Abstract
Retrograde trafficking mediates the transport of endocytic membranes from endosomes to the trans-Golgi network (TGN). Dysregulation of these pathways can result in multiple ailments, including late-onset Alzheimer disease. One of the key retrograde transport regulators, the retromer complex, is tightly controlled by many factors, including the C-terminal Eps15 homology domain (EHD) proteins. This mini-review focuses on recent findings and discusses the regulation of the retromer complex by EHD proteins and the novel EHD1 interaction partner, Rabankyrin-5 (Rank-5).
Endocytic Transport
Endocytic transport is essential for various cellular functions such as the regulation of membrane homeostasis, expression of cell surface receptors and signal transduction.Citation1,Citation2 It is also exploited by certain pathogens and protein toxins for their entry into the cell and subsequent cytotoxic activities.Citation3,Citation4 Therefore, elucidation of the underlying mechanisms of this trafficking is crucial for identifying new therapeutic targets and for successful drug delivery.
Endocytic pathways are highly regulated by numerous proteins, including Rab GTPases,Citation5-Citation8 soluble NSF attachment protein receptor (SNAREs) proteinsCitation9,Citation10 and the C-terminal Eps15 homology domain (EHD) proteins.Citation11,Citation12 Rab proteins are key regulators of endocytic trafficking. They are reversibly associated with specific membrane organelles in a manner dependent on their GTP-bound status, and they mediate distinct endocytic steps via recruitment of coat components,Citation14 molecular motors,Citation15-Citation17 and tethering factors.Citation18,Citation19 Ultimately vesicle fusion to target membranes is facilitated by SNARE-induced activity.Citation20,Citation21
Another family of endocytic regulators, the EHD proteins, has drawn intense interest in recent years. The four mammalian EHDs, EHD1–4, regulate multiple steps in the endocytic trafficking pathways.Citation12 In contrast to Rabs, EHD proteins contain an ATP-binding G domain.Citation22 The structure of EHDs suggests a function in the scission of vesicles upon ATP hydrolysis.Citation23 This is supported by a recent study demonstrating that EHD1 promotes clathrin- and dynamin-dependent synaptic vesicle budding,Citation24 in coordination with amphiphysin.Citation25
Retrograde Transport and Retromer
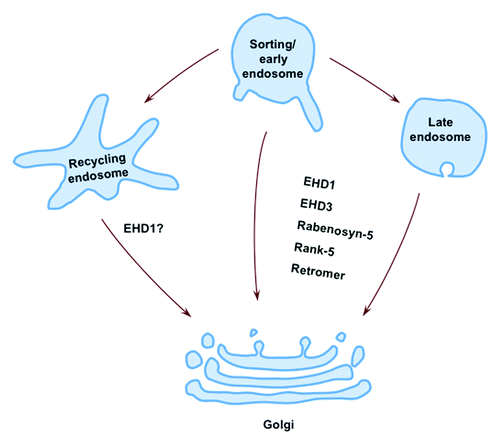
Following their transport through the TGN, newly synthesized proteins are dispatched to the plasma membrane (and/or secreted) or targeted to endocytic organelles via anterograde transport. To maintain membrane homeostasis in the TGN, proteins and lipids need to be replenished by the process of retrograde transport, which returns membranes from early, late, and recycling endosomes to the TGN ().Citation26,Citation27 Retrograde transport is important for many cellular functions, including lysosome biogenesisCitation28-Citation30 and development.Citation31-Citation36 Dysregulation of this pathway is associated with a variety of diseases including late-onset Alzheimer disease and other neuronal disorders.Citation37
Figure 1. Retrograde transport and EHD proteins. Retrograde transport of endocytic membranes occurs from early endosomes, late endosomes and/or recycling endosomes to the TGN. EHD1 and EHD3 interact with the retromer complex and regulate retromer-mediated membrane transport. For simplicity, only EHD proteins and their interaction partners are shown.
Protein sorting into the retrograde pathway is regulated by multiple factors, and the better known ones include clathrin and epsinR,Citation38 AP-1,Citation39 oculocerebrorenal syndrome of lowe (OCRL),Citation40 Golgi-associated γ-ear-containing ARF binding protein 3 (GGA3),Citation41 phosphofurin acidic cluster sorting protein 1 (PACS1),Citation41 Rab9, TIP47,Citation42,Citation43 SNX3Citation33,Citation44-Citation47 and the retromer complex, a key regulator of retrograde transport.Citation48,Citation49
Retromer is an evolutionary conserved heteropentameric complex that consists of two subcomplexes: a sorting nexin (SNX) dimer comprised of SNX1/2Citation50,Citation51 or SNX5/6,Citation52,Citation53 and a cargo recognition heterotrimer comprised of vacuolar protein sorting (Vps) 26/29/35.Citation54 The retromer SNX proteins contain a phox homology (PX) domain that can bind to the phosphatidylinositol 3-monophosphate/3,5-bisphosphate,Citation55,Citation56 and a Bin-Amphiphysin-Rvs (BAR) domain that detects membrane curvature and/or tubulates membranes.Citation57-Citation59 Retromer-mediated tubule formation occurs with the highest frequency during the Rab5-to-Rab7 endosomal transition,Citation60,Citation61 and the scission of the tubules is regulated by the WASH (Wiskott-Aldrich Syndrome protein family Homolog) complex.Citation62-Citation64
The cargo-recognition complex trimer interacts with retromer cargos via Vps35.Citation28,Citation65 The other two subunits, Vps29 and Vps26, bind to the C- and N-termini of Vps35, respectively.Citation66 Unlike the SNX proteins, the cargo-recognition complex does not contain lipid-binding domains. Its recruitment to the endosome requires the small GTPase Rab7, and can be inhibited by TBC1D5, a member of the Rab GAP family.Citation67,Citation68 Rab5 is also involved in the recruitment of Vps26/29/35, possibly via its effect on the phosphatidylinositol 3-kinase.Citation67 The mechanism of mammalian retromer recruitment and assembly is still not fully understood.
