Abstract
Chronic stress is known to have a profound negative impact on human health and has been suggested to influence a number of disease states. However, the mechanisms underlying the deleterious effects of stress remain largely unknown. Stress is known to promote the release of epinephrine, a catecholamine stress hormone that binds to β2-adrenergic receptors (β2ARs) with high affinity. Our previous work has demonstrated that chronic stimulation of a β2AR-β-arrestin-1-mediated signaling pathway by infusion of isoproterenol suppresses p53 levels and impairs genomic integrity. In this pathway, β-arrestin-1, which is activated via β2ARs, facilitates the AKT-mediated activation of Mdm2 and functions as a molecular scaffold to promote the binding and degradation of p53 by the E3-ubiquitin ligase, Mdm2. Here, we show that chronic restraint stress in mice recapitulates the effects of isoproterenol infusion to reduce p53 levels and results in the accumulation of DNA damage in the frontal cortex of the brain, two effects that are abrogated by the β-blocker, propranolol and by genetic deletion of β-arrestin-1. These data suggest that the β2AR-β-arrestin-1 signaling pathway may represent an attractive therapeutic target to prevent some of the negative consequences of stress in the treatment of stress-related disorders.
Stress Response Pathways
More than 70 y ago, Hans SelyeCitation1 first recognized a clinical constellation of “nonspecific disease features” that occur in response to acute or chronic stress and referred to these collectively as “the stress response.” The stress response is mediated primarily by two systems: the hypothalamic-pituitary-adrenocortical (HPA) axis and the autonomic nervous system (ANS). Secreted hormones from these systems (corticosteroids and catecholamines, respectively) can affect every system of the body that expresses the relevant receptors. The stress response in the ANS is primarily mediated by stimulation of the sympathetic nervous system (SNS) and the subsequent release of the catecholamine hormone, epinephrine and the neurotransmitter, norepinephrine. Epinephrine binds to the β2-subtype of adrenergic receptors (β2ARs) with high affinity.
The catabolic, lipogenic, anti-reproductive and immunosuppressive effects of HPA axis activation allow an organism to resist and recover from stress and challenges to homeostasis. SNS activation and secretion of epinephrine facilitate the “fight-or-flight” response. However, the stress response in humans also engenders unwanted effects.Citation2 Because of the nature of chronic psychosocial stress, aspects of the stress response may continue for an extended period, leading to prolonged secretion of stress hormones and consequent potentially undesirable effects to affected individuals. Although chronic stress represents a major risk factor for a number of human diseases [e.g., Alzheimer disease,Citation3 type 2 diabetes,Citation4 cardiovascular disease,Citation5 cancerCitation6 and post-traumatic stress disorder (PTSD)Citation7,Citation8], its clinical importance remains controversial due, at least in part, to difficulties associated with conducting standardized human behavioral studies.
Accumulation of DNA Damage via β2AR-Mediated Stress Response Pathways
β2ARs are prototypical G-protein-coupled receptors (GPCRs)Citation9-Citation11 that are identified throughout the body, including in the brain, lung, skeletal muscle and bone marrow.Citation12,Citation13 The β2ARs regulate numerous physiological processes, such as glycogenolysis in the liverCitation14 or relaxation of vascular smooth muscle.Citation15 Stimulation of the β2AR leads to Gs-dependent adenylyl cyclase activation and cAMP production, followed by the activation of protein kinase A (PKA).Citation11,Citation16 Desensitization of the β2AR is conferred by G-protein-coupled receptor kinase (GRK)-dependent phosphorylation,Citation17 followed by the recruitment of β-arrestins,Citation18 which are known to function as independent signal transducers in addition to their roles in receptor desensitization.Citation19,Citation20 The E3-ubiquitin ligase Mdm2 catalyzes the transient ubiquitination of β-arrestins, which is required for clathrin-mediated β2AR internalization.Citation21
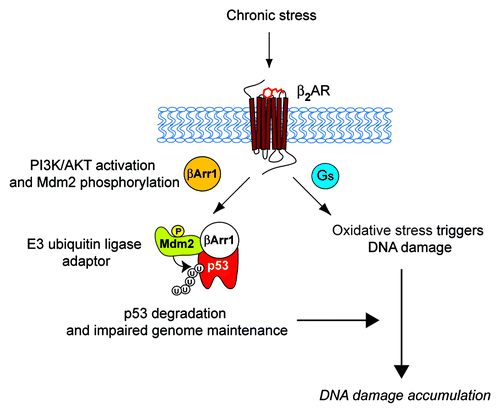
Recently, we elucidated a novel molecular mechanism by which chronic stimulation of β2AR activates Gs-PKA and β-arrestin-1-mediated signaling pathways, which trigger DNA damage and degrade p53, respectively. The activation of these two pathways synergistically leads to accumulation of DNA damage ().Citation22 Stimulation of the β2AR leads to β-arrestin-1-mediated activation of AKT and phosphorylation of Mdm2 at Ser 166, which results in the activation of Mdm2. Mdm2 activation then leads to β-arrestin-1-facilitated ubiquitination and degradation of p53, during which β-arrestin-1 acts as an E3-ubiquitin ligase adaptor. Meanwhile, stimulation of β2AR leads to the production of reactive oxygen species by NAD(P)H oxidase,Citation23 activation of adenylyl cyclase and PKA signaling (the downstream targets of the β2AR-G-protein signaling cascade) and the promotion of oxidative stress via the suppression of antioxidative mechanisms.Citation24 Thus, upon chronic secretion of catecholamines and stimulation of β2ARs, G-protein and β-arrestin cascades lead to increased oxidative stress and decreased p53 levels, respectively, thus lowering genome maintenance and increasing DNA damage and de novo genomic rearrangements.Citation22 β-arrestin-1 plays a dual role in this process. First, cytosolic β-arrestin-1 mediates the activation of PI3K/AKT upon β2AR stimulation. Second, nuclear β-arrestin-1 scaffolds Mdm2 to p53, facilitating the ubiquitination and degradation of p53.
