Abstract
Ubiquitylation is currently recognized as a major posttranslational modification that regulates diverse cellular processes. Pirh2 is a ubiquitin E3 ligase that regulates the turnover and functionality of several proteins involved in cell proliferation and differentiation, cell cycle checkpoints, and cell death. Here we review the role of Pirh2 as a regulator of the DNA damage response through the ubiquitylation of p53, Chk2, p73, and PolH. By ubiquitylating these proteins, Pirh2 regulates cell cycle checkpoints and cell death in response to DNA double-strand breaks or the formation of bulky DNA lesions. We also discuss how Pirh2 affects cell proliferation and differentiation in unstressed conditions through ubiquitylation and degradation of c-Myc, p63, and p27kip1. Finally, we link these different functions of Pirh2 to its role as a tumor suppressor in mice and as a prognosis marker in various human cancer subtypes.
Introduction
Ubiquitylation has recently emerged as a key posttranslational modification in various cellular responses, regulating important cellular processes such as cell growth and proliferation, apoptosis, and the response to DNA damage. Pirh2 (p53-induced protein with a RING-H2 domain, also known as Rchy1) is an ubiquitin E3 ligase that was initially identified as a protein that interacts with the androgen receptor.Citation1 It was subsequently identified as a transcriptional target of p53 and a regulator of p53 turnover following DNA damage.Citation2 It has since been implicated in the ubiquitylation and degradation of several key proteins involved in the regulation of cell cycle, cell death, and proliferation such as p73, p63, p27kip1, and c-Myc.Citation3-Citation6 We have recently identified the tumor suppressor and cell cycle regulator Chk2 as an additional target of Pirh2.Citation7 In what follows we show that through its E3 ligase activity Pirh2 acts as a key regulator of cell proliferation and cell cycle progression under both normal and stressed conditions.
Pirh2 and p53
The tumor suppressor p53 is a transcription factor that plays key roles in the regulation of cellular responses to stress such as DNA damage, oncogene activation and hypoxia.Citation8 In response to DNA damage p53 activates numerous downstream transcriptional targets involved in cell cycle arrest and apoptosis such as p21, Puma, Bax, and Noxa.Citation9 The levels and activity p53 in the cell are largely regulated through its posttranslational modifications such as ubiquitylation, phosphorylation, and acetylation. Monoubiquitylation of p53 can result in its export from the nucleus and its mitochondrial translocation, thus preventing it from acting as a transcription factor while allowing it to induce mitochondrial mediated apoptosis.Citation10 Polyubiquitylation of p53 leads to its proteasomal degradation and several E3 ligases for p53 ubiquitylation have been identified including Mdm2, Cop1, ARF-BP1, synoviolin, p300/CBP, E4F1, and Pirh2.Citation11 Similar to Mdm2, Pirh2 is a transcriptional target of p53 and its transcription is upregulated in a cell type and DNA damage sp.ecific manner.Citation2 Pirh2 binds to p53 and mediates its polyubiquitylation in concert with the E2 ligase ubcH5b.Citation2 Overexpression of Pirh2 leads to a decrease in p53-mediated functions including apoptosis and cell cycle arrest.Citation2 Studies of the interaction between Pirh2 and p53 showed that Pirh2 preferentially interacts with and leads to the degradation of p53 in its active tetrameric form rather than its monomeric form.Citation12 Despite the data showing Pirh2 ubiquitylation of p53 and other key cellular proteins, the in vivo functions of this E3 ligase remained elusive. Recently we reported that in contrast to Mdm2 knockouts, Pirh2−/− mice were viable and displayed normal development.Citation6 Analysis of Pirh2−/− mice supports a role for Pirh2 in mediating degradation of activated tetrameric p53, as under untreated conditions, these mutants displayed only a mild increase in the basal levels of the p53 protein and no significant transactivation of p53 targets was observed.Citation6 In contrast, irradiated cells derived from various organs of Pirh2−/− mice displayed a greater increase in the expression levels of p53 and its transcriptional targets involved in cell cycle arrest (e.g., p21) and apoptosis (e.g., Puma, Noxa, and Bax). Furthermore, cells derived from Pirh2−/− mice were more radiosensitive, owing to their increased p53 activity.Citation6 These results support an important physiological role for Pirh2 in the recovery from DNA damage.
