Abstract
Tunneling nanotubes are actin-based cytoplasmic extensions that function as intercellular channels in a wide variety of cell types.There is a renewed and keen interest in the examination of modes of intercellular communication in cells of all types, especially in the field of cancer biology. Tunneling nanotubes –which in the literature have also been referred to as “membrane nanotubes,” “’intercellular’ or ‘epithelial’ bridges,” or “cytoplasmic extensions” – are under active investigation for their role in facilitating direct intercellular communication. These structures have not, until recently, been scrutinized as a unique and previously unrecognized form of direct cell-to-cell transmission of cellular cargo in the context of human cancer. Our recent study of tunneling nanotubes in human malignant pleural mesothelioma and lung adenocarcinomas demonstrated efficient transfer of cellular contents, including proteins, Golgi vesicles, and mitochondria, between cells derived from several well-established cancer cell lines. Further, we provided effective demonstration that such nanotubes can form between primary malignant cells from human patients. For the first time, we also demonstrated the in vivo relevance of these structures in humans, having effectively imaged nanotubes in intact solid tumors from patients. Here we provide further analysis and discussion on our findings, and offer a prospective ‘road map’ for studying tunneling nanotubes in the context of human cancer. We hope that further understanding of the mechanisms, methods of transfer, and particularly the role of nanotubes in tumor-stromal cross-talk will lead to identification of new selective targets for cancer therapeutics.
Tunneling nanotubes (TnTs) are long, non-adherent actin-based cytoplasmic extensions that function as intercellular bridges and connect a wide variety of cell types.Citation1-Citation13These unique conduits have attracted increasing interest as an under-recognized mechanism of cell-to-cell communication and transfer of cellular contents. What remains to be established, among many things, is the role of TnTs in establishing direct cytoplasmic connections between cancer and/or stromal cells. We postulate that TnT formation between malignant cells and cells of the surrounding tumor matrix may facilitate tumor development, progression and organization in preparation for tumoral invasion and metastasis.
We have recently shown that intercellular transfer of cellular cargo such as proteins, Golgi vesicles, and mitochondria takes place between malignant mesothelioma cells via TnTs.Citation8 This seminal work provided the first evidence that TnTs can form between malignant tumor cells of mesothelioma and lung cancer. Presence of TnTs in these cancer cells was established using cells derived from both human patient specimens as well as from established cancer cell lines. We also provided the first direct evidence demonstrating that TnTs are not just an in vitro phenomenon, but rather a functionally relevant entity that exists in solid tumors in patients, and thus may play a critical role in tumor formation and advancement. The existence of TnTs in solid tumors opens up a new avenue for targeted therapy and can be investigated as means of delivering small molecule inhibitors or biological agents where delivery of such agents is traditionally difficult to achieve. In support of these possibilities, a greater attention has been paid to the importance of the cellular characteristics of cancer development. TnTs have been proposed as one of the potential candidates that merits further study.Citation14 We contemplate and propose that the field of cancer biology and therapeutics is primed for the exploration of the mechanisms and roles of TnTs in facilitating both development and recurrence of cancer.
In order to move forward in using TnTs as potential therapeutic targets and to understand tumor biology, we need to address some key question such as ‘How do we proceed from the observation of intercellular transfer between cancer cells to discovery of potential effects of TnTs in altering cancer cell transformation and activity?’ We propose that this road to novel paradigms in therapeutics should be marked by its end goal: in the case of cancer, to identify potential selective targets that will improve upon the current standards of treatment of solid tumor malignancies. The first approach should be to characterize the mechanisms of TnT formation and maintenance, and to clearly understand how and why TnTs spontaneously self-terminate once cell-to-cell communication has been completed. An important aspect of this approach will be the identification of signals sent and received by cells located at considerable distances, and how such signals are transmitted, directly resulting in de novo formation of TnTs. Second, once established, what are the methods by which TnTs allow intercellular trafficking of critical cargo of various sizes and purpose? Are TnTs mere conduits, or do they actively facilitate this cell-to-cell transfer in a more specific and selective manner than is currently known? Third, it will be critical to study formation of TnTs among non-malignant cell populations, and to determine whether or not such formation is identical to that seen in cancer cell populations. Fourth, cross-talk between stromal and cancer cells via TnTs may provide impetus for cells deficient in vital bioproducts to be supplemented by surrounding cells.