Figure 1. Schematic diagram of β2AR-dependent regulation of DNA damage in the catecholamine-mediated stress response. The stress response and activation of the sympathetic nervous system (SNS) release catecholamine stress hormones, such as epinephrine, which activate β2AR and its downstream signaling pathways. This initiates activation of Gs-PKA signaling and cytosolic β-arrestin-1 (βArr1: orange)-mediated activation of PI3K/AKT signaling leading to phosphorylation and activation of Mdm2. βArr1 in the nucleus (white) facilitates the Mdm2-mediated nuclear export and degradation of p53, compromising genome maintenance.Citation22 Meanwhile, stimulation of β2AR leads to production of reactive oxygen species by NAD(P)H oxidase,Citation23 and activation of adenylyl cyclase and PKA signaling promotes oxidative stress by suppressing antioxidative mechanisms.Citation24 Thus, these two independent G-protein and β-arrestin-mediated pathways synergistically affect the accumulation of DNA damage. P, phosphorylation at ser 166 on Mdm2; U, ubiquitination.
A Behavioral Stress Model Leads to a β2AR-β-Arrestin-1 Signaling Cascade-Mediated Accumulation of DNA Damage
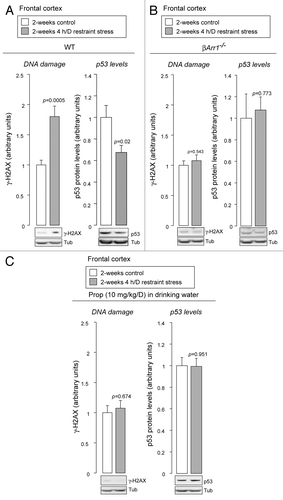
In our previous study, chronic administration of catecholamines was used to mimic the conditions under which stress leads to the stimulation of β2ARs.Citation22 To examine β2AR-β-arrestin-1 signaling cascades under more physiological conditions, we utilized a chronic restraint stress model, which has been shown to promote secretion of epinephrine and norepinephrine.Citation25 After 2 wk of restraint stress, we observe an accumulation of DNA damage and reduced levels of p53 in the brain frontal cortex (), which is a site particularly susceptible to chronic stress.Citation26 These responses are mediated through a β2AR-β-arrestin-1 signaling cascade, because genetic deletion of β-arrestin-1 abrogates these effects (). Furthermore, administering propranolol, a blood-brain-barrier permeant β-blocker,Citation27 also prevents these effects, indicating that βARs stimulated by endogenous β-adrenergic catecholamines are responsible ().
Figure 2. Behavioral stress leads to accumulation of DNA damage and reduced levels of p53. (A) Restraint stress leads to accumulation of DNA damage and lowering levels of p53. After restraint stress, wild-type mice (WT) were sacrificed, brains were removed, and brain frontal cortex was dissected. Their homogenates were analyzed by immunoblotting with indicated antibodies. DNA damage was examined by phosphorylation of histone H2AX (γ-H2AX), one of the earliest indicators of DNA damage.Citation63 Mean ± SEM. Student’s t-test, two-tailed. n = 10 for each condition. (B) Genetic deletion of β-arrestin-1 abrogates the DNA damage accumulation and p53 degradation induced by restraint stress. After restraint stress, β-arrestin-1-knockout mice (βArr1−/−) were sacrificed, brains were removed, and brain frontal cortex was dissected. Mean ± SEM. Student’s t-test, two-tailed. n = 5 for each condition. (C) Propranolol, a β-blocker, inhibits the accumulation of DNA damage and p53 degradation induced by restraint stress. Propranolol (Prop) was administered to WT, starting 2 d before the first exposure to restraint stress. Mean ± SEM. Student’s t-test, two-tailed. n = 10 for each condition.