Pirh2 and the p53 Homologs p63 and p73
The p63 and p73 proteins are homologs of p53.Citation13 They share several of the functional domains of p53 including its DNA-binding domain, oligomerization domain, and transactivation domain.Citation14,Citation15 Both p63 and p73 act as transcription factors to activate pro-apoptotic proteins and cell cycle regulators.Citation16 They play a role in G1/S cell cycle arrest by upregulating p21.Citation15 In addition, p63 and p73 play important roles in development.Citation17 The p63 protein is involved in development of limbs and all organs forming stratified epithelium such as the skin, mammary and salivary glands.Citation17,Citation18 In addition, p63 has also been involved in tumor suppression.Citation19 Mice that are p73-deficient display abnormal neuronal development.Citation18,Citation20 They were also found to develop tumors.Citation19,Citation21
Recently, it was found that p63 is a target of Pirh2 ubiquitylation. Pirh2 can ubiquitylate p63 and its dominant negative form ΔN-p63, leading to their proteasomal degradation.Citation4 ΔN-p63 was found to promote epidermal cell proliferation whereas keratinocyte differentiation requires restraining of proliferation.Citation22 Pirh2 overexpression in HaCaT cells promoted their in vitro differentiation into keratinocytes through the downregulation of ΔN-p63.Citation4 This study suggests an important role for Pirh2 in controlling cell proliferation in the context of cell differentiation.
The p73 protein is responsive to DNA damage.Citation15 Previous studies indicated that p73 is the target of HECT ubiquitin ligase Itch and F-box protein FBox45 that regulate its proteosomal degradation.Citation23,Citation24 The activity of these E3 ligases is downregulated following DNA damage, leading to accumulation of p73 protein.Citation23,Citation24 Because of the high homology between p73 and p53 it was hypothesized that p73 might also be a target of Pirh2 ubiquitylation. Indeed Pirh2 was found to polyubiquitylate p73 using K11, K29, K48, and K63 linkages in vivo and predominantly K63 linkages in vitro. This ubiquitylation of p73 leads to its proteasomal degradation and is downregulated following DNA damage.Citation25,Citation26 Pirh2 was found to be important for p73-mediated G1 cell cycle arrest and loss of Pirh2 protein was shown to increase p73-dependent and p53-independent cell death following exposure to the DNA damaging agent doxorubicin.Citation25,Citation26 By regulating the levels of p53 homologs p63 and p73, Pirh2 is able to induce G1/S cell cycle arrest or induce apoptosis in response to DNA damage in a p53-independent manner.
Pirh2 and p27kip1
The tumor suppressor p27kip1 is a cyclin-dependent kinase (CDK) inhibitor that regulates the transition from G0/G1 phases to the S phase of the cell cycle during normal cell proliferation.Citation27 Levels of p27kip1 are tightly regulated throughout the cell cycle. They are usually high during G0/G1 phase and drop sharply just before cell entry into the S phase.Citation28 Levels of p27kip1 are regulated trancriptionally, translationally and posttranslationally through phosphorylation and ubiquitylation.Citation27,Citation28 The ubiquitin ligase Skp2 polyubiquitylates p27kip1 and mediates its degradation. However, in its absence, p27kip1 was still degraded, prompting investigators to look for other ubiquitin ligases that may regulate p27kip1 turnover. Pirh2 was found to interact with p27kip1 and to mediate its polyubiquitylation and proteasomal degradation.Citation29 Knockdown of Pirh2 prevented serum-starved cells to be released from G0 cell cycle arrest following serum stimulation.Citation29 Therefore, Pirh2 can regulate normal cellular proliferation in response to mitogenic signals.
