Apoptosis is a highly conserved program of cell death in multicellular organisms that is central to their homeostasis. Defects in apoptosis are associated with cancer as well as neurodegenerative and autoimmune diseases.Citation1 There are two ways apoptosis can be induced: intrinsic and extrinsic. The intrinsic pathway is triggered via changes in the mitochondria, the extrinsic pathway via stimulation of the so-called death receptors (DRs). All members of the DR family are expressed on the cell membrane and characterized by the presence of a death domain (DD) playing a central role in the transduction of the apoptotic signal. So far the DR family comprises TNF-R1, CD95/Fas/APO-1, DR3, TNF-related apoptosis-inducing ligand-receptor (TRAIL-R1), TRAIL-R2 and DR6.Citation1 The central event in DR signaling is the formation of the death-inducing signaling complex (DISC). The DISC comprises oligomerized, probably trimerized receptors, the adaptor protein FADD, procaspase-8, procaspase-10 and c-FLIP.Citation1
Procaspase-8 activation at the DISC is a central initiation event in CD95-induced apoptosis. The N-terminal part of procaspase-8 has two death effector domains (DED) followed by the large catalytic subunit p18 and a small catalytic subunit p10. Elegant biochemical studies have shown that procaspase-8 activation at the DISC involves dimerization, oligomerization and cleavage.Citation2-Citation4 The cleavage of procaspase-8 at the DISC occurs via several cleavage products, leading to the generation of the active caspase-8 heterotetramer p102-p182.Citation5
Despite the fascinating progress in understanding caspase-8 activation at the DISC, several issues have remained open. The question of how the DISC could provide a platform for procaspase-8 activation to support its dimerization has been especially intriguing. Indeed, according to the previous models of the DISC, one FADD molecule recruits one procaspase-8, and the dimer formation between two procaspase-8 molecules is somehow supported by mutual orientation of FADDs or even neighboring receptor complexes. This point was not clarified. Furthermore, it has been demonstrated that procaspase-8 could be activated both by intermolecular and interdimer mechanisms. According to the intermolecular mechanism the procaspase-8 activation occurs within a dimer. In the interdimer mechanism one procaspase-8 dimer could activate a neighboring dimer of procaspase-8.Citation2,Citation6 The question of how two dimers of procaspase-8 could be formed and interact in the context of “the one FADD to one procaspase-8” model was also puzzling.
In our recent study, we analyzed the stoichiometry of CD95 DISC molecules using contemporary experimental and theoretical approaches, i.e., quantitative mass spectrometry, western blot and mathematical modeling, and found a novel feature of the structural organization of the DISC.Citation7 Our data showed that FADD is present in lower amounts at the DISC compared with procaspase-8, and procaspase-8 could form chains at the DISC via interactions between its DEDs. Furthermore, in these chains, procaspase-8 can form dimers and consequently might be activated. Independently of these findings, MacFarlane and coworkers have employed a combination of quantitative mass spectrometry, structural modeling and mutation studies in a reconstituted DISC model and demonstrated a crucial role for caspase-8 DED chain assembly following ligation of another important member of the DR family, TRAIL-R.Citation8 These findings enabled us to expand this knowledge beyond the CD95 system and to conclude that DED-chain formation serves as a general basis for caspase-8 activation in DR complexes. The discovery of chains allowed understanding of the DISC puzzle: how dimers of procaspase-8 could be formed in the context of the DISC as a prerequisite for caspase-8 activation. Furthermore, procaspase-8 chains as the platform for procaspase-8 activation also allow interdimer activation of procaspase-8, i.e., activation of one dimer by another dimer, since it has now become clear how dimers of procaspase-8 could be located in close proximity.