Recently, Feng et al.Citation28 reported that chronic restraint stress decreases p53 protein levels and promotes tumorigenesis in mice exposed to ionizing radiation (IR). Interestingly, the authors found that the HPA axis-dependent glucocorticoids induce activation of SGK1 (serum- and glucocorticoid-induced protein kinase 1), followed by phosphorylation of Mdm2 at Ser 166, which facilitates p53 degradation. Because β-adrenergic catecholamines also lead to phosphorylation of Mdm2 at Ser 166,Citation22 both the SNS and the HPA axis, with β-adrenergic catecholamines and glucocorticoids, respectively, may synergistically affect Mdm2 function, thus lowering p53 levels.Citation29
β-Blockers
β-blockers, which are antagonists of β1- and/or β2-adrenergic receptors, are widely prescribed therapeutic agents for the chronic treatment of heart failure, in which they act via poorly understood mechanisms to afford cardioprotection.Citation30 Interestingly, clinical and epidemiological studies suggest that β-blockers have additional therapeutic benefits. For example, chronic β-blocker therapy is associated with lower incidences of prostate cancerCitation31 and reduced metastasis, tumor recurrence and specific mortality in breast cancer.Citation32 Treatment with the β-blocker propranolol has been recently used as an effective therapy for an infantile vascular tumor, hemangiomas, causing regression.Citation33 Behavioral stress in mice increases ovarian tumor growth, and this effect is inhibited by administering the β-blocker propranolol or RNA interference (RNAi) against β2AR.Citation34 In a mouse stroke model for middle cerebral artery occlusion (MCAO), administering the β2AR-selective β-blocker ICI 118,551 or genetic deletion of β2AR decrease post-ischemic brain injury.Citation35 In psychiatric disorders, treatment with the β-blocker propranolol decreases PTSD after traumaCitation8 and anxiety-like behavior by repeated social defeat.Citation7 These studies implicate diverse effects of β-blockers that potentially involve inhibition of either or both G-protein and β-arrestin signaling cascades.
The data we present here demonstrate that administering the non-subtype selective β-blocker propranolol prevents behavioral stress-induced accumulation of DNA damage and degradation of p53 (). These effects are, at least in part, mediated through inhibition of a β2AR-β-arrestin-1 signaling cascade, because genetic deletion of β-arresin-1 shows effects similar to β-blockade (). β-blockers have been developed as antagonists for βARs, mainly targeting G-protein signaling. Our data highlight the therapeutic potential of β-blockers, also targeting effects of β-arrestin-1 signaling that affects p53 levels and genome integrity during chronic stress.
β-Arrestin-1, p53 and Mdm2
The role of β-arrestin-1 as a negative regulator of p53Citation22,Citation36 is in contrast to a previously reported role for β-arrestin-2, which has been shown to anchor Mdm2 in the cytosol and thereby stabilize nuclear p53.Citation37,Citation38 These strikingly different results may simply reflect the distinct cellular localization of these isoforms (β-arrestin-2 is confined to the cytosol). The fact that the addition of a nuclear export signal to β-arrestin-1 abolished its effect on p53Citation22 strongly supports this interpretation. Alternatively, these differences may be indicative of a very specific, and yet to be fully appreciated, role for the different β-arrestin isoforms downstream of specific receptors in different physiologically relevant pathways.Citation39,Citation40 Furthermore, the identification of β-arrestin-1 as an E3-ubiquitin ligase adaptor in the nucleus, adds to the growing list of nuclear roles of β-arrestin-1.Citation41 It has been shown that nuclear β-arrestin-1 regulates transcription of p27, c-fos and Bcl-2 by scaffolding the transcription factor CREB (cAMP response element-binding) and the acetyl transferase p300, which acetylates histone H4 and activates gene expression.Citation42,Citation43 β-arrestin-1 also sequesters the polycomb group (PcG) recruiter YY1, relieving PcG-mediated gene repression.Citation44
p53, the “guardian of the genome,”Citation45 regulates cell cycle arrest, DNA repair and/or apoptosis incurred under both basal and genotoxic conditions via transcription-dependent and -independent mechanisms.Citation46 Although we have investigated a role of p53 in genome maintenance, it is conceivable that other aspects of p53 function are also affected by the prolonged decreases in p53 levels caused by catecholamines. These include, but are not limited to, the induction of cell death by PUMA (p53-upregulated modulator of apoptosis),Citation47,Citation48 the regulation of metabolic pathways by TIGAR (TP53-induced glycolysis and apoptosis regulator)Citation49 and SCO2 (synthesis of cytochrome c oxidase 2),Citation50 centrosome duplicationCitation51 and the regulation of maternal reproduction through LIF (leukemia-inhibitory factor).Citation52 Furthermore, p63, a p53 homolog, has been shown to protect the female germ line during meiotic arrest.Citation53 It remains to be determined whether catecholamine hormones affect the stability of p53 homologs.