One such potential example comes from recent studies demonstrating intercellular transfer of mitochondria from ‘positive’ cells to mitochondria-deficient cells for supplementation or reprogramming of recipient cells.Citation15,Citation16 The signals which stimulate TnT formation by tumor cells and guide intercellular contact between distant tumor cells in the matrix may indeed originate from stromal cells, providing further support to the idea that histologically ‘benign’ cells of the tumor matrix are crucial to tumor maintenance and metastasis.Citation17,Citation18
Differentiating unique aspects of TnTs in transformed vs. non-malignant cells will provide insight into whether or not this is a selective process that can be targeted for cancer therapy. Severing communication in medias res (“in the middle of things” in Latin), and/or inhibiting communication between tumors cells by suppressing TnT formation in the first place, may be potential goals of new or existing targeted cancer therapies. Actin-depolymerizing agents, such as Latrunculin A or cytochalasin B or D, have been used in prior in vitro studies to disrupt actin-based cell extensions, including TnTs. However, translating this finding into cancer treatments targeted at disrupting TnTs would not be feasible in the context of administration to human patients. Our demonstration that the widely used medications metformin and everolimus (an mTor inhibitor) suppress TnT formation provide an initial glimpse of the molecular pathways critical to TnT formation, as well as potential approaches to treating human tumors reliant on TnTs. These two drugs have potential real-world application in cancer therapy as adjuncts to combination therapy. Everolimus is already in use for treatment of advanced breast cancerCitation19 and metastatic renal cell carcinoma;Citation20 metformin is also being actively investigated as a potential anti-cancer therapy in light of its ability to decrease rates of cancer incidence in diabetic patients, as well as improve survival in cancer patients with diabetes.Citation21,Citation22 In addition to reduction of ATP production in mitochondria, activation of AMPK, and inhibition of gluconeogenesis by metformin, a recent report shows this drug causes a decrease in levels of reactive oxygen species and in DNA damageCitation23 – all of which are metabolic processes which potentially impact TnTs.
Targeting TnTs is one potential approach to augment, or enhance, the anti-tumor effect from a cancer biology perspective, and it may be true that such an approach would only be effective in specific subsets of patients rather than a universal approach. As tumor biology is extremely complex, any drugs targeting TnTs would be added as an additional approach to current standards of care. Again, we emphasize carefully that the challenge in this endeavor is to determine whether or not there exist components of TnTs which are truly cancer-specific, and serve as true selective targets for disruption that will minimize impact on TnTs connecting “normal” cells essential to physiologic functioning.
Cell-to-cell signaling in cancer is an issue which has not been well understood, and TnTs provide a plausible fundamental and common mechanism by which this interface of signals takes place between cancer cells. As we recently commented in our publication, the paradigm of gap junctions or microvesicles as proprietors of intercellular communication is most effective for cells in relatively close proximity. We propose that the concept of TnTs augments and supplements, rather than displaces, that paradigm, as TnTs can connect cells located a considerable distance apart. This fact takes on particular importance in light of the fact that tumors are heterogeneous three-dimensional structures composed of many other cell types, including inflammatory and structural stromal cells. It is believed that cancer cells comprise as little as 10% of solid tumors,Citation24,Citation25 and thus may not be situated in close enough proximity to allow communication via gap junctions or microvesicles. In our study, it was not uncommon to detect TnTs connecting mesothelioma or lung cancer cells more than 100 µm apart in vitro. In fact, we have noticed such TnT formation in many cancer cell lines and primary cancer cells derived from breast, ovarian, prostate, pancreatic, and colon cancer, to name several examples. Our visualization of TnTs in solid tumors from human patients provided further support of this notion. TnTs may in effect be a normal part of cellular communication; in the world of tumor biology, they may even potentiate tumor and stromal cell cross-talk. This cross-talk takes on considerable importance in light of studies which support the idea that tumors with a higher proportion of stromal cells actually may be more aggressive and have worse prognosis for patients.Citation26 We are cognizant that cancer cells do not have exclusive domain over TnT formation. However, the question of whether or not all TnTs are the same, both structurally and functionally, across cell types remains to be examined and answered. The vast interplay that occurs among cell types to facilitate tumor viability may occur by nanotubes, as just one potential mechanism.
