Pancreatic β-cells, located within the islets of Langerhans, are key players in the control of blood glucose homeostasis. The amount of insulin secreted by these cells is precisely adjusted to avoid hypo- or hyperglycaemic episodes and prevent the appearance of diabetes mellitus. Pregnancy and obesity are associated with diminished insulin sensitivity of target tissues and a consequent rise in insulin needs. The increased insulin demand is normally compensated by expansion of β-cells and improvement in their secretory activity.Citation1 A better understanding of these adaptive events could open new therapeutic perspectives for prevention and/or treatment of type 2 and gestational diabetes. MicroRNAs are gene expression regulators that reduce translation and/or stability of target mRNAs by binding to their 3′-untranslated regions. These tiny RNA molecules govern different physiological and pathological processes and participate to the regulation of β-cell functions.Citation2 We recently provided evidence that microRNAs contribute to compensatory β-cell mass expansion in response to insulin resistance associated with pregnancy and obesity.Citation3
We found that in rat islets β-cell expansion during pregnancy is coupled to changes in the level of some microRNAs.Citation3 These changes were reproduced in dissociated rat islet cells by transfecting oligonucleotides that either mimic or block each microRNA. This revealed that knockdown of miR-338-3p, a microRNA downregulated in the islets of pregnant rats, does not interfere with the secretory properties of β-cells, but boosts their proliferation and improves their survival under pro-apoptotic conditions. Interestingly, the level of miR-338-3p is also diminished in the islets of animal models characterized by obesity, insulin resistance and compensatory β-cell mass expansion, the db/db mice and mice fed a high-fat diet. These observations point to a broader involvement of miR-338-3p in compensatory β-cell mass expansion. The decrease of miR-338-3p observed in pregnancy and obesity can be reproduced by activating the cAMP/PKA-dependent pathway. Our data suggest that, during pregnancy, miR-338-3p levels are controlled by the unconventional estradiol receptor GPR30 that is coupled to the cAMP signaling pathway. Indeed, estradiol levels increase during gestation and islet GPR30 expression is strongly upregulated concomitantly with β-cell mass expansion. Downregulation of miR-338-3p in obese mice is unlikely to be triggered by the same mechanism, because the expression of GPR30 is unchanged in the islets of these animals. An attractive candidate for the control of miR-338-3p expression in obese mice is glucagon-like peptide 1 (GLP1), a hormone that stimulates β-cell proliferation and survival via a cAMP-dependent mechanism. Indeed, we showed that rat and human islets exposed to a GLP1 analog contain reduced levels of miR-338-3p. Moreover, previous studies reported increased GLP1 production in islets of type 2 diabetic donors and of obese animals.Citation4,Citation5
Our study raises a number of questions that remain unsolved so far. A point that deserves further investigations is the possible contribution of the miR-338-3p hosting gene to the control of the β-cell mass. Most microRNAs are generated from independent transcriptional units located outside protein-coding regions, but a fraction of them is excised from introns of protein-coding transcripts. This is the case of miR-338-3p, which is transcribed from an intron of the gene coding for the apoptosis-associated tyrosine kinase (AATK). AATK family members are predominantly expressed in brains, where they induce cell death and neurite extension.Citation6 At present, no information is available about the function of AATK in β-cells. The expression of AATK is downregulated by cAMP-raising agents and by exposure to estradiol and GLP1 analogs (our unpublished observations). Moreover, we found that during gestation, the decrease in AATK levels in rat islets parallels that of miR-338-3p and coincides with the changes in β-cell mass. This may indicate a cooperative action of miR-338-3p and its hosting gene in the control of β-cell mass expansion under insulin resistance conditions.
