Polycomb group proteins are epigenetic gene silencers that have been implicated in cancer development and stem cell maintenance.Citation1 Biological and genetic studies indicate that PcG proteins exist in at least two separate protein complexes, polycomb-repressive complex 2 (PRC2) and polycomb-repressive complex 1 (PRC1), which act in concert to promote and maintain gene repression.Citation1 EZH2, the catalytically active component of PRC2, plays an important role in many biological processes through its ability to trimethylate lysine 27 in histone H3. The core enzymatic activity of PRC1 is an E3 ubiquitin ligase activity contributed by RING1B (and enhanced by BMI1), which ubiquitinates histone H2A at lysine 119.Citation1
PcG proteins are displaced from the promoters of one set of target genes while being recruited to the promoters of another set of target genes during lineage specification.Citation1 How extracellular signaling regulates this process is not well-understood. In 2005, Cha and colleagues found Akt mediated phosphorylation of EZH2 results in a decrease of lysine 27 trimethylation and derepression of silenced genes.Citation2 Recently, we reported that phosphorylation of BMI1 at serine 316 by Akt impairs its function by triggering its dissociation from the Ink4a-Arf locus, which results in decreased histone H2A ubiquitination and the inability of BMI1 to promote cell growth and tumorigenesis.Citation3 It is very likely that other PcG proteins, including EZH1 and MEL18, are downstream targets of Akt. Thus, Akt appears to regulate the activities of both the PRC2 and PRC1 complexes in the cell ().
Figure 1. PI3K-Akt signaling regulates polycomb group protein and hematopoietic stem cell self-renewal.
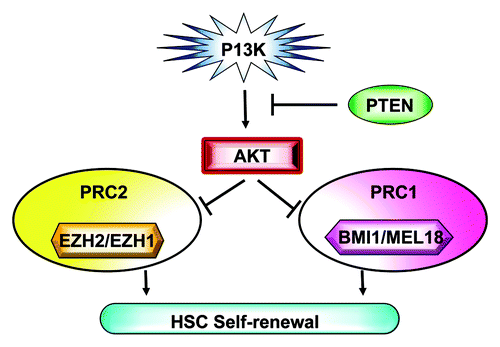
PcG proteins are required for the maintenance of embryonic as well as a broad range of adult stem cells, including hematopoietic stem cells (HSCs).Citation1 HSCs have both the capacity to self-renew and to differentiate into many different mature blood cell types. Understanding the molecular mechanisms governing stem cell self-renewal remains the holy grail of stem cell biology and holds great promise for the development of stem cell-based therapies aimed at treating debilitating and life-threatening diseases such as cancer. Self-renewal requires the integration of survival signals and proliferation controls with the maintenance of an undifferentiated state. This demands a complex crosstalk between extrinsic signals from the microenvironment and the cell-intrinsic regulators of HSCs to maintain an undifferentiated state.Citation4 However, how the crosstalk is coordinated has not been well-defined at the molecular level. Activation of the PI3K-Akt signaling impairs HSC self-renewal,Citation5 and we demonstrated that BMI1 is a critical downstream target of PI3K-Akt signaling in HSCs and that Akt-mediated phophorylation of BMI1 inhibits its ability to promote hematopoietic stem/progenitor cell (HSPC) self-renewal.Citation3 At virtually the same time, the van Lohuizen lab reported that Akt-mediated BMI1 phosphorylation enhances its oncogenic potential in an Ink4a and Arf-independent mouse model of human prostate cancer.Citation6 They identified three sites of serine phosphorylation [Ser251, Ser253 and Ser255 (SDSGS)], none of which were consensus Akt phosphorylation sites (RXRXXS or T).Citation6 Nonetheless, it appears that the inhibition of cell proliferation and oncogenesis by Akt-mediated phosphorylation of BMI1, occurs in a site-specific and possibly context-dependent manner.
Similar to BMI1, a component of Polycomb-repressive complex 1 (PRC1), PRC2 and its components play important roles in hematopoiesis. Ezh2-deficient embryos died of anemia because of insufficient expansion of HSCs/progenitor cells. Deletion of Ezh2 in adult BM, however, did not significantly compromise hematopoiesis.Citation7 On the contrary, Ezh1 maintains repopulating HSCs in a slow-cycling, undifferentiated state, protecting them from senescence. Ezh1 ablation induces significant loss of adult HSCs, with concomitant impairment of their self-renewal capacity due to a potent senescence response, suggesting that Ezh1 is an important histone methyltransferase for adult HSC maintenance.Citation8 Therefore, it is possible that Akt-mediated phosphorylation of EZH2 or EZH1 may limit HSC function as well.
Hematopoietic stem cells (HSCs) give rise to all blood and immune cells and are used in clinical transplantation protocols to treat a wide variety of diseases. The ability to increase the number of HSCs either in vivo or in vitro would provide new treatment options, but the amplification of HSCs has been difficult to achieve. Although hematopoietic stem cells (HSCs) will rapidly expand after in vivo transplantation, experience from in vitro studies indicates that control of HSPC self-renewal and differentiation in culture remains difficult. Most hematopoietic cell growth factors activate Akt in ex vivo cultures, promoting HSC differentiation. Therefore, treating HSCs with Akt inhibitors may expand HSCs ex vivo. Our understanding of the crosstalk between PI3K-Akt signaling and polycomb group proteins may facilitate the development of innovative clinical strategies that can enhance ex vivo HSC expansion.
References
- Bracken AP, et al. Nat Rev Cancer 2009; 9:773 - 84; http://dx.doi.org/10.1038/nrc2736; PMID: 19851313
- Cha TL, et al. Science 2005; 310:306 - 10; http://dx.doi.org/10.1126/science.1118947; PMID: 16224021
- Liu Y, et al. Sci Signal 2012; 5:ra77; http://dx.doi.org/10.1126/scisignal.2003199; PMID: 23092893
- Orford KW, et al. Nat Rev Genet 2008; 9:115 - 28; http://dx.doi.org/10.1038/nrg2269; PMID: 18202695
- Yilmaz OH, et al. Nature 2006; 441:475 - 82; http://dx.doi.org/10.1038/nature04703; PMID: 16598206
- Nacerddine K, et al. J Clin Invest 2012; 122:1920 - 32; http://dx.doi.org/10.1172/JCI57477; PMID: 22505453
- Mochizuki-Kashio M, et al. Blood 2011; 118:6553 - 61; http://dx.doi.org/10.1182/blood-2011-03-340554; PMID: 22042701
- Hidalgo I, et al. Cell Stem Cell 2012; 11:649 - 62; http://dx.doi.org/10.1016/j.stem.2012.08.001; PMID: 23122289