Our genomes are attacked constantly by reactive oxygen species generated as by-products of metabolic processes or induced by exogenous sources, such as UV light and cigarette smoke. Tens of thousands of DNA lesions are estimated to occur daily in each human cell. To cope with these genomic insults and preserve their genetic information, cells have evolved a set of conserved mechanisms collectively called the DNA damage response (DDR). These highly orchestrated signaling and DNA repair networks are tightly controlled by various post-translational modifications (PTMs), including, predominantly, phosphorylation and ubiquitination.Citation1
Double-strand breaks (DSBs) are the most deleterious type of DNA lesion. Under physiological conditions, the majority of DNA breakages that enter or arise in mitosis originate during S phase, when DNA replication machinery approaches unstable or challenging DNA structures, such as fragile sites.Citation2 Once a DSB occurs, the protein kinases ataxia-telangiectasia mutated protein (ATM) and DNA-dependent protein kinase (DNA-PK) activate the DDR by phosphorylating H2AX to generate γ-H2AX. This factor recruits mediator of DNA damage checkpoint 1 (MDC1), which mediates the DDR in the vicinity of a DSB. MDC1 promotes the phosphorylation-dependent recruitment of DDR-sensor proteins, such as the Mre11-Rad50-Nbs1 complex (MRN).Citation1 This formation is deemed the “proximal” DDR, and its components are early markers of DSBs. MDC1 also functions as a platform for the integration of ubiquitination events. As a second PTM, ubiquitination orchestrates the “distal” DDR. Phosphorylated MDC1 recruits the E3 ubiquitin ligase, RNF8, which mobilizes two parallel ubiquitin chain cascades: Lys48-linked ubiquitin and Lys63-linked ubiquitin.Citation3 These chains are essential for the recruitment of “caretaker” gene products and DNA repair proteins, such as 53BP1, BRCA1 and Rad51, which constitute the fully activated DDR.Citation4,Citation5 Precise spatiotemporal synchronization of proximal and distal DDR events is essential for efficient DNA repair.
The fully activated DDR, coordinated by phosphorylation and ubiquitination, can occur during G1, S or G2 phases of the cell cycle. In contrast, only the proximal DDR exists in mitosis.Citation6 This truncated DDR can sense and mark DNA damage but cannot execute DNA repair, owing to its inability to activate ubiquitin signaling cascades. Cells in mitosis preserve their damaged DNA until mitotic exit, when the DDR resumes full activation, and the lesion can be repaired.Citation7 Inactivation of DNA repair in mitosis likely occurs because of compacted and condensed mitotic chromosomes that obstruct RNF8 and the other distal DDR components to the proximal DDR assembly. In addition, the complexity of mitotic processes and the limited duration of mitosis could explain why chromosomal segregation and cytokinesis preclude DNA repair.
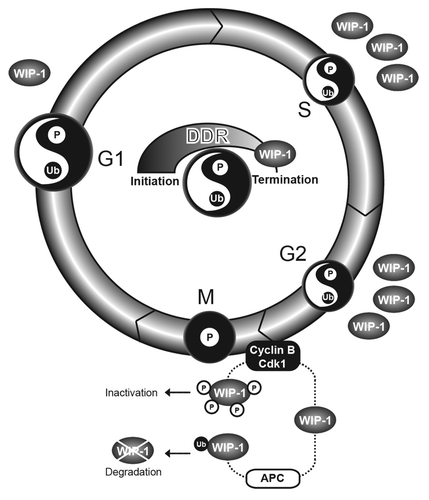
In a recent issue of Cell Cycle, Macurek et al.Citation8 described the maintenance of the proximal DDR during mitosis and the mechanisms by which damaged mitotic DNA is marked and preserved but not repaired. These authors identified the strict, cell cycle-dependent regulation of Wip1/PPM1D, a phosphatase that dephosphorylates γ-H2AX and other elements of the DDR.Citation9 Wip1 protein expression and enzymatic activity are low during G1, increase gradually as cells enter S phase and peak during G2. When cells enter mitosis, Wip1 phosphatase activity is downregulated rapidly by ubiquitin-dependent Wip1 proteasomal degradation coordinated by APC/CCdc20 (). Macurek et al.Citation8 demonstrated that the Wip1 catalytic domain is phosphorylated at multiple sites during mitosis by cyclin B/Cdk1 and other unidentified kinases. Phosphorylation of Wip1 inactivates its phosphatase activity. Endogenous Wip1 phosphorylation may regulate its phosphatase activity or may only be a prerequisite for ubiquitination and consequent proteasomal degradation. Nevertheless, the work of Macurek et al.Citation8 provides evidence that phosphorylation and proteasomal degradation occur cooperatively to inactivate and remove Wip1 from the cellular milieu during mitosis. Wip1 is essential for the timely inactivation of the DDR. After damaged DNA is repaired, the removal of Wip1 as the cell proceeds to mitosis allows only the proximal DDR to assemble on existing DNA lesions. Macurek et al.Citation8 elucidate the function of Wip1 phosphatase during the cell cycle and underscore the importance of Wip1 downregulation in the preservation of a truncated DDR during mitosis. Additional research is warranted to address the molecular details of distal DDR inactivation during mitosis.