Mdm2 is one of the most well-established regulators of p53.Citation54 The catalytic activity of Mdm2 is regulated by a variety of interacting molecules, such as the acetyl transferase, p300,Citation55 or the tumor suppressor, ARF.Citation56,Citation57 In our previous study,Citation22 we showed that with chronic administration of catecholamines to mimic stress-induced β2AR-stimulated conditions, β-arrestin-1 plays a key role in the Mdm2-p53 cascade, facilitating the binding of Mdm2 and p53 in the nucleus and the resulting degradation of p53. This is reminiscent of the role of β-arrestins in the cytosol, where they serve as adaptors for Mdm2-dependent ubiquitination and degradation of its substrates.Citation58-Citation60 In addition, recent studies have shown that β-arrestins and related molecules serve as adaptors for other E3-ubiquitin ligases.Citation61 These studies indicate that β-arrestins may have a general role as scaffolding molecules in diverse ubiquitination pathways.
Conclusions
Our results provide a plausible explanation of the mechanism through which chronic stress and prolonged activation of the SNS can influence genomic integrity. Catecholamine hormones secreted during chronic stress activate β2ARs and trigger both G-protein and β-arrestin-1 signaling cascades. The former influences oxidative stress,Citation23,Citation24 and the latter influences genome maintenance by regulating p53.Citation22,Citation36 The demonstration of the effects of stress hormones on DNA damage represents a conceptual as well as a tangible advance. Such a process could not only explain the development of some human disorders and conditions, such as aging or cancer, but also suggests a model supporting potential prophylactic and/or therapeutic interventions. Consistent with this hypothesis, our current study showed that the β-blocker propranolol inhibited DNA damage accumulation in response to stress. Considering the pleiotropic effects of β2ARs, selectively targeting β-arrestin-1, one of its signaling arms, could represent an attractive therapeutic approach to prevent/block/ameliorate the negative consequences of stress.
In addition to the therapeutic relevance of our findings, the evidence that the accumulation of DNA damage induced by behavioral stress involves the β2AR-β-arrestin-1 system provides support for the concept that the β-arrestin-1 signaling cascade may play an important role in helping to maintain the integrity of the genome.
Materials and Methods
Reagents
Propranolol was purchased from Sigma. Antibodies were obtained from the following companies: anti-γ-H2AX monoclonal antibody was from Molecular Probes, anti-tubulin antibody from Sigma, anti-p53 (FL-393) antibody was from Santa Cruz.
Restraint stress
WT (C57BL/6) or βArr1-knockout (βArr1−/−)Citation62 mice were restrained in 50-ml conical tubes, which were uniformly perforated for ventilation, for 4 h/d for 2 wk. Propranolol (10 mg/kg/day) was administered in the drinking water and was kept in a light-protected bottle, starting 2 d before the first exposure to restraint stress. After restraint stress, animals were sacrificed, brains were removed, and brain frontal cortex was dissected. For protein preparation, the brain frontal cortex was lysed and sonicated in RIPA buffer (50 mM Tris pH 7.4, 500 mM NaCl, 1% SDS, 1% Triton X100, 1 mM EDTA, Halt protease and phosphatase inhibitor cocktail). All animals used in these studies were adult male mice 8–12 wk of age. All mouse strains were backcrossed to the C57BL/6 background ≥ 10 generations. Animals were handled according to approved protocols and animal welfare regulations of the Institutional Review Board at Duke University Medical Center.
Immunoblotting
Immunoblotting was performed as described in reference Citation22.
Acknowledgments
R.J.L. is a Howard Hughes Medical Institute investigator. This work was supported by HL16037 and HL70631 (R.J.L.), RO1-MH79201 and RO1-MH60451 (M.G.C) and F32- MH093092 (B.D.S). We thank D. Addison and Q. Lennon for secretarial assistance.
