Cell cycle progression through the G1 phase is tightly controlled by cyclin-dependent kinases (CDKs). Activity of CDKs is negatively regulated by two unrelated families of CDK inhibitors (CDKi), namely, INK and Cip/Kip. The Cip/Kip family of CDKi consists of three members, p21Cip1/WAF1, p27Kip1 and p57Kip2. In the context of human cancers, these three proteins are considered suppressors of tumorigenesis and tumor progression. Therefore, their levels of expression in both normal and cancerous cells are under complex transcriptional and post-translational regulations, including ubiquitination and proteasomal degradation.Citation1
The upstream regulators leading to degradation of p21Cip1/WAF1 and p27Kip1 proteins have been extensively studied. For both p21Cip1/WAF1 and p27Kip1 proteins, Ser/Thr phosphorylation serves as a pivotal event that exports them out of nucleus and promotes their degradation in both cytoplasm and nucleus. Although multiple Ser/Thr kinases can phosphorylate p21Cip1/WAF1 and p27Kip1, Akt has attracted substantial attention because of its frequent activation in many types of cancer and its close association with several oncogenic receptor tyrosine kinases (RTKs), such as EGFR and HER2. In HER2-overexpressing breast cancer cells, Akt is often hyperactive and phosphorylates p21Cip1/WAF1 at Thr145Citation2 and p27Kip1 at Thr157/Thr198,Citation3-Citation5 resulting in their nuclear export and proteasomal degradation. Akt-mediated destabilization of p21Cip1/WAF1 and p27Kip1 underlies the aggressive growth and progression of HER2-driven breast cancer.
Emerging evidence suggests that p57Kip2 plays an important role in embryonic development, hematopoietic stem cell quiescence and inhibition of cell cycle progression. Unlike p21Cip1/WAF1 and p27Kip1, the upstream pathways leading to p57Kip2 intracellular trafficking and stability are still not well understood. Nevertheless, it has been reported that TGF-β1 stimulates p57Kip2 phosphorylation at Thr310, leading to its ubiquitination and proteasomal degradation. Stress-activated protein kinase p38 phosphorylates p57Kip2 at Thr143 and enhances p57Kip2 association with and inhibition of CDK2.Citation6 Subunit 6 of the COP9 signalosome complex (CSN6) associates with p57Kip2 and Skp2, a component of the E3 ligase. This association, in turn, leads to Skp2-mediated p57Kip2 ubiquitination and subsequent degradation.Citation7 Interestingly, CSN6 can be phosphorylated by Akt at Ser60, which renders CSN6 more stabilized.
The elegant study by Zhao et al. showed for the first time that Akt interacts with and phosphorylates p57Kip2 at Ser282 and Thr310, resulting in p57Kip2 nuclear export, ubiquitination and proteasome-mediated degradation.Citation8 HER2-overexpressing breast cancer cells were found to express lower levels of p57Kip2 than those with normal HER2 expression. Constitutively active Akt induced p57Kip2 degradation, whereas a dominant-negative Akt mutant and PI3K inhibition led to p57Kip2 stabilization. The authors further showed that Akt-mediated phosphorylation and stabilization of CSN6 contributed to p57Kip2 degradation. Consistent with these observations, restoration of p57Kip2 in HER2-amplified, p57Kip2-deficient breast cancer cells led to reduced cell growth in vitro and the inability to form xenografts in nude mice, indicating that p57Kip2 antagonizes HER2-mediated breast cancer cell growth and possibly HER2-related tumorigenesis. Analysis of patient breast carcinomas revealed for the first time that in patients with HER2-overexpressing tumors, low p57Kip2 levels correlated with poor overall survival.
A significant implication for the novel Akt→p57Kip2 signaling axis is that it may play an important role in embryonic development in addition to cancer. Unlike p21Cip1/WAF1 and p27Kip1, p57Kip2-knockout mice uniquely displayed developmental defects and a phenotype that resembles the Beckwith-Wiedeman syndrome, a childhood overgrowth syndrome. The potential involvement of the Akt→p57Kip2 link in embryonic development is worthy of investigations in future studies.