Questions that should be addressed using the TnT models include the role of TnTs in frontline local invasion and metastasis of malignant cells. We demonstrated using the in vitro modified wound healing/scratch assay technique that TnTs form in significant numbers at the invasion front of mesothelioma cells.Citation8 Are TnTs in this instance facilitating crosstalk to coordinate tumor cell invasion, to ensure that aggressively proliferating cells are, in essence, synchronized? If this is the case in vitro, then this scenario may very well also occur in vivo. From the standpoint of cancer recurrence following attempt at definitive treatment, can TnT formation between residual malignant cells result in communication between distant cells? Would this allow scattered distant cells an opportunity to mobilize and allow tumor recovery and eventually regrowth? Finally, the issue of resistance to chemotherapy is of significant biologic and clinical importance. If, as been demonstrated with antibiotic resistance spread between bacterial cells communicating via TnTs,Citation12 cancer cells propagate chemoresistance by sending counteractive resistant signals or genetic codes of resistance via TnTs, targeting these nanotubes will help us understand if chemoresistance is an avoidable phenomenon, rather than inevitable. Angiogenesis is a significant subject of cancer research; the study of TnTs in the context of the angiogenesis phenomenon may help differentiate mechanisms of sustainable peritumoral blood vessel formation that propagate tumor existence, and thus may lead to identification of new druggable anti-angiogenic targets. Finally, we also consider the possibility of harnessing TnTs as potential “Trojan horses” which can be exploited to enhance drug delivery to tumors. Can TnTs in the periphery of cells, and thus located among the most highly proliferating cells of a solid tumor, take up small molecule inhibitors and essentially transport them throughout the tumor, leading to increased susceptibility of tumor cells or stromal cells not accessible by conventional methods of drug delivery? These are all questions pertinent to the world of cancer research which can be addressed by studying the mechanisms and function of TnTs in the context of cancer.
As this topic attracts increasing interest from researchers spanning many fields of biology in general, we recognize that one of the potential barriers to study – from a logistical rather than scientific perspective – is the wide disparity in nomenclature used to define TnTs in the published literature. Comprehensive literature searches on this topic are complicated by the fact that studies on TnTs and associated structures have been published using a rather large number of disparate names, including ‘cellular bridges’,Citation27 ‘intercellular bridges’,Citation28,Citation29 ‘intercellular membrane bridges’,Citation30 ‘tubular bridges’,Citation1,Citation31 ‘epithelial bridges’,Citation32 ‘intercellular conduits’,Citation33 ‘bridging conduits’,Citation34,Citation35 ‘intercellular membrane nanotubes (ICNs)’,Citation36 ‘membrane nanotubes’,Citation4,Citation13,Citation37-Citation40 and ‘intercellular nanotubes’Citation12. We also note that the structures known as ‘cytonemes’ should likely be considered distinct from TnTs, but nonetheless should be included in discussion of cytoplasmic extensions which facilitate intercellular communication and transfer. The term ‘cellular bridges’ in particular might apply to a number of variations of open-ended actin-based extensions which facilitate intercellular communication, but might paint a broader stroke than intended. Critical review of research papers using these names reveals that many of the studied structures are either ‘true’ TnTs, or some variation thereof.