In our study, following blockade of miR-338-3p, we detected differences in the expression of many genes involved in proliferation and apoptosis.Citation3 However, the direct targets of miR-338-3p are still not identified. The tumor necrosis factor receptor II (TNFRII, also called TNFrsf1β) is predicted by computational programs to be a direct target of miR-338-3p. Indeed, modulation of miR-338-3p levels affects the expression of luciferase reporter constructs containing the 3′UTR sequence of TNFRII (our unpublished results). Moreover, we found that TNRFII is strongly upregulated in rat islets at day 14 of gestation. TNFRII occurs as a membrane-attached protein or in a soluble truncated form generated by proteolytic cleavage or by alternative splicing.Citation7 Interestingly, serum levels of the soluble variant of TNFRII are higher in healthy than in type 2 diabetic subjects and correlate with insulin sensitivity.Citation8 Among other effects, the reduction of islet miR-338-3p expression occurring under conditions of insulin resistance may possibly enable the production of larger amounts of the soluble form of TNFRII. This could potentially render β-cells more resistant to apoptosis favoring their compensatory expansion. Indeed, overexpression of the soluble form of TNFRII protects a fibroblast cell line against TNFα-induced apoptosis,Citation7 and other studies have identified TNFRII as a neuroprotective factor.Citation9
Our study revealed a central role for microRNAs in compensatory β-cell mass expansion associated with pregnancy and obesity. Some important aspects of the mode of action of miR-338-3p are still missing and deserve further investigations. However, a better understanding of the molecular events controlled by this microRNA promises to open new perspectives for the design of better pharmacological treatments for type 2 and gestational diabetes.
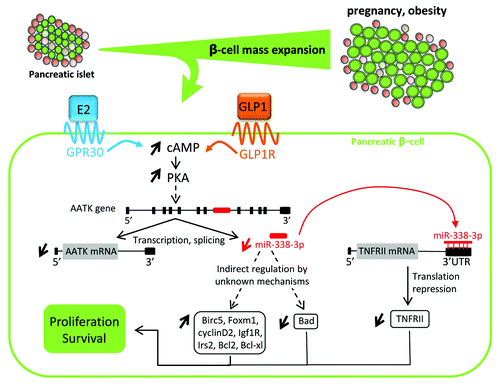
Figure 1. Model explaining the regulation of miR-338-3p expression and its mode of action. Under insulin resistance conditions associated with pregnancy and obesity, the augmented insulin needs are covered by an increase in the β-cell mass and in hormone secretion. The rise in cAMP levels resulting from occupation of GPR30 and GLP1 receptors leads to protein kinase A activation. This causes reduction of AATK gene transcription and decreased production of miR-338-3p that is generated from the seventh intron of the AATK mRNA. The presence of reduced amounts of miR-338-3p promotes the expression of proliferative and/or anti-apoptotic genes, such as Birc5, Foxm1, cyclin D2, Igf1R, Irs2, Bcl2 and Bcl-xl, and reduces the expression of the pro-apoptotic gene Bad. miR-338-3p will also directly inhibit TNFRII mRNA translation by binding to its 3′UTR. Collectively, these events will result in β-cell proliferation and improved survival, permitting adaptive β-cell mass expansion to meet the increased insulin needs during pregnancy and obesity.
References
- Rieck S, et al. Trends Endocrinol Metab 2010; 21:151 - 8; http://dx.doi.org/10.1016/j.tem.2009.11.001; PMID: 20015659
- Guay C, et al. Diabetes Obes Metab 2012; 14:Suppl 3 12 - 21; http://dx.doi.org/10.1111/j.1463-1326.2012.01654.x; PMID: 22928560
- Jacovetti C, et al. J Clin Invest 2012; 122:3541 - 51; http://dx.doi.org/10.1172/JCI64151; PMID: 22996663
- Marchetti P, et al. Diabetologia 2012; 55:3262 - 72; http://dx.doi.org/10.1007/s00125-012-2716-9; PMID: 22965295
- Ellingsgaard H, et al. Nat Med 2011; 17:1481 - 9; http://dx.doi.org/10.1038/nm.2513; PMID: 22037645
- Tomomura M, et al. Neuroscience 2007; 148:510 - 21; http://dx.doi.org/10.1016/j.neuroscience.2007.05.048; PMID: 17651901
- Lainez B, et al. Int Immunol 2004; 16:169 - 77; http://dx.doi.org/10.1093/intimm/dxh014; PMID: 14688072
- Fernández-Real JM, et al. Eur J Endocrinol 2006; 154:723 - 30; http://dx.doi.org/10.1530/eje.1.02145; PMID: 16645020
- Fontaine V, et al. J Neurosci 2002; 22:RC216; PMID: 11917000