References
- Selye H. A Syndrome Produced by Diverse Nocuous Agents. Nature 1936; 138:32; http://dx.doi.org/10.1038/138032a0
- Charmandari E, Tsigos C, Chrousos G. Endocrinology of the stress response. Annu Rev Physiol 2005; 67:259 - 84; http://dx.doi.org/10.1146/annurev.physiol.67.040403.120816; PMID: 15709959
- Norton MC, Smith KR, Østbye T, Tschanz JT, Schwartz S, Corcoran C, et al, Cache County Investigators. Early parental death and remarriage of widowed parents as risk factors for Alzheimer disease: the Cache County study. Am J Geriatr Psychiatry 2011; 19:814 - 24; http://dx.doi.org/10.1097/JGP.0b013e3182011b38; PMID: 21873837
- Pouwer F, Kupper N, Adriaanse MC. Does emotional stress cause type 2 diabetes mellitus? A review from the European Depression in Diabetes (EDID) Research Consortium. Discov Med 2010; 9:112 - 8; PMID: 20193636
- Figueredo VM. The time has come for physicians to take notice: the impact of psychosocial stressors on the heart. Am J Med 2009; 122:704 - 12; http://dx.doi.org/10.1016/j.amjmed.2009.05.001; PMID: 19635269
- Antoni MH, Lutgendorf SK, Cole SW, Dhabhar FS, Sephton SE, McDonald PG, et al. The influence of bio-behavioural factors on tumour biology: pathways and mechanisms. Nat Rev Cancer 2006; 6:240 - 8; http://dx.doi.org/10.1038/nrc1820; PMID: 16498446
- Wohleb ES, Hanke ML, Corona AW, Powell ND, Stiner LM, Bailey MT, et al. β-Adrenergic receptor antagonism prevents anxiety-like behavior and microglial reactivity induced by repeated social defeat. J Neurosci 2011; 31:6277 - 88; http://dx.doi.org/10.1523/JNEUROSCI.0450-11.2011; PMID: 21525267
- Vaiva G, Ducrocq F, Jezequel K, Averland B, Lestavel P, Brunet A, et al. Immediate treatment with propranolol decreases posttraumatic stress disorder two months after trauma. Biol Psychiatry 2003; 54:947 - 9; http://dx.doi.org/10.1016/S0006-3223(03)00412-8; PMID: 14573324
- Cerione RA, Strulovici B, Benovic JL, Lefkowitz RJ, Caron MG. Pure beta-adrenergic receptor: the single polypeptide confers catecholamine responsiveness to adenylate cyclase. Nature 1983; 306:562 - 6; http://dx.doi.org/10.1038/306562a0; PMID: 6316161
- Dixon RA, Kobilka BK, Strader DJ, Benovic JL, Dohlman HG, Frielle T, et al. Cloning of the gene and cDNA for mammalian beta-adrenergic receptor and homology with rhodopsin. Nature 1986; 321:75 - 9; http://dx.doi.org/10.1038/321075a0; PMID: 3010132
- Lefkowitz RJ. Seven transmembrane receptors: something old, something new. Acta Physiol (Oxf) 2007; 190:9 - 19; http://dx.doi.org/10.1111/j.1365-201X.2007.01693.x; PMID: 17428228
- Rainbow TC, Parsons B, Wolfe BB. Quantitative autoradiography of beta 1- and beta 2-adrenergic receptors in rat brain. Proc Natl Acad Sci USA 1984; 81:1585 - 9; http://dx.doi.org/10.1073/pnas.81.5.1585; PMID: 6324206
- Regard JB, Sato IT, Coughlin SR. Anatomical profiling of G protein-coupled receptor expression. Cell 2008; 135:561 - 71; http://dx.doi.org/10.1016/j.cell.2008.08.040; PMID: 18984166
- Arnold A, McAuliff JP, Colella DF, O’Connor WV, Brown TG Jr.. The beta-2 receptor mediated glycogenolytic responses to catecholamines in the dog. Arch Int Pharmacodyn Ther 1968; 176:451 - 7; PMID: 5718970
- Lands AM, Arnold A, McAuliff JP, Luduena FP, Brown TG Jr.. Differentiation of receptor systems activated by sympathomimetic amines. Nature 1967; 214:597 - 8; http://dx.doi.org/10.1038/214597a0; PMID: 6036174
- Mukherjee C, Caron MG, Coverstone M, Lefkowitz RJ. Identification of adenylate cyclase-coupled beta-adrenergic receptors in frog erythrocytes with (minus)-[3-H] alprenolol. J Biol Chem 1975; 250:4869 - 76; PMID: 238972