Pirh2 and c-Myc
Although Pirh2−/− mice display increased levels of the tumor suppressor p53 in response to DNA damage, we found that about 25% of Pirh2−/− mice display sp.ontaneous solid tumor development.Citation6 This unexpected increased cancer predisposition was explained by the observation that the expression level of the oncogene c-Myc was elevated in various tissues derived from Pirh2−/− mice. c-Myc was then found to be a target of Pirh2 polyubiquitylation and protesomal degradation. Interestingly, overexpression of c-Myc in Pirh2−/− mice was associated with lymphoproliferation and sp.lenomegaly caused by accumulation of plasma cells.Citation6 Increased proliferation in activated Pirh2−/− B and T-cells was observed, although there was a concomitant increase in apoptosis in these cells, probably caused by c-Myc-induced accumulation and activation of p19/ARF and p53.Citation6 Interestingly, double mutant Pirh2−/−p53−/− mice die significantly faster than their single mutant counterparts and 60% of these mice develop tumors. This could be caused by the unrestricted c-Myc-induced cell proliferation in the absence of p53.Citation6 Studies of the expression of PIRH2 in human cancer indicated that decreased PIRH2 mRNA levels were associated with poor outcome in patients with breast, ovarian, and squamous cell lung carcinoma.Citation6 Conversely, increased levels of PIRH2 expression were reported for lung cancerCitation30 and were also found to correlate with disease progression in prostate cancer.Citation31 Thus, this E3 ligase might play different roles in cancer progression and tumorigenesis depending on cell and tissue type. Because its targets include both tumor suppressors (e.g., p53) and oncogenes (e.g., c-Myc), it is possible that PIRH2 itself acts as a tumor suppressor in some contexts and as an oncoprotein in others.
Pirh2 and DNA Polymerase Eta
DNA polymerase Eta (polH) is required for translesion DNA synthesis following exposure to UV irradiation. It was found that Pirh2 monoubiquitylates polH.Citation32 This ubiquitylation of polH suppresses its interaction with PCDNA and prevents it from acting as a polymerase that bypasses residual DNA lesions during the S phase of the cell cycle. This results in increased cellular sensitivity to UV light.Citation32 Thus, Pirh2 can regulate cellular responses to different types of DNA damage including DNA double strand breaks caused by ionizing radiation and bulky DNA lesions caused by UV irradiation.
Pirh2 and Chk2
Chk2 is a serine-threonine protein kinase involved in the regulation of cell cycle checkpoints. Chk2 is also a tumor suppressor and multi-organ cancer susceptibility gene. Indeed, germline mutations in the human CHK2 gene have been observed in different types of familial cancers including breast and prostate cancer.Citation33 Li-Fraumeni syndrome is an autosomal dominant hereditary disease which is typically associated with p53 mutations. Li-Fraumeni patients usually develop multiple tumors over their lifetime. CHK2 mutations that destabilize the CHK2 protein and impair its function have been identified in a subset of Li-Fraumeni patients.Citation34 In response to DNA double strand breaks, CHK2 is phosphorylated and activated by its upstream kinase ATM.Citation35 It then acts to phosphorylate multiple downstream substrates involved in cell cycle checkpoint, DNA repair, and apoptosis such as p53 and Brca1.Citation33 CHK2 is involved in the G1-S, intra-S, and G2-M cell cycle checkpoints in response to DNA double strand break formation.Citation36 Our most recent study of Pirh2−/− mice revealed an increase in Chk2 levels both under unstressed