EHD and Retromer
EHD proteins interact with and regulate retromer-mediated retrograde transport to the Golgi. EHD1 colocalizes and interacts with Vps26 and Vps35, and affects retromer-mediated retrieval of mannose-6-phosphate receptor (M6PR).Citation69 The C-terminal EH domain and nucleotide-binding of EHD1 is required for maintaining normal retromer localization and M6PR transport. However, no direct binding between EHD1 and retromer has been detected, suggesting the involvement of additional factors, possibly through recruitment by the EH domain.
Another EHD family member, EHD3, regulates endosome-to-Golgi trafficking and affects Golgi morphology.Citation29 Depletion of EHD3 or its interaction partner Rabenosyn-5, a divalent Rab4/5 effector, alters the localization of SNX1 and SNX2 to enlarged early endosomes, and disrupts the retrograde transport of Shiga Toxin B subunit.Citation29 As a consequence, the biosynthetic transport of cathepsin D, a lysosomal lumenal hydrolase, is impaired upon EHD3 or Rabenosyn-5 knock-down. Although EHD1 and EHD3 are both required for the retromer-mediated transport to the Golgi, EHD3 might play a more prominent role at the early endosome.Citation70 The underlying mechanism by which EHDs regulate the retromer complex remains to be elucidated.
Rabankyrin-5 (Rank-5) and Retromer
The wide variety of EHD-based functions is further enhanced by their interactions with multiple binding partners. These interactions generally occur between the EHD EH domain and a region of the binding partner that contains one or more asparagine-proline-phenylalanine (NPF) motif flanked by acidic residues.Citation71,Citation72 An example is the recently identified new EHD1 binding partner, Rank-5, that plays a role in retromer-mediated retrograde transport.Citation73
Rank-5, a Rab5 effector, is required for macropinocytosis and early endosome fusion.Citation74 It is comprised of an N-terminal BTB (Bric-a-brac, Tramtrack and Broad complex)/POZ (Pox virus and zinc finger) domain, followed by 21 consecutive ankyrin repeats, and a C-terminal FYVE (Fab1, YOTB, Vac1, EEA1)-finger domain. While there is a NPFED motif located within the fifth ankyrin domain, we were initially unsure if this site would be exposed for binding; however, subsequent studies confirmed a direct interaction (ref. Citation73 and see ). It is noteworthy that Rank-5 specifically binds to EHD1, but not the other EHDs, including EHD3. The latter EHD protein is the closest paralog of EHD1, sharing 86% sequence identity, suggesting the fine-tuning of individual EHD protein functions by their selective binding partners, despite the high level of homology among family members.
Figure 2. Schematic diagram of Rank-5 domain architecture. Rank-5 contains a C-terminal BTB domain and an N-terminal FYVE domain, with 21 consecutive ankyrin repeats in between. The NPFED motif localizes to the fifth ankyrin repeat.

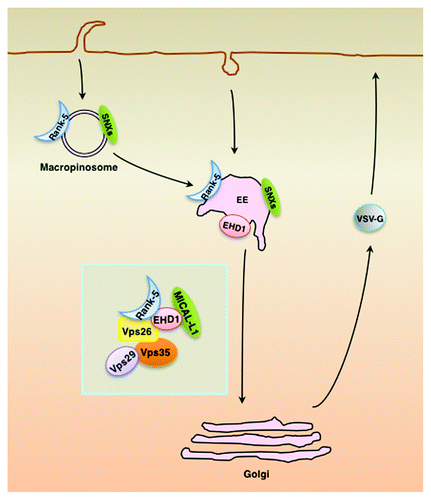
Rank-5 interacts with retromer to regulate its subcellular localization, and binding to EHD1 is required for Rank-5 regulation of retromer, since mutating the NPF motif did not rescue retromer mislocalization upon Rank-5 knockdown.Citation73 The regulatory roles of Rank-5 at distinct steps of the membrane transport pathways are summarized in the model shown in . Rank-5 promotes the formation of macropinosomes and plays a role in homotypic early-endosome fusion.Citation74 Through binding to EHD1, Rank-5 is recruited to the retromer and influences the latter’s localization and retromer-mediated membrane transport. Depletion of Rank-5 or EHD1 compromises vesicular stomatitis virus-G (VSV-G) secretion, possibly indirectly by affecting Golgi membrane maintenance.Citation73
Figure 3. Proposed model for Rank-5 function. Rank-5 is required for the formation of macropinosomes and plays a role in homotypic early-endosome fusion. Through binding to EHD1 and MICAL-L1, Rank-5 is recruited to the retromer and influences retromer-mediated retrograde transport. Furthermore, depletion of either Rank-5 or EHD1 affects VSV-G secretion, possibly through its effect on the homeostasis of the TGN. EE, early endosomes.