We acknowledge that since the study of TnTs is still relatively in its earliest stages of development, the various structures being reported may in fact be structurally or functionally different. For example, some forms of TnTs connecting malignant cells may vary from that of immune cells, such as macrophages or dendritic cells. However, at this stage not enough is known to make that distinction. This great variability in assigned nomenclature from groups working on these structures has the potential to prevent thorough review and awareness of advancements in this field. As most, if not all, of these papers cite the Rustom et al. paper from 2004Citation6 as the first significant documentation of tunneling nanotubes/nanotubules connecting cells, as well as the use of this term, we respectfully propose consolidation, or at least inclusion, of the terms “Tunneling Nanotubes,” and the abbreviation “TNT” or “TnT,” as standard nomenclature in future publications. The inclusion of the term ‘tunneling’ will aid readers in making the distinction from other, ‘non-tunneling’ forms of nanotube connections. In addition, as researchers make new discoveries regarding the composition and function of such cell extensions, distinctions or differences from known characteristics of TnTs should be clearly acknowledged and stated for clarification. In this way, perhaps confusion can be avoided and literature searches will reveal more comprehensive analysis of new data in this exciting field.
In conclusion, the study of TnTs in the formation, communication, organization, and spread of solid tumors is in its infancy. We hope that ongoing studies in our laboratory and others will lead to increased understanding and elucidation of the mechanisms by which tumor cell interactions take place. Identification of unique aspects of TnTs which anchor and facilitate cell-to-cell transfer of cell components between cancer cells may lead to identification of new specific and selective targets for cancer therapy. TnTs open up a new area of cancer biology and therapeutics that encompasses intercellular communications between cancer cells, between stromal cells, cross-talk between stromal and tumor cells, and interaction within the tumor microenvironment.
Figure 1. Tunneling nanotubes (TnTs) connecting mesothelioma cells (MSTO-211H cell line). All images were taken with Leica TCS SP5 with 40x/1.25NA or 100x/1.4NA oil objectives. (A) TnT connecting MSTO-211H cells, contrasted with shorter, adherent lamellopodia and actin stress fibers in the background. Blue = DAPI, green = actin (phalloidin stain), red = mitochondria (MitoTracker Red). (B) TnTs connecting MSTO-211H cells. Blue = DAPI, green = Golgi apparatus (GM130 antibody), red = mitochondria (MitoTracker Red). (C) TnT between MSTO-211H cells. Blue = Hoechst 3342, red = MitoTracker Red. (D) Ultrafine TnT connecting MSTO-211H cells. Blue = DAPI, red = RFP.
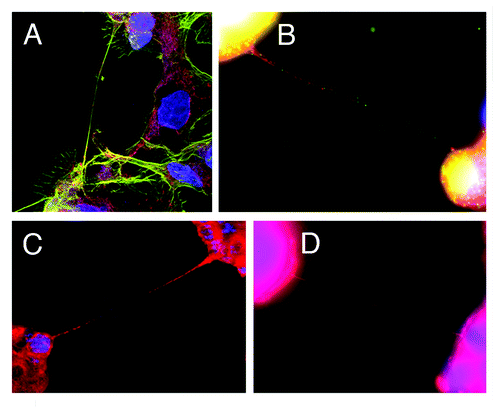
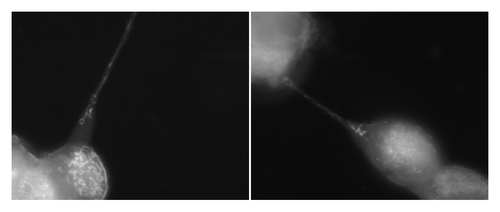
Figure 2. Tunneling nanotubes serve as a conduit for intercellular transport of organelles, include mitochondria. Numerous mitochondria were visualized in transit between MSTO-211H (biphasic mesothelioma) cells stained with MitoTracker Red. Images were captured using Leica inverted microscope, 100x/1.4NA oil objective.
Acknowledgments
This research has been funded by the Baker Street Foundation, the Minnesota Medical Foundation, and the Karen Wyckoff Rein in Sarcoma Foundation.