- Benovic JL, Strasser RH, Caron MG, Lefkowitz RJ. Beta-adrenergic receptor kinase: identification of a novel protein kinase that phosphorylates the agonist-occupied form of the receptor. Proc Natl Acad Sci USA 1986; 83:2797 - 801; http://dx.doi.org/10.1073/pnas.83.9.2797; PMID: 2871555
- Lohse MJ, Benovic JL, Codina J, Caron MG, Lefkowitz RJ. beta-Arrestin: a protein that regulates beta-adrenergic receptor function. Science 1990; 248:1547 - 50; http://dx.doi.org/10.1126/science.2163110; PMID: 2163110
- Luttrell LM, Ferguson SS, Daaka Y, Miller WE, Maudsley S, Della Rocca GJ, et al. Beta-arrestin-dependent formation of beta2 adrenergic receptor-Src protein kinase complexes. Science 1999; 283:655 - 61; http://dx.doi.org/10.1126/science.283.5402.655; PMID: 9924018
- Reiter E, Ahn S, Shukla AK, Lefkowitz RJ. Molecular mechanism of β-arrestin-biased agonism at seven-transmembrane receptors. Annu Rev Pharmacol Toxicol 2012; 52:179 - 97; http://dx.doi.org/10.1146/annurev.pharmtox.010909.105800; PMID: 21942629
- Shenoy SK, McDonald PH, Kohout TA, Lefkowitz RJ. Regulation of receptor fate by ubiquitination of activated beta 2-adrenergic receptor and beta-arrestin. Science 2001; 294:1307 - 13; http://dx.doi.org/10.1126/science.1063866; PMID: 11588219
- Hara MR, Kovacs JJ, Whalen EJ, Rajagopal S, Strachan RT, Grant W, et al. A stress response pathway regulates DNA damage through β2-adrenoreceptors and β-arrestin-1. Nature 2011; 477:349 - 53; http://dx.doi.org/10.1038/nature10368; PMID: 21857681
- Moniri NH, Daaka Y. Agonist-stimulated reactive oxygen species formation regulates beta2-adrenergic receptor signal transduction. Biochem Pharmacol 2007; 74:64 - 73; http://dx.doi.org/10.1016/j.bcp.2007.03.016; PMID: 17451656
- Yan L, Vatner DE, O’Connor JP, Ivessa A, Ge H, Chen W, et al. Type 5 adenylyl cyclase disruption increases longevity and protects against stress. Cell 2007; 130:247 - 58; http://dx.doi.org/10.1016/j.cell.2007.05.038; PMID: 17662940
- Yamauchi N, Shibasaki T, Wakabayashi I, Demura H. Brain beta-endorphin and other opioids are involved in restraint stress-induced stimulation of the hypothalamic-pituitary-adrenal axis, the sympathetic nervous system, and the adrenal medulla in the rat. Brain Res 1997; 777:140 - 6; PMID: 9449422
- Arnsten AF. Stress signalling pathways that impair prefrontal cortex structure and function. Nat Rev Neurosci 2009; 10:410 - 22; http://dx.doi.org/10.1038/nrn2648; PMID: 19455173
- Cahill L, Prins B, Weber M, McGaugh JL. Beta-adrenergic activation and memory for emotional events. Nature 1994; 371:702 - 4; http://dx.doi.org/10.1038/371702a0; PMID: 7935815
- Feng Z, Liu L, Zhang C, Zheng T, Wang J, Lin M, et al. Chronic restraint stress attenuates p53 function and promotes tumorigenesis. Proc Natl Acad Sci USA 2012; 109:7013 - 8; http://dx.doi.org/10.1073/pnas.1203930109; PMID: 22509031
- Hu W, Feng Z, Levine AJ. The Regulation of Multiple p53 Stress Responses is Mediated through MDM2. Genes Cancer 2012; 3:199 - 208; http://dx.doi.org/10.1177/1947601912454734; PMID: 23150753
- Rajagopal S, Rajagopal K, Lefkowitz RJ. Teaching old receptors new tricks: biasing seven-transmembrane receptors. Nat Rev Drug Discov 2010; 9:373 - 86; http://dx.doi.org/10.1038/nrd3024; PMID: 20431569
- Perron L, Bairati I, Harel F, Meyer F. Antihypertensive drug use and the risk of prostate cancer (Canada). Cancer Causes Control 2004; 15:535 - 41; http://dx.doi.org/10.1023/B:CACO.0000036152.58271.5e; PMID: 15280632