How EHD1 and Rank-5 regulate retromer function is still largely unknown. EHD1 localizes to the same complex with the retromer cargo-recognition units, but has little or no interaction with the SNX dimer.Citation69,Citation73 This led us to suggest that EHD1 might act on the Vps subcomplex and indirectly affect SNX localization and SNX1-decorated tubules. Since no direct interaction has been identified either between EHD1 or Rank-5 and the retromer, this highlights the importance of elucidating the other proteins in the complex to further understand the mechanisms of the EHD-mediated retromer regulation.
Conclusion/Summary
Much progress has been made in understanding the regulation of the retromer complex. The recent studies demonstrating the involvement of EHD proteins in retromer-based transport raise many new questions. For example, where is the site of EHD action? Do EHD proteins act in the recruitment of retromer components or the scission of retromer-containing tubules? Do EHD paralogs play distinct roles in the regulation of retrograde transport? Are recycling endosomes involved (ref. Citation13 and )? Are there other binding partners that participate in this process? How do Rab5, EHDs and their common interaction partners orchestrate the regulation of retromer? Further investigation will be required to significantly enhance our understanding of the role of EHDs on retromer regulation.
References
- Platta HW, Stenmark H. Endocytosis and signaling. Curr Opin Cell Biol 2011; 23:393 - 403; http://dx.doi.org/10.1016/j.ceb.2011.03.008; PMID: 21474295
- Sorkin A, von Zastrow M. Endocytosis and signalling: intertwining molecular networks. Nat Rev Mol Cell Biol 2009; 10:609 - 22; http://dx.doi.org/10.1038/nrm2748; PMID: 19696798
- Johannes L, Decaudin D. Protein toxins: intracellular trafficking for targeted therapy. Gene Ther 2005; 12:1360 - 8; http://dx.doi.org/10.1038/sj.gt.3302557; PMID: 15902276
- Sandvig K, van Deurs B. Entry of ricin and Shiga toxin into cells: molecular mechanisms and medical perspectives. EMBO J 2000; 19:5943 - 50; http://dx.doi.org/10.1093/emboj/19.22.5943; PMID: 11080141
- Grosshans BL, Ortiz D, Novick P. Rabs and their effectors: achieving specificity in membrane traffic. Proc Natl Acad Sci U S A 2006; 103:11821 - 7; http://dx.doi.org/10.1073/pnas.0601617103; PMID: 16882731
- Hutagalung AH, Novick PJ. Role of Rab GTPases in membrane traffic and cell physiology. Physiol Rev 2011; 91:119 - 49; http://dx.doi.org/10.1152/physrev.00059.2009; PMID: 21248164
- Pfeffer S, Aivazian D. Targeting Rab GTPases to distinct membrane compartments. Nat Rev Mol Cell Biol 2004; 5:886 - 96; http://dx.doi.org/10.1038/nrm1500; PMID: 15520808
- Stenmark H. Rab GTPases as coordinators of vesicle traffic. Nat Rev Mol Cell Biol 2009; 10:513 - 25; http://dx.doi.org/10.1038/nrm2728; PMID: 19603039
- Jahn R, Scheller RH. SNAREs--engines for membrane fusion. Nat Rev Mol Cell Biol 2006; 7:631 - 43; http://dx.doi.org/10.1038/nrm2002; PMID: 16912714
- Südhof TC, Rothman JE. Membrane fusion: grappling with SNARE and SM proteins. Science 2009; 323:474 - 7; http://dx.doi.org/10.1126/science.1161748; PMID: 19164740
- Grant BD, Caplan S. Mechanisms of EHD/RME-1 protein function in endocytic transport. Traffic 2008; 9:2043 - 52; http://dx.doi.org/10.1111/j.1600-0854.2008.00834.x; PMID: 18801062
- Naslavsky N, Caplan S. EHD proteins: key conductors of endocytic transport. Trends Cell Biol 2011; 21:122 - 31; http://dx.doi.org/10.1016/j.tcb.2010.10.003; PMID: 21067929
- McKenzie JE, Raisley B, Zhou X, Naslavsky N, Taguchi T, Caplan S, et al. Retromer guides STxB and CD8-M6PR from early to recycling endosomes, EHD1 guides STxB from recycling endosome to Golgi. Traffic 2012; 13:1140 - 59; http://