References
- Zani BG, Indolfi L, Edelman ER. Tubular bridges for bronchial epithelial cell migration and communication. PLoS One 2010; 5:e8930; http://dx.doi.org/10.1371/journal.pone.0008930; PMID: 20126618
- Watkins SC, Salter RD. Functional connectivity between immune cells mediated by tunneling nanotubules. Immunity 2005; 23:309 - 18; http://dx.doi.org/10.1016/j.immuni.2005.08.009; PMID: 16169503
- Ventelä S, Toppari J, Parvinen M. Intercellular organelle traffic through cytoplasmic bridges in early spermatids of the rat: mechanisms of haploid gene product sharing. Mol Biol Cell 2003; 14:2768 - 80; http://dx.doi.org/10.1091/mbc.E02-10-0647; PMID: 12857863
- Sowinski S, Jolly C, Berninghausen O, Purbhoo MA, Chauveau A, Köhler K, et al. Membrane nanotubes physically connect T cells over long distances presenting a novel route for HIV-1 transmission. Nat Cell Biol 2008; 10:211 - 9; http://dx.doi.org/10.1038/ncb1682; PMID: 18193035
- Sherer NM, Lehmann MJ, Jimenez-Soto LF, Horensavitz C, Pypaert M, Mothes W. Retroviruses can establish filopodial bridges for efficient cell-to-cell transmission. Nat Cell Biol 2007; 9:310 - 5; http://dx.doi.org/10.1038/ncb1544; PMID: 17293854
- Rustom A, Saffrich R, Markovic I, Walther P, Gerdes HH. Nanotubular highways for intercellular organelle transport. Science 2004; 303:1007 - 10; http://dx.doi.org/10.1126/science.1093133; PMID: 14963329
- Onfelt B, Nedvetzki S, Benninger RK, Purbhoo MA, Sowinski S, Hume AN, et al. Structurally distinct membrane nanotubes between human macrophages support long-distance vesicular traffic or surfing of bacteria. J Immunol 2006; 177:8476 - 83; PMID: 17142745
- Lou E, Fujisawa S, Morozov A, Barlas A, Romin Y, Dogan Y, et al. Tunneling nanotubes provide a unique conduit for intercellular transfer of cellular contents in human malignant pleural mesothelioma. PLoS One 2012; 7:e33093; http://dx.doi.org/10.1371/journal.pone.0033093; PMID: 22427958
- Gurke S, Barroso JF, Hodneland E, Bukoreshtliev NV, Schlicker O, Gerdes HH. Tunneling nanotube (TNT)-like structures facilitate a constitutive, actomyosin-dependent exchange of endocytic organelles between normal rat kidney cells. Exp Cell Res 2008; 314:3669 - 83; http://dx.doi.org/10.1016/j.yexcr.2008.08.022; PMID: 18845141
- Gousset K, Schiff E, Langevin C, Marijanovic Z, Caputo A, Browman DT, et al. Prions hijack tunnelling nanotubes for intercellular spread. Nat Cell Biol 2009; 11:328 - 36; http://dx.doi.org/10.1038/ncb1841; PMID: 19198598
- Eugenin EA, Gaskill PJ, Berman JW. Tunneling nanotubes (TNT) are induced by HIV-infection of macrophages: a potential mechanism for intercellular HIV trafficking. Cell Immunol 2009; 254:142 - 8; http://dx.doi.org/10.1016/j.cellimm.2008.08.005; PMID: 18835599
- Dubey GP, Ben-Yehuda S. Intercellular nanotubes mediate bacterial communication. Cell 2011; 144:590 - 600; http://dx.doi.org/10.1016/j.cell.2011.01.015; PMID: 21335240
- Arkwright PD, Luchetti F, Tour J, Roberts C, Ayub R, Morales AP, et al. Fas stimulation of T lymphocytes promotes rapid intercellular exchange of death signals via membrane nanotubes. Cell Res 2010; 20:72 - 88; http://dx.doi.org/10.1038/cr.2009.112; PMID: 19770844
- Kwok R. Cell biology: The new cell anatomy. Nature 2011; 480:26 - 8; http://dx.doi.org/10.1038/480026a; PMID: 22129705
- Cho YM, Kim JH, Kim M, Park SJ, Koh SH, Ahn HS, et al. Mesenchymal stem cells transfer mitochondria to the cells with virtually no mitochondrial function but not with pathogenic mtDNA mutations. PLoS One 2012; 7:e32778; http://dx.doi.org/10.1371/journal.pone.0032778; PMID: 22412925
- Acquistapace A, Bru T, Lesault PF, Figeac F, Coudert AE, le Coz O, et al. Human mesenchymal stem cells reprogram adult cardiomyocytes toward a progenitor-like state through partial cell fusion and mitochondria transfer. Stem Cells 2011; 29:812 - 24; http://dx.doi.org/10.1002/stem.632; PMID: 21433223
- Morozov A, Downey RJ, Healey J, Moreira AL, Lou E, Franceschino A, et al. Benign mesenchymal stromal cells in human sarcomas. Clinical cancer research: an official journal of the American Association for Cancer Research 2010; 16:5630-40.