- Powe DG, Entschladen F. Targeted therapies: Using β-blockers to inhibit breast cancer progression. Nat Rev Clin Oncol 2011; 8:511 - 2; http://dx.doi.org/10.1038/nrclinonc.2011.123; PMID: 21808268
- Léauté-Labrèze C, Dumas de la Roque E, Hubiche T, Boralevi F, Thambo JB, Taïeb A. Propranolol for severe hemangiomas of infancy. N Engl J Med 2008; 358:2649 - 51; http://dx.doi.org/10.1056/NEJMc0708819; PMID: 18550886
- Thaker PH, Han LY, Kamat AA, Arevalo JM, Takahashi R, Lu C, et al. Chronic stress promotes tumor growth and angiogenesis in a mouse model of ovarian carcinoma. Nat Med 2006; 12:939 - 44; http://dx.doi.org/10.1038/nm1447; PMID: 16862152
- Han RQ, Ouyang YB, Xu L, Agrawal R, Patterson AJ, Giffard RG. Postischemic brain injury is attenuated in mice lacking the beta2-adrenergic receptor. Anesth Analg 2009; 108:280 - 7; http://dx.doi.org/10.1213/ane.0b013e318187ba6b; PMID: 19095863
- Sood R, Ritov G, Richter-Levin G, Barki-Harrington L. Selective increase in the association of the β2 adrenergic receptor, β Arrestin-1 and p53 with Mdm2 in the ventral hippocampus one month after underwater trauma. Behav Brain Res 2012; 240C:26 - 8; PMID: 23174211
- Wang P, Gao H, Ni Y, Wang B, Wu Y, Ji L, et al. Beta-arrestin 2 functions as a G-protein-coupled receptor-activated regulator of oncoprotein Mdm2. J Biol Chem 2003; 278:6363 - 70; http://dx.doi.org/10.1074/jbc.M210350200; PMID: 12488444
- Boularan C, Scott MG, Bourougaa K, Bellal M, Esteve E, Thuret A, et al. beta-arrestin 2 oligomerization controls the Mdm2-dependent inhibition of p53. Proc Natl Acad Sci USA 2007; 104:18061 - 6; http://dx.doi.org/10.1073/pnas.0705550104; PMID: 17984062
- Ahn S, Wei H, Garrison TR, Lefkowitz RJ. Reciprocal regulation of angiotensin receptor-activated extracellular signal-regulated kinases by beta-arrestins 1 and 2. J Biol Chem 2004; 279:7807 - 11; http://dx.doi.org/10.1074/jbc.C300443200; PMID: 14711824
- Kovacs JJ, Hara MR, Davenport CL, Kim J, Lefkowitz RJ. Arrestin development: emerging roles for beta-arrestins in developmental signaling pathways. Dev Cell 2009; 17:443 - 58; http://dx.doi.org/10.1016/j.devcel.2009.09.011; PMID: 19853559
- Beaulieu JM, Caron MG. Beta-arrestin goes nuclear. Cell 2005; 123:755 - 7; http://dx.doi.org/10.1016/j.cell.2005.11.010; PMID: 16325568
- Kang J, Shi Y, Xiang B, Qu B, Su W, Zhu M, et al. A nuclear function of beta-arrestin1 in GPCR signaling: regulation of histone acetylation and gene transcription. Cell 2005; 123:833 - 47; http://dx.doi.org/10.1016/j.cell.2005.09.011; PMID: 16325578
- Shi Y, Feng Y, Kang J, Liu C, Li Z, Li D, et al. Critical regulation of CD4+ T cell survival and autoimmunity by beta-arrestin 1. Nat Immunol 2007; 8:817 - 24; http://dx.doi.org/10.1038/ni1489; PMID: 17618287
- Yue R, Kang J, Zhao C, Hu W, Tang Y, Liu X, et al. Beta-arrestin1 regulates zebrafish hematopoiesis through binding to YY1 and relieving polycomb group repression. Cell 2009; 139:535 - 46; http://dx.doi.org/10.1016/j.cell.2009.08.038; PMID: 19879840
- Levine AJ, Oren M. The first 30 years of p53: growing ever more complex. Nat Rev Cancer 2009; 9:749 - 58; http://dx.doi.org/10.1038/nrc2723; PMID: 19776744
- Sengupta S, Harris CC. p53: traffic cop at the crossroads of DNA repair and recombination. Nat Rev Mol Cell Biol 2005; 6:44 - 55; http://dx.doi.org/10.1038/nrm1546; PMID: 15688066
- Yu J, Zhang L, Hwang PM, Kinzler KW, Vogelstein B. PUMA induces the rapid apoptosis of colorectal cancer cells. Mol Cell 2001; 7:673 - 82; http://dx.doi.org/10.1016/S1097-2765(01)00213-1; PMID: 11463391