conditions and following DNA damage.Citation7 Despite the similar level of expression and activation of Atm in the absence or presence of Pirh2, γ-irradiation resulted in higher phosphorylation levels of p53 and E2F1, two Chk2 substrates in Pirh2−/− cells compared with wildtype controls. This finding suggested the possibility that accumulated Chk2 in Pirh2−/− cells might be due to defective ubiquitylation of this kinase. Chk2 was previously reported to be a target of ubiquitylation and its stability was reported to be regulated by its phosphorylation at Ser456 following DNA damage.Citation37 Mdm2 and PCAF were both found to be important for Chk2 polyubiquitylation.Citation38 However, the fact that Mdm2 and PCAF E3 ligase mutants are still able to induce Chk2 polyubiquitylation suggests that these E3 ligases do not directly ubiquitylate Chk2.Citation38 Ionizing radiation-induced Chk2 autophosphorylation at Ser379 was also found to be required for its ubiquitylation by a Cul1-containig complex.Citation39 Our data indicated that Chk2 and Pirh2 interact and that Pirh2 polyubiquitylates Chk2 both in vivo and in vitro in the presence of ubcH5b. Similar to what was previously observed, Chk2 phosphorylation at Ser460 in mouse cells prevented Chk2 ubiquitylation and mutation of this serine residue to alanine resulted in increased ubiquitylation of Chk2 by Pirh2. This suggests a strong role for Chk2 phosphorylation for the regulation of its stability, especially following DNA damage.Citation7 USP28, a deubiquitylating enzyme that is a downstream target of ATM in response to DNA damage, is needed to stabilize Chk2 in response to DNA damage.Citation40 We found that USP28 counteracts Pirh2 ubiquitylation of Chk2.Citation7 To examine the effect of Pirh2 deficiency on Chk2 physiological activity we examined cell cycle checkpoint responses of Pirh2−/− and Pirh2−/−Chk2−/− mouse embryonic fibroblasts following ionizing radiation. We found that loss of Pirh2 elicited a stronger G1-S and G2-M cell cycle arrest compared with what was observed in WT cells. Interestingly, concomitant loss of Chk2 completely rescued this phenotype suggesting that increased Chk2 levels and activity were responsible for this hyperactivation of the cell cycle checkpoints.Citation7
Conclusion
Recent studies have highlighted the role of the E3 ubiquitin ligase Pirh2 in the regulation of cell cycle checkpoints, cell proliferation, differentiation, and apoptosis. Pirh2 is especially important in the regulation of cellular homeostasis following the DNA damage response as it ubiquitylates and leads to the degradation of several transducers and effectors in this pathway including Chk2, p53, p73, p63, and polH. Pirh2 also has important roles in keeping cell proliferation in check through its regulation of c-Myc, p27kip1 and ΔN-p63. Depending on the cellular context Pirh2 can act as a tumor suppressor that inhibits excessive cell proliferation and promotes cell differentiation or an oncogene that hinders DNA damage responses and drives tumorigenesis. We expect that identification of novel substrates of the E3 ligase substrates will help further understand the cellular and physiological functions of this protein. ()
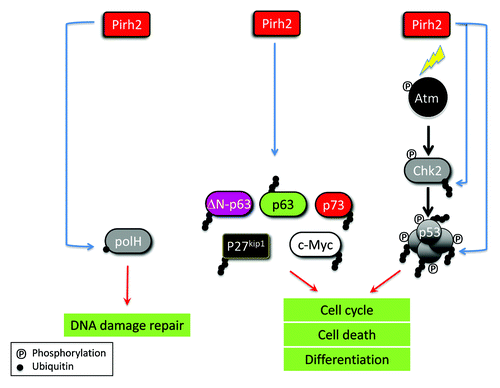
Figure 1. Involvement of Pirh2 in the regulation of different cellular processes through ubiquitylation of several key proteins. The E3 ligase Pirh2 ubiquitylates several substrates including c-Myc, Chk2,S p53, PolH, p63, DN-p63, p73, and p27kip1 (See text). Through these ubiquitylation events, Pirh2 contributes to the regulation of DNA damage repair, cell cycle, cell death, and differentiation.