Figure 1. Wip1 inactivation and degradation lead to truncated DDR in mitosis. Wip1 phosphatase terminates the DDR. Here, the concept of yin-yang is used to represent the harmony of phosphorylation (p = Yin-dark) and ubiquitination (Ub = Yang-white) events in the coordination of the fully activated DDR during G1, S, and G2. Only the fully activated DDR can execute DNA repair. The domination of Yin (P) in the DDR results from the truncated and inactive DDR during mitosis (M), which can identify, but not repair, DNA damage. The inactivation and degradation of Wip1 enables the existence of truncated DDR (Yin) in mitosis. Once the cell exits mitosis, Wip1 expression gradually increases.
References
- Ciccia A, Elledge SJ. The DNA damage response: making it safe to play with knives. Mol Cell 2010; 40:179 - 204; http://dx.doi.org/10.1016/j.molcel.2010.09.019; PMID: 20965415
- Lukas C, Savic V, Bekker-Jensen S, Doil C, Neumann B, Pedersen RS, et al. 53BP1 nuclear bodies form around DNA lesions generated by mitotic transmission of chromosomes under replication stress. Nat Cell Biol 2011; 13:243 - 53; http://dx.doi.org/10.1038/ncb2201; PMID: 21317883
- Ramadan K. p97/VCP- and Lys48-linked polyubiquitination form a new signaling pathway in DNA damage response. Cell Cycle 2012; 11:1062 - 9; http://dx.doi.org/10.4161/cc.11.6.19446; PMID: 22391235
- Al-Hakim A, Escribano-Diaz C, Landry MC, O’Donnell L, Panier S, Szilard RK, et al. The ubiquitous role of ubiquitin in the DNA damage response. DNA Repair (Amst) 2010; 9:1229 - 40; http://dx.doi.org/10.1016/j.dnarep.2010.09.011; PMID: 21056014
- Meerang M, Ritz D, Paliwal S, Garajova Z, Bosshard M, Mailand N, et al. The ubiquitin-selective segregase VCP/p97 orchestrates the response to DNA double-strand breaks. Nat Cell Biol 2011; 13:1376 - 82; http://dx.doi.org/10.1038/ncb2367; PMID: 22020440
- Giunta S, Jackson SP. Give me a break, but not in mitosis: the mitotic DNA damage response marks DNA double-strand breaks with early signaling events. Cell Cycle 2011; 10:1215 - 21; http://dx.doi.org/10.4161/cc.10.8.15334; PMID: 21412056
- Giunta S, Belotserkovskaya R, Jackson SP. DNA damage signaling in response to double-strand breaks during mitosis. J Cell Biol 2010; 190:197 - 207; http://dx.doi.org/10.1083/jcb.200911156; PMID: 20660628
- Macurek L, Benada J, Müllers E, Halim VA, Krejčíková K, Burdová K, et al. Downregulation of Wip1 phosphatase modulates the cellular threshold of DNA damage signaling in mitosis. Cell Cycle 2012; 12; PMID: 23255129
- Lowe J, Cha H, Lee MO, Mazur SJ, Appella E, Fornace AJ Jr.. Regulation of the Wip1 phosphatase and its effects on the stress response. Front Biosci 2012; 17:1480 - 98; http://dx.doi.org/10.2741/3999; PMID: 22201816