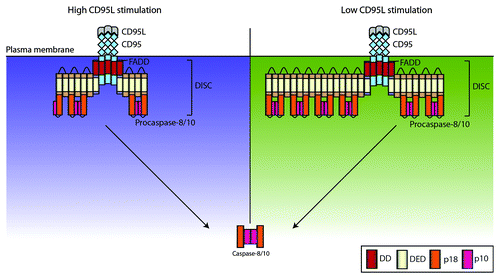
In our study we draw special attention to the dynamics of the procaspase-8 chains.Citation7 The mathematical modeling predicted that the chains have a variable length depending upon stimulation strength (). This prediction was confirmed by mass spectrometry analysis. We found that strong stimulation results in shorter chains, while weak stimulation leads to longer chains. The generation of longer chains upon a low number of activated receptors might be important for efficient apoptosis induction to ensure enough active caspase-8 in the cell (). Indeed, if the length of the chains is the same upon low and high stimulation, the amount of active caspase-8 generated would be reduced proportionally to the decrease in the stimulation strength. However, longer chains upon low stimulation strength should provide an additional source for more active caspase-8 in the cell. Interestingly, the modeling also predicted that the length of procaspase-8 chains has to be restricted. Indeed, a spontaneous activation of only very few receptors might lead to apoptosis via generation of a few long chains of procaspase-8 molecules. Upon chain length restriction, however, the model predicted no caspase-8 activation upon very low CD95L concentration as has been shown before.Citation9,Citation10 Therefore, we found that the DISC is a highly dynamic system, and its stoichiometry crucially depends upon CD95 stimulation strength.
Figure 1. Caspase-8 chain length at the DISC depends on CD95L stimulation strength. We developed an agent-based model of procaspase-8 activation at the DISC by chain formation via DED interaction of procaspase-8 molecules. The model predicted that lower CD95L stimulation should result in longer chains at the DISC (right side, depicted in green) and high CD95L stimulation strength should result in shorter chains (left side, depicted in purple). This prediction was confirmed by AQUA peptide-based mass spectrometry analysis.Citation7
Despite the progress in understanding procaspase-8 activation via the DED chain mechanism by MacFarlane’s and our groups, from these findings a number of new questions arose. What is the role of the DR aggregation in mediating the DED chain formation? How do c-FLIP proteins inhibit caspase-8 activation in a DED chain? How is the activation of procaspase-8 regulated in the chain? These intriguing questions will be elucidated in future studies.
Taken together, using a combination of experimental methods with mathematical modeling, we unraveled a new view on procaspase-8 activation at the DISC and its dynamics. This powerful methodology could be further applied to the study of other death signaling platforms such as the necrosome, ripoptosome and other receptor complexes and provide further fascinating insights into the mechanisms of life and death decisions.
References
- Krammer PH, et al. Nat Rev Immunol 2007; 7:532 - 42; http://dx.doi.org/10.1038/nri2115; PMID: 17589543
- Hughes MA, et al. Mol Cell 2009; 35:265 - 79; http://dx.doi.org/10.1016/j.molcel.2009.06.012; PMID: 19683492
- Oberst A, et al. J Biol Chem 2010; 285:16632 - 42; http://dx.doi.org/10.1074/jbc.M109.095083; PMID: 20308068
- Fuentes-Prior P, et al. Biochem J 2004; 384:201 - 32; http://dx.doi.org/10.1042/BJ20041142; PMID: 15450003
- Lavrik IN, et al. Cell Death Differ 2012; 19:36 - 41; http://dx.doi.org/10.1038/cdd.2011.155; PMID: 22075988
- Chang DW, et al. EMBO J 2003; 22:4132 - 42; http://dx.doi.org/10.1093/emboj/cdg414; PMID: 12912912
- Schleich K, et al. Mol Cell 2012; 47:306 - 19; http://dx.doi.org/10.1016/j.molcel.2012.05.006; PMID: 22683265
- Dickens LS, et al. Mol Cell 2012; 47:291 - 305; http://dx.doi.org/10.1016/j.molcel.2012.05.004; PMID: 22683266
- Bentele M, et al. J Cell Biol 2004; 166:839 - 51; http://dx.doi.org/10.1083/jcb.200404158; PMID: 15364960
- Lavrik IN, et al. J Biol Chem 2007; 282:13664 - 71; http://dx.doi.org/10.1074/jbc.M700434200; PMID: 17347143