Embryonic stem cells (ESCs) are valuable tools for regenerative medicine, being capable of self-renewing in culture indefinitely while retaining their pluripotency, i.e., the ability to generate any cell of the adult organism. At the heart of these capabilities is a complex transcriptional network, which carefully guards an uncommitted state, yet permits ESCs to remain poised for differentiation. So far, many ESC transcription factors, including the core pluripotency proteins, Oct4, Sox2 and Nanog (OSN), have been identified and extensively characterized. These molecules participate in highly interrelated pathways to activate their own expression, as well as downstream self-renewal regulators, via co-recruitment to enhancer regions in the vicinity of these genes. However, we know much less about the individual roles played by each of these factors within multi-protein complexes, the identity of their potential coregulators, or how they functionally connect to the general transcription and chromatin remodeling machineries. In this context, we recently investigated the protein-protein interactions required for the function of one particular molecule, the orphan nuclear receptor Esrrb. In doing so, we uncovered novel and essential roles for the nuclear receptor coactivator Ncoa3 in pluripotent cells,Citation1 making this the first characterization of a nuclear receptor coactivator in stem cell biology.
Esrrb has recently been found to act both as a powerful enhancer of ESC self-renewal,Citation1-Citation4 a crucial downstream target of the Wnt/GSK3/Tcf3 signaling pathwayCitation3 and also able to substitute for Nanog in pluripotent cells.Citation2 Additionally, an important role for Esrrb is apparent in the induction of pluripotency via somatic cell reprogramming.Citation5,Citation6 Despite these findings, however, nothing is known of the mechanisms by which Esrrb function is conferred in ESCs. To address this, we based our investigations on previous knowledge gained from studying estrogen-related receptors (ERRs) in somatic cells, where their activity canonically requires the recruitment of a protein coactivator to a conserved portion of their ligand-binding domain (LBD), termed the AF-2 region. We theorized that Esrrb might function similarly in ESCs and through a combination of mutagenesis and functional assays, demonstrated that Esrrb’s ability to sustain ESC self-renewal crucially resides in its LBD/AF-2 domain.Citation1 In turn, we demonstrated that Ncoa3 is the coactivator specifically recruited to this AF-2 region and is an essential mediator of Esrrb function in pluripotent cells.
Widely known as the oncogene, AIB1 in cancer or SRC-3/ACTR in somatic cells, Ncoa3 is reported to bind to many nuclear receptors as well as some transcription factors, where it induces the strong expression of specific target genes. Correspondingly in ESCs, we found that Ncoa3 is critical for controlling Esrrb-dependent activation of important self-renewal regulators such as Esrrb, Klf4, Nanog and Sox2. Depleting Ncoa3 in ESCs leads to downregulation of these genes and triggers differentiation,Citation1 mirroring the effect of shRNA-mediated Esrrb knockdown itself. Through a combination of Ncoa3 ChIP-sequencing and DNA microarray techniques, we furthermore investigated the genome-wide relevance of the Esrrb-Ncoa3 partnership in pluripotent cells. Sites co-bound by these proteins alongside the OSN triad include ESC-associated genes as well as, interestingly, a high proportion of germ cell targets including some relevant to reprogramming events in the germline.Citation7 Accordingly, depletion of Ncoa3 significantly hinders the generation of induced pluripotent stem cells (iPSCs) in vitro.Citation1 Together, these observations suggest that Esrrb and Ncoa3 might also act in synergy during the formation and/or reprogramming of primordial germ cells (PGCs) in vivo.
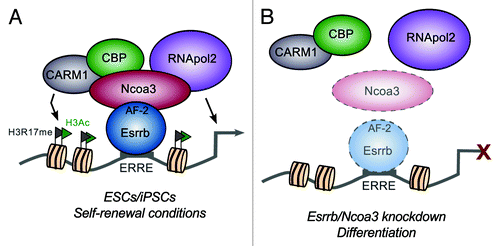
Our collective findings lead onto a key question: what is the functional role for Esrrb and Ncoa3 underlying their essential nature in the ESC network? Genome-wide, both ESC and PGC-relevant target genes are also enriched for marks of active enhancers, H3K4me1/H3K27ac and p300 recruitment, suggesting a link between the presence of Esrrb-Ncoa3 and transcriptional activation. Moreover, we discovered that Ncoa3 interacts with RNA polymerase II itself and is required for the association between Esrrb and the general transcription machinery.Citation1 Complementing our data, a concurrent study reports that Ncoa3 also recruits the chromatin-modifying proteins CARM1 and CBP to the Nanog locus,Citation8 thus ensuring the maintenance of active histone modifications at this gene. Overall, these results point toward a crucial role for Ncoa3 in potentiating transcription in ESCs by locally facilitating an “open” chromatin state as well as providing a scaffold upon which to recruit the basal transcription machinery itself (). Loss of Esrrb/Ncoa3 upon differentiation or shRNA-mediated depletion would therefore inhibit the transcription of ESC-associated genes, consequently triggering a collapse of the network and the exit of self-renewal ().
Figure 1. Proposed model depicting the role of Esrrb and Ncoa3 at ESC-specific enhancers. (A) In pluripotent cells, Esrrb binds to ERR response elements (ERREs) at active enhancer regions, which also contain bound core proteins such as Nanog and Oct4 (not shown). AF-2-mediated recruitment of Ncoa3 is, in turn, essential for Esrrb activity, with Ncoa3 binding both epigenetic and basal transcription machinery complexes to bring about strong activation of target genes. (B) Upon conditions where Esrrb and/or Ncoa3 proteins are downregulated (faint color), their loss might lead to alterations in chromatin structure and destabilization of RNA polymerase II (RNApol2), thus triggering differentiation.
In conclusion, these findings establish Ncoa3 as a key member of pluripotency transcriptional circuitries, emphasizing the importance of this coactivator not just in somatic and cancer cells, but also in early developmental contexts. An interesting future avenue is to characterize the upstream signaling pathways that may regulate Ncoa3 in pluripotent cells, as this molecule is a well-known target for numerous post-translational modifications. Accordingly, increased Ncoa3 stability notably correlates with GSK3 inhibition in ESCs.Citation8 Ncoa3 modification in response to one or more external signaling pathways could therefore alter its protein stability, cellular location or even its transcriptional activity, thus serving as an elegant way to fine-tune the cellular state of ESCs and iPSCs.
References
- Percharde M, et al. Genes Dev 2012; 26:2286 - 98; http://dx.doi.org/10.1101/gad.195545.112; PMID: 23019124
- Festuccia N, et al. Cell Stem Cell 2012; 11:477 - 90; http://dx.doi.org/10.1016/j.stem.2012.08.002; PMID: 23040477
- Martello G, et al. Cell Stem Cell 2012; 11:491 - 504; http://dx.doi.org/10.1016/j.stem.2012.06.008; PMID: 23040478
- Zhang X, et al. J Biol Chem 2008; 283:35825 - 33; http://dx.doi.org/10.1074/jbc.M803481200; PMID: 18957414
- Feng B, et al. Nat Cell Biol 2009; 11:197 - 203; http://dx.doi.org/10.1038/ncb1827; PMID: 19136965
- Buganim Y, et al. Cell 2012; 150:1209 - 22; http://dx.doi.org/10.1016/j.cell.2012.08.023; PMID: 22980981
- Gillich A, et al. Cell Stem Cell 2012; 10:425 - 39; http://dx.doi.org/10.1016/j.stem.2012.01.020; PMID: 22482507
- Wu Z, et al. J Biol Chem 2012; 287:38295 - 304; http://dx.doi.org/10.1074/jbc.M112.373092; PMID: 22977234