Acknowledgments
This work was supported by grants from the Canadian Institute of Health Research and the National Cancer Institute of Canada.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- Beitel LK, Elhaji YA, Lumbroso R, Wing SS, Panet-Raymond V, Gottlieb B, Pinsky L, Trifiro MA. Cloning and characterization of an androgen receptor N-terminal-interacting protein with ubiquitin-protein ligase activity. J Mol Endocrinol 2002; 29:41 - 60; http://dx.doi.org/10.1677/jme.0.0290041; PMID: 12200228
- Leng RP, Lin Y, Ma W, Wu H, Lemmers B, Chung S, Parant JM, Lozano G, Hakem R, Benchimol S. Pirh2, a p53-induced ubiquitin-protein ligase, promotes p53 degradation. Cell 2003; 112:779 - 91; http://dx.doi.org/10.1016/S0092-8674(03)00193-4; PMID: 12654245
- Jung YS, Qian Y, Chen X. Pirh2 RING-finger E3 ubiquitin ligase: its role in tumorigenesis and cancer therapy. FEBS Lett 2012; 586:1397 - 402; http://dx.doi.org/10.1016/j.febslet.2012.03.052; PMID: 22673504
- Jung YS, Qian Y, Yan W, Chen X. Pirh2 E3 ubiquitin ligase modulates keratinocyte differentiation through p63. J Invest Dermatol 2013; 133:1178 - 87; http://dx.doi.org/10.1038/jid.2012.466; PMID: 23235527
- Yan W, Chen X, Zhang Y, Zhang J, Jung YS, Chen X. Arsenic suppresses cell survival via Pirh2-mediated proteasomal degradation of ΔNp63 protein. J Biol Chem 2013; 288:2907 - 13; http://dx.doi.org/10.1074/jbc.M112.428607; PMID: 23271742
- Hakem A, Bohgaki M, Lemmers B, Tai E, Salmena L, Matysiak-Zablocki E, Jung YS, Karaskova J, Kaustov L, Duan S, et al. Role of Pirh2 in mediating the regulation of p53 and c-Myc. PLoS Genet 2011; 7:e1002360; http://dx.doi.org/10.1371/journal.pgen.1002360; PMID: 22125490
- Bohgaki M, Hakem A, Halaby MJ, Bohgaki T, Li Q, Bissey PA, Shloush J, Kislinger T, Sanchez O, Sheng Y, et al. The E3 ligase PIRH2 polyubiquitylates CHK2 and regulates its turnover. Cell Death Differ 2013; 20:812 - 22; http://dx.doi.org/10.1038/cdd.2013.7; PMID: 23449389
- Zilfou JT, Lowe SW. Tumor suppressive functions of p53. Cold Spring Harb Perspect Biol 2009; 1:a001883; http://dx.doi.org/10.1101/cshperspect.a001883; PMID: 20066118
- Bell HS, Ryan KM. Targeting the p53 family for cancer therapy: ‘big brother’ joins the fight. Cell Cycle 2007; 6:1995 - 2000; http://dx.doi.org/10.4161/cc.6.16.4614; PMID: 17721076
- Marchenko ND, Wolff S, Erster S, Becker K, Moll UM. Monoubiquitylation promotes mitochondrial p53 translocation. EMBO J 2007; 26:923 - 34; http://dx.doi.org/10.1038/sj.emboj.7601560; PMID: 17268548
- Brooks CL, Gu W. p53 regulation by ubiquitin. FEBS Lett 2011; 585:2803 - 9; http://dx.doi.org/10.1016/j.febslet.2011.05.022; PMID: 21624367
- Sheng Y, Laister RC, Lemak A, Wu B, Tai E, Duan S, Lukin J, Sunnerhagen M, Srisailam S, Karra M, et al. Molecular basis of Pirh2-mediated p53 ubiquitylation. Nat Struct Mol Biol 2008; 15:1334 - 42; http://dx.doi.org/10.1038/nsmb.1521; PMID: 19043414
- Levrero M, De Laurenzi V, Costanzo A, Gong J, Wang JY, Melino G. The p53/p63/p73 family of transcription factors: overlapping and distinct functions. J Cell Sci 2000; 113:1661 - 70; PMID: 10769197