- Cooke VG, LeBleu VS, Keskin D, Khan Z, O’Connell JT, Teng Y, et al. Pericyte depletion results in hypoxia-associated epithelial-to-mesenchymal transition and metastasis mediated by met signaling pathway. Cancer Cell 2012; 21:66 - 81; http://dx.doi.org/10.1016/j.ccr.2011.11.024; PMID: 22264789
- Baselga J, Campone M, Piccart M, Burris HA 3rd, Rugo HS, Sahmoud T, et al. Everolimus in postmenopausal hormone-receptor-positive advanced breast cancer. N Engl J Med 2012; 366:520 - 9; http://dx.doi.org/10.1056/NEJMoa1109653; PMID: 22149876
- Motzer RJ, Escudier B, Oudard S, Hutson TE, Porta C, Bracarda S, et al, RECORD‐1 Study Group. Phase 3 trial of everolimus for metastatic renal cell carcinoma : final results and analysis of prognostic factors. Cancer 2010; 116:4256 - 65; http://dx.doi.org/10.1002/cncr.25219; PMID: 20549832
- Garrett CR, Hassabo HM, Bhadkamkar NA, Wen S, Baladandayuthapani V, Kee BK, et al. Survival advantage observed with the use of metformin in patients with type II diabetes and colorectal cancer. Br J Cancer 2012; 106:1374 - 8; http://dx.doi.org/10.1038/bjc.2012.71; PMID: 22421948
- Bo S, Ciccone G, Rosato R, Villois P, Appendino G, Ghigo E, et al. Cancer mortality reduction and metformin: a retrospective cohort study in type 2 diabetic patients. Diabetes Obes Metab 2012; 14:23 - 9; http://dx.doi.org/10.1111/j.1463-1326.2011.01480.x; PMID: 21812892
- Algire C, Moiseeva O, Deschênes-Simard X, Amrein L, Petruccelli L, Birman E, et al. Metformin reduces endogenous reactive oxygen species and associated DNA damage. Cancer Prev Res (Phila) 2012; 5:536 - 43; http://dx.doi.org/10.1158/1940-6207.CAPR-11-0536; PMID: 22262811
- Dvorak HF. Tumors: wounds that do not heal. Similarities between tumor stroma generation and wound healing. N Engl J Med 1986; 315:1650 - 9; PMID: 3537791
- Elenbaas B, Weinberg RA. Heterotypic signaling between epithelial tumor cells and fibroblasts in carcinoma formation. Exp Cell Res 2001; 264:169 - 84; http://dx.doi.org/10.1006/excr.2000.5133; PMID: 11237532
- de Kruijf EM, van Nes JG, van de Velde CJ, Putter H, Smit VT, Liefers GJ, et al. Tumor-stroma ratio in the primary tumor is a prognostic factor in early breast cancer patients, especially in triple-negative carcinoma patients. Breast Cancer Res Treat 2011; 125:687 - 96; http://dx.doi.org/10.1007/s10549-010-0855-6; PMID: 20361254
- McKinney MC, Stark DA, Teddy J, Kulesa PM. Neural crest cell communication involves an exchange of cytoplasmic material through cellular bridges revealed by photoconversion of KikGR. Developmental dynamics: an official publication of the American Association of Anatomists 2011; 240:1391-401.
