunol 2000; 164:256 - 64; PMID: 10605019
- Kageyama R, Ohtsuka T, Kobayashi T. The Hes gene family: repressors and oscillators that orchestrate embryogenesis. Development 2007; 134:1243 - 51; http://dx.doi.org/10.1242/dev.000786; PMID: 17329370
- van Es JH, van Gijn ME, Riccio O, van den Born M, Vooijs M, Begthel H, et al. Notch/gamma-secretase inhibition turns proliferative cells in intestinal crypts and adenomas into goblet cells. Nature 2005; 435:959 - 63; http://dx.doi.org/10.1038/nature03659; PMID: 15959515
- Zanotti S, Smerdel-Ramoya A, Canalis E. HES1 (hairy and enhancer of split 1) is a determinant of bone mass. J Biol Chem 2011; 286:2648 - 57; http://dx.doi.org/10.1074/jbc.M110.183038; PMID: 21084301