- Amelio I, Grespi F, Annicchiarico-Petruzzelli M, Melino G. p63 the guardian of human reproduction. Cell Cycle 2012; 11:4545 - 51; http://dx.doi.org/10.4161/cc.22819; PMID: 23165243
- Zawacka-Pankau J, Kostecka A, Sznarkowska A, Hedström E, Kawiak A. p73 tumor suppressor protein: a close relative of p53 not only in structure but also in anti-cancer approach?. Cell Cycle 2010; 9:720 - 8; http://dx.doi.org/10.4161/cc.9.4.10668; PMID: 20160513
- Allocati N, Di Ilio C, De Laurenzi V. p63/p73 in the control of cell cycle and cell death. Exp Cell Res 2012; 318:1285 - 90; http://dx.doi.org/10.1016/j.yexcr.2012.01.023; PMID: 22326462
- McKeon F, Melino G. Fog of war: the emerging p53 family. Cell Cycle 2007; 6:229 - 32; http://dx.doi.org/10.4161/cc.6.3.3876; PMID: 17297295
- Yang A, Walker N, Bronson R, Kaghad M, Oosterwegel M, Bonnin J, Vagner C, Bonnet H, Dikkes P, Sharpe A, et al. p73-deficient mice have neurological, pheromonal and inflammatory defects but lack spontaneous tumours. Nature 2000; 404:99 - 103; http://dx.doi.org/10.1038/35003607; PMID: 10716451
- Flores ER, Sengupta S, Miller JB, Newman JJ, Bronson R, Crowley D, Yang A, McKeon F, Jacks T. Tumor predisposition in mice mutant for p63 and p73: evidence for broader tumor suppressor functions for the p53 family. Cancer Cell 2005; 7:363 - 73; http://dx.doi.org/10.1016/j.ccr.2005.02.019; PMID: 15837625
- Garcia V, Silva J, Dominguez G, García JM, Peña C, Rodriguez R, Provencio M, España P, Bonilla F. Overexpression of p16INK4a correlates with high expression of p73 in breast carcinomas. Mutat Res 2004; 554:215 - 21; http://dx.doi.org/10.1016/j.mrfmmm.2004.04.008; PMID: 15450420
- Tomasini R, Mak TW, Melino G. The impact of p53 and p73 on aneuploidy and cancer. Trends Cell Biol 2008; 18:244 - 52; http://dx.doi.org/10.1016/j.tcb.2008.03.003; PMID: 18406616
- Lee H, Kimelman D. A dominant-negative form of p63 is required for epidermal proliferation in zebrafish. Dev Cell 2002; 2:607 - 16; http://dx.doi.org/10.1016/S1534-5807(02)00166-1; PMID: 12015968
- Peschiaroli A, Scialpi F, Bernassola F, Pagano M, Melino G. The F-box protein FBXO45 promotes the proteasome-dependent degradation of p73. Oncogene 2009; 28:3157 - 66; http://dx.doi.org/10.1038/onc.2009.177; PMID: 19581926
- Rossi M, De Laurenzi V, Munarriz E, Green DR, Liu YC, Vousden KH, Cesareni G, Melino G. The ubiquitin-protein ligase Itch regulates p73 stability. EMBO J 2005; 24:836 - 48; http://dx.doi.org/10.1038/sj.emboj.7600444; PMID: 15678106
- Jung YS, Qian Y, Chen X. The p73 tumor suppressor is targeted by Pirh2 RING finger E3 ubiquitin ligase for the proteasome-dependent degradation. J Biol Chem 2011; 286:35388 - 95; http://dx.doi.org/10.1074/jbc.M111.261537; PMID: 21852228
- Wu H, Zeinab RA, Flores ER, Leng RP. Pirh2, a ubiquitin E3 ligase, inhibits p73 transcriptional activity by promoting its ubiquitination. Mol Cancer Res 2011; 9:1780 - 90; http://dx.doi.org/10.1158/1541-7786.MCR-11-0157; PMID: 21994467
- Chu IM, Hengst L, Slingerland JM. The Cdk inhibitor p27 in human cancer: prognostic potential and relevance to anticancer therapy. Nat Rev Cancer 2008; 8:253 - 67; http://dx.doi.org/10.1038/nrc2347; PMID: 18354415