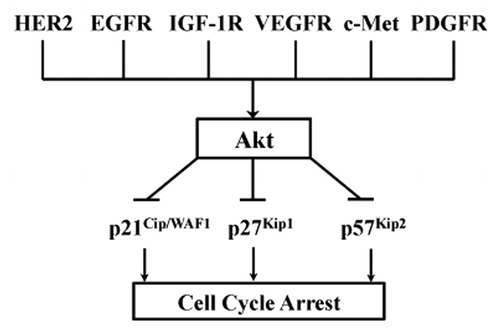
In light of the new findings reported by Zhao et al. combined with those published previously,Citation2-Citation5 we now know that Akt phosphorylates all three members of the Cip/Kip family of CDKi (). This important discovery indeed revised the signaling landscape for the HER2→Akt signaling module, in that Akt plays a central role in lifting CDKi-mediated cell cycle arrest and therefore facilitates proliferation of HER2-amplified breast cancer. An immediate significant implication of these findings is that the newly discovered Akt→p57Kip2 signaling axis may have a broad impact on different subtypes of breast cancers, such as triple-negative and basal-like breast cancer, given the fact that Akt can also be activated by other RTKs besides HER2, such as EGFR, IGF-1R, VEGFR, c-Met and PDGFR. Another potential implication is that the negative regulation of p57Kip2 by Akt may also exist in other types of cancer, since Akt activation is a common phenomenon in a number of human cancers. Indeed, future investigations are needed to broadly explore the impact of the Akt→p57Kip2 signaling axis on cancer and embryonic development.
Figure 1. Akt at the converging crossroad connecting multiple receptor tyrosine kinases to all three members of the Cip/Kip family of CDKi. Akt is known to be activated by several RTKs that are frequently activated in human cancers. These RTKs include HER2, EGFR, IGF-1R, VEGFR, c-Met, PDGFR and several others. It is also known that there are a number of proteins serving as the downstream effectors of Akt, such as, mTOR and two CDKi, p21Cip1/WAF1 and p27Kip1. Importantly, the study by Zhao et al. provided the first evidence that defines p57Kip2 as the substrate of Akt, thus making Akt a central common Ser/Thr kinase that negatively regulates all three members of the Cip/Kip family of CDKi that contribute to cell cycle arrest. Consequently, these reported findings potentially place Akt at the converging point that connects multiple RTKs to all three members of the Cip/Kip family of CDKi, in order to unblock cell cycle arrest and support uncontrolled cell proliferation in cancer cells.
References
- Lu Z, Hunter T. Ubiquitylation and proteasomal degradation of the p21(Cip1), p27(Kip1) and p57(Kip2) CDK inhibitors. Cell Cycle 2010; 9:2342 - 52; http://dx.doi.org/10.4161/cc.9.12.11988; PMID: 20519948
- Zhou BP, Liao Y, Xia W, Spohn B, Lee MH, Hung MC. Cytoplasmic localization of p21Cip1/WAF1 by Akt-induced phosphorylation in HER-2/neu-overexpressing cells. Nat Cell Biol 2001; 3:245 - 52; http://dx.doi.org/10.1038/35060032; PMID: 11231573
- Liang J, Zubovitz J, Petrocelli T, Kotchetkov R, Connor MK, Han K, et al. PKB/Akt phosphorylates p27, impairs nuclear import of p27 and opposes p27-mediated G1 arrest. Nat Med 2002; 8:1153 - 60; http://dx.doi.org/10.1038/nm761; PMID: 12244302
- Shin I, Yakes FM, Rojo F, Shin NY, Bakin AV, Baselga J, et al. PKB/Akt mediates cell-cycle progression by phosphorylation of p27(Kip1) at threonine 157 and modulation of its cellular localization. Nat Med 2002; 8:1145 - 52; http://dx.doi.org/10.1038/nm759; PMID: 12244301
- Viglietto G, Motti ML, Bruni P, Melillo RM, D’Alessio A, Califano D, et al. Cytoplasmic relocalization and inhibition of the cyclin-dependent kinase inhibitor p27(Kip1) by PKB/Akt-mediated phosphorylation in breast cancer. Nat Med 2002; 8:1136 - 44; http://dx.doi.org/10.1038/nm762; PMID: 12244303
- Joaquin M, Gubern A, González-Nuñez D, Josué Ruiz E, Ferreiro I, de Nadal E, et al. The p57 CDKi integrates stress signals into cell-cycle progression to promote cell survival upon stress. EMBO J 2012; 31:2952 - 64; http://dx.doi.org/10.1038/emboj.2012.122; PMID: 22569127
- Chen B, Zhao R, Su CH, Linan M, Tseng C, Phan L, et al. CDK inhibitor p57 (Kip2) is negatively regulated by COP9 signalosome subunit 6. Cell Cycle 2012; 11:4633 - 41; http://dx.doi.org/10.4161/cc.22887; PMID: 23187808
- Zhao R, Yang HY, Shin J, Phan L, Fang L, Yeung SC, et al. CDK inhibitor p57 (Kip2) is downregulated by Akt during HER2-mediated tumorigenicity. Cell Cycle 2013; 12; PMID: 23421998