- Nakano K, Vousden KH. PUMA, a novel proapoptotic gene, is induced by p53. Mol Cell 2001; 7:683 - 94; http://dx.doi.org/10.1016/S1097-2765(01)00214-3; PMID: 11463392
- Bensaad K, Tsuruta A, Selak MA, Vidal MN, Nakano K, Bartrons R, et al. TIGAR, a p53-inducible regulator of glycolysis and apoptosis. Cell 2006; 126:107 - 20; http://dx.doi.org/10.1016/j.cell.2006.05.036; PMID: 16839880
- Matoba S, Kang JG, Patino WD, Wragg A, Boehm M, Gavrilova O, et al. p53 regulates mitochondrial respiration. Science 2006; 312:1650 - 3; http://dx.doi.org/10.1126/science.1126863; PMID: 16728594
- Fukasawa K, Choi T, Kuriyama R, Rulong S, Vande Woude GF. Abnormal centrosome amplification in the absence of p53. Science 1996; 271:1744 - 7; http://dx.doi.org/10.1126/science.271.5256.1744; PMID: 8596939
- Hu W, Feng Z, Teresky AK, Levine AJ. p53 regulates maternal reproduction through LIF. Nature 2007; 450:721 - 4; http://dx.doi.org/10.1038/nature05993; PMID: 18046411
- Suh EK, Yang A, Kettenbach A, Bamberger C, Michaelis AH, Zhu Z, et al. p63 protects the female germ line during meiotic arrest. Nature 2006; 444:624 - 8; http://dx.doi.org/10.1038/nature05337; PMID: 17122775
- Kruse JP, Gu W. Modes of p53 regulation. Cell 2009; 137:609 - 22; http://dx.doi.org/10.1016/j.cell.2009.04.050; PMID: 19450511
- Grossman SR, Perez M, Kung AL, Joseph M, Mansur C, Xiao ZX, et al. p300/MDM2 complexes participate in MDM2-mediated p53 degradation. Mol Cell 1998; 2:405 - 15; http://dx.doi.org/10.1016/S1097-2765(00)80140-9; PMID: 9809062
- Zhang Y, Xiong Y, Yarbrough WG. ARF promotes MDM2 degradation and stabilizes p53: ARF-INK4a locus deletion impairs both the Rb and p53 tumor suppression pathways. Cell 1998; 92:725 - 34; http://dx.doi.org/10.1016/S0092-8674(00)81401-4; PMID: 9529249
- Pomerantz J, Schreiber-Agus N, Liégeois NJ, Silverman A, Alland L, Chin L, et al. The Ink4a tumor suppressor gene product, p19Arf, interacts with MDM2 and neutralizes MDM2’s inhibition of p53. Cell 1998; 92:713 - 23; http://dx.doi.org/10.1016/S0092-8674(00)81400-2; PMID: 9529248
- Girnita L, Shenoy SK, Sehat B, Vasilcanu R, Girnita A, Lefkowitz RJ, et al. beta-Arrestin is crucial for ubiquitination and down-regulation of the insulin-like growth factor-1 receptor by acting as adaptor for the MDM2 E3 ligase. J Biol Chem 2005; 280:24412 - 9; http://dx.doi.org/10.1074/jbc.M501129200; PMID: 15878855
- Salcedo A, Mayor F Jr., Penela P. Mdm2 is involved in the ubiquitination and degradation of G-protein-coupled receptor kinase 2. EMBO J 2006; 25:4752 - 62; http://dx.doi.org/10.1038/sj.emboj.7601351; PMID: 17006543
- Lakshmikanthan V, Zou L, Kim JI, Michal A, Nie Z, Messias NC, et al. Identification of betaArrestin2 as a corepressor of androgen receptor signaling in prostate cancer. Proc Natl Acad Sci USA 2009; 106:9379 - 84; http://dx.doi.org/10.1073/pnas.0900258106; PMID: 19458261
- Lin CH, MacGurn JA, Chu T, Stefan CJ, Emr SD. Arrestin-related ubiquitin-ligase adaptors regulate endocytosis and protein turnover at the cell surface. Cell 2008; 135:714 - 25; http://dx.doi.org/10.1016/j.cell.2008.09.025; PMID: 18976803
- Conner DA, Mathier MA, Mortensen RM, Christe M, Vatner SF, Seidman CE, et al. beta-Arrestin1 knockout mice appear normal but demonstrate altered cardiac responses to beta-adrenergic stimulation. Circ Res 1997; 81:1021 - 6; http://dx.doi.org/10.1161/01.RES.81.6.1021; PMID: 9400383
- Bonner WM, Redon CE, Dickey JS, Nakamura AJ, Sedelnikova OA, Solier S, et al. GammaH2AX and cancer. Nat Rev Cancer 2008; 8:957 - 67; http://dx.doi.org/10.1038/nrc2523; PMID: 19005492