- Lu Z, Hunter T. Ubiquitylation and proteasomal degradation of the p21(Cip1), p27(Kip1) and p57(Kip2) CDK inhibitors. Cell Cycle 2010; 9:2342 - 52; http://dx.doi.org/10.4161/cc.9.12.11988; PMID: 20519948
- Hattori T, Isobe T, Abe K, Kikuchi H, Kitagawa K, Oda T, Uchida C, Kitagawa M. Pirh2 promotes ubiquitin-dependent degradation of the cyclin-dependent kinase inhibitor p27Kip1. Cancer Res 2007; 67:10789 - 95; http://dx.doi.org/10.1158/0008-5472.CAN-07-2033; PMID: 18006823
- Duan W, Gao L, Druhan LJ, Zhu WG, Morrison C, Otterson GA, Villalona-Calero MA. Expression of Pirh2, a newly identified ubiquitin protein ligase, in lung cancer. J Natl Cancer Inst 2004; 96:1718 - 21; http://dx.doi.org/10.1093/jnci/djh292; PMID: 15547185
- Logan IR, Gaughan L, McCracken SR, Sapountzi V, Leung HY, Robson CN. Human PIRH2 enhances androgen receptor signaling through inhibition of histone deacetylase 1 and is overexpressed in prostate cancer. Mol Cell Biol 2006; 26:6502 - 10; http://dx.doi.org/10.1128/MCB.00147-06; PMID: 16914734
- Jung YS, Hakem A, Hakem R, Chen X. Pirh2 E3 ubiquitin ligase monoubiquitinates DNA polymerase eta to suppress translesion DNA synthesis. Mol Cell Biol 2011; 31:3997 - 4006; http://dx.doi.org/10.1128/MCB.05808-11; PMID: 21791603
- Antoni L, Sodha N, Collins I, Garrett MD. CHK2 kinase: cancer susceptibility and cancer therapy - two sides of the same coin?. Nat Rev Cancer 2007; 7:925 - 36; http://dx.doi.org/10.1038/nrc2251; PMID: 18004398
- Lee SB, Kim SH, Bell DW, Wahrer DC, Schiripo TA, Jorczak MM, Sgroi DC, Garber JE, Li FP, Nichols KE, et al. Destabilization of CHK2 by a missense mutation associated with Li-Fraumeni Syndrome. Cancer Res 2001; 61:8062 - 7; PMID: 11719428
- Matsuoka S, Huang M, Elledge SJ. Linkage of ATM to cell cycle regulation by the Chk2 protein kinase. Science 1998; 282:1893 - 7; http://dx.doi.org/10.1126/science.282.5395.1893; PMID: 9836640
- Lavin MF. Ataxia-telangiectasia: from a rare disorder to a paradigm for cell signalling and cancer. Nat Rev Mol Cell Biol 2008; 9:759 - 69; http://dx.doi.org/10.1038/nrm2514; PMID: 18813293
- Kass EM, Ahn J, Tanaka T, Freed-Pastor WA, Keezer S, Prives C. Stability of checkpoint kinase 2 is regulated via phosphorylation at serine 456. J Biol Chem 2007; 282:30311 - 21; http://dx.doi.org/10.1074/jbc.M704642200; PMID: 17715138
- Kass EM, Poyurovsky MV, Zhu Y, Prives C. Mdm2 and PCAF increase Chk2 ubiquitination and degradation independently of their intrinsic E3 ligase activities. Cell Cycle 2009; 8:430 - 7; http://dx.doi.org/10.4161/cc.8.3.7624; PMID: 19176998
- Lovly CM, Yan L, Ryan CE, Takada S, Piwnica-Worms H. Regulation of Chk2 ubiquitination and signaling through autophosphorylation of serine 379. Mol Cell Biol 2008; 28:5874 - 85; http://dx.doi.org/10.1128/MCB.00821-08; PMID: 18644861
- Zhang D, Zaugg K, Mak TW, Elledge SJ. A role for the deubiquitinating enzyme USP28 in control of the DNA-damage response. Cell 2006; 126:529 - 42; http://dx.doi.org/10.1016/j.cell.2006.06.039; PMID: 16901786