- Caneparo L, Pantazis P, Dempsey W, Fraser SE. Intercellular bridges in vertebrate gastrulation. PLoS One 2011; 6:e20230; http://dx.doi.org/10.1371/journal.pone.0020230; PMID: 21647454
- Vidulescu C, Clejan S, O’connor KC. Vesicle traffic through intercellular bridges in DU 145 human prostate cancer cells. J Cell Mol Med 2004; 8:388 - 96; http://dx.doi.org/10.1111/j.1582-4934.2004.tb00328.x; PMID: 15491514
- Jung SH, Park J-Y, Joo J-H, Kim Y-M, Ha K-S. Extracellular ultrathin fibers sensitive to intracellular reactive oxygen species: formation of intercellular membrane bridges. Exp Cell Res 2011; 317:1763 - 73; http://dx.doi.org/10.1016/j.yexcr.2011.02.010; PMID: 21356206
- Xue Y, Xing Z, Hellem S, Arvidson K, Mustafa K. Endothelial cells influence the osteogenic potential of bone marrow stromal cells. Biomed Eng Online 2009; 8:34; http://dx.doi.org/10.1186/1475-925X-8-34; PMID: 19919705
- Zani BG, Edelman ER. Cellular bridges: Routes for intercellular communication and cell migration. Commun Integr Biol 2010; 3:215 - 20; http://dx.doi.org/10.4161/cib.3.3.11659; PMID: 20714396
- Xu W, Santini PA, Sullivan JS, He B, Shan M, Ball SC, et al. HIV-1 evades virus-specific IgG2 and IgA responses by targeting systemic and intestinal B cells via long-range intercellular conduits. Nat Immunol 2009; 10:1008 - 17; http://dx.doi.org/10.1038/ni.1753; PMID: 19648924
- Kadiu I, Gendelman HE. Macrophage bridging conduit trafficking of HIV-1 through the endoplasmic reticulum and Golgi network. J Proteome Res 2011; 10:3225 - 38; http://dx.doi.org/10.1021/pr200262q; PMID: 21563830
- Kadiu I, Gendelman HE. Human immunodeficiency virus type 1 endocytic trafficking through macrophage bridging conduits facilitates spread of infection. Journal of neuroimmune pharmacology: the official journal of the Society on NeuroImmune Pharmacology 2011; 6:658-75.
- Kabaso D, Bobrovska N, Góźdź W, Gongadze E, Kralj-Iglič V, Zorec R, et al. The transport along membrane nanotubes driven by the spontaneous curvature of membrane components. Bioelectrochemistry 2012; http://dx.doi.org/10.1016/j.bioelechem.2012.02.009; PMID: 22502994
- Chauveau A, Aucher A, Eissmann P, Vivier E, Davis DM. Membrane nanotubes facilitate long-distance interactions between natural killer cells and target cells. Proc Natl Acad Sci U S A 2010; 107:5545 - 50; http://dx.doi.org/10.1073/pnas.0910074107; PMID: 20212116
- Iglic A, Lokar M, Babnik B, Slivnik T, Veranic P, Hägerstrand H, et al. Possible role of flexible red blood cell membrane nanodomains in the growth and stability of membrane nanotubes. Blood Cells Mol Dis 2007; 39:14 - 23; http://dx.doi.org/10.1016/j.bcmd.2007.02.013; PMID: 17475520
- He K, Shi X, Zhang X, Dang S, Ma X, Liu F, et al. Long-distance intercellular connectivity between cardiomyocytes and cardiofibroblasts mediated by membrane nanotubes. Cardiovasc Res 2011; 92:39 - 47; http://dx.doi.org/10.1093/cvr/cvr189; PMID: 21719573
- Kabaso D, Lokar M, Kralj-Iglič V, Veranič P, Iglič A. Temperature and cholera toxin B are factors that influence formation of membrane nanotubes in RT4 and T24 urothelial cancer cell lines. Int J Nanomedicine 2011; 6:495 - 509; http://dx.doi.org/10.2147/IJN.S16982; PMID: 21468353