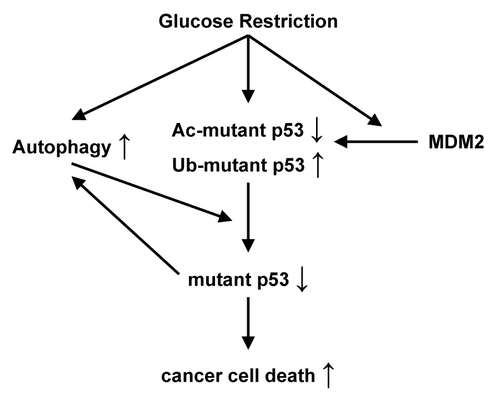
While the wild type form of p53 possesses strong tumor-suppressive activities, the p53 proteins that are commonly mutated in cancer often endow more malignant properties to the cancers they inhabit.Citation1,Citation2 There are several lines of evidence supporting such oncogenic gain of function of mutant p53. Compared with p53-null mice, knock-in mice harboring mutant p53 proteins display different and more metastatic tumor spectra. Such mutant proteins are frequently present at far higher levels than the wild-type protein in tumors; in fact, the p53 protein present in the knock-in mice accumulates in tumors despite being inherently unstable in normal tissues,Citation3 suggesting that stabilization of mutant p53 protein is required for its oncogenic activity. Consistently, knockdown of mutant p53 protein in human cancer cell lines leads to reduced cell proliferation, invasion, motility, tumorigenicity and resistance to anticancer drugs.Citation1,Citation2 Since epidemiological studies indicate that high levels of mutant p53 proteins correlate with tumor aggressiveness and poorer outcomes, it is important to understand how mutant p53 is stabilized in tumors and how it can be eliminated.
The Avantaggiati group in a recent issue of Cell Cycle have recently provided important new insight into this question.Citation4 They demonstrated that glucose restriction (GR) results in deacetylation and destabilization of endogenous mutant p53, but not of wild-type p53 protein. As protein degradation is mediated primarily by two pathways; the 26S proteasome and autophagy, the authors sought to identify which pathway is responsible for the degradation. They found that while the proteasome inhibitor MG132 treatment does not abolish GR-induced mutant p53 degradation, knockdown of autophagy genes such as Beclin-1, ATG5, ATG7 or pharmacological inhibition of autophagy prevents the degradation. Further, mutant p53 physically interacts with components of the autophagy machinery in a GR-dependent manner, suggesting that mutant p53 is a substrate for autophagic degradation. Interestingly, a C-terminal acetylation-mimicking mutant version of p53 (G245A-6KQ) is resistant to GR-dependent degradation. Taken together, these findings suggest that GR induces posttranslational modifications of lysines within mutant p53 proteins, which subsequently target them for autophagy-dependent degradation.
The authors next examined the effects of GR-induced degradation of mutant p53 on autophagy and cancer cell death. As indicated by two markers of autophagy (LC3 conversion and p62 degradation), GR activates this process, and the subsequent mutant p53 protein degradation leads to a maximal induction of autophagy and cell death. Consistent with their previous observations, expression of G245A-6KQ mutant p53 confers at least partial resistance to GR-induced cell death. Next, the authors used two mutant p53 mouse models to investigate the effects of a low carbohydrate (LC) diet on p53 stability and tumorigenicity in vivo. In line with their ex vivo data, in knock-in mice harboring the tumor-derived p53 mutation (A135V) placed on an LC diet, p53 protein is destabilized in mammary glands, ovaries and adipose tissues, while p53 in wild-type mice is stabilized. In xenografted mice, mutant p53 expressing cancer cells show enhanced tumorigenicity compared with those that are either p53-null or bearing wild-type p53.Citation2 Strikingly, Rodriguez et al. found that an LC diet leads to a marked decrease in size of xenografted tumors with mutant p53, while this diet does not decrease tumors arising from the GR-resistant mutant p53G245A-6KQ expressing cells—it actually increases their growth.
The findings of Rodriguez et al.Citation4 raise several interesting questions. First, virtually every residue with the ~200 amino acid DNA-binding domain of p53 has been found to be mutated in different tumors, albeit with differing frequencies. Autophagy is activated when the proteasome fails to eliminate misfolded and aggregated proteins,Citation5 and different mutant p53 proteins vary in their propensity for aggregation.Citation6 Is there a correlation between mutant p53 proteins’ tendency to aggregate and their ability to be degraded after GR? Second, MDM2, the prime E3 ligase that ubiquitinates wild-type p53, cannot bind to p53 in Nutlin-3a-treated cells.Citation7 Since the authors found that while mutant p53 is ubiquitinated after GR, Nutlin-3a actually blocks GR-induced mutant p53 degradation, what is the role of MDM2 in the setting of their study? Last, but not least, AMP-activated protein kinase (AMPK) has been known to regulate autophagyCitation8 and may also be involved in GR-induced mutant p53 degradation. Is AMPK critical for the upstream signaling pathway to GR-induced mutant p53 degradation? Answers to these and other questions will provide the next chapters in this exciting story. ()
References
- Brosh R, Rotter V. When mutants gain new powers: news from the mutant p53 field. Nat Rev Cancer 2009; 9:701 - 13; PMID: 19693097
- Freed-Pastor WA, Prives C. Mutant p53: one name, many proteins. Genes Dev 2012; 26:1268 - 86; http://dx.doi.org/10.1101/gad.190678.112; PMID: 22713868
- Terzian T, Suh YA, Iwakuma T, Post SM, Neumann M, Lang GA, et al. The inherent instability of mutant p53 is alleviated by Mdm2 or p16INK4a loss. Genes Dev 2008; 22:1337 - 44; http://dx.doi.org/10.1101/gad.1662908; PMID: 18483220
- Rodriguez OC, Choudhury S, Kolukula V, Vietsch EE, Catania J, Preet A, et al. Dietary downregulation of mutant p53 levels via glucose restriction: mechanisms and implications for tumor therapy. Cell Cycle 2012; 11:4436 - 46; http://dx.doi.org/10.4161/cc.22778; PMID: 23151455
- Kirkin V, McEwan DG, Novak I, Dikic I. A role for ubiquitin in selective autophagy. Mol Cell 2009; 34:259 - 69; http://dx.doi.org/10.1016/j.molcel.2009.04.026; PMID: 19450525
- Xu J, Reumers J, Couceiro JR, De Smet F, Gallardo R, Rudyak S, et al. Gain of function of mutant p53 by coaggregation with multiple tumor suppressors. Nat Chem Biol 2011; 7:285 - 95; http://dx.doi.org/10.1038/nchembio.546; PMID: 21445056
- Vassilev LT, Vu BT, Graves B, Carvajal D, Podlaski F, Filipovic Z, et al. In vivo activation of the p53 pathway by small-molecule antagonists of MDM2. Science 2004; 303:844 - 8; http://dx.doi.org/10.1126/science.1092472; PMID: 14704432
- Mihaylova MM, Shaw RJ. The AMPK signalling pathway coordinates cell growth, autophagy and metabolism. Nat Cell Biol 2011; 13:1016 - 23; http://dx.doi.org/10.1038/ncb2329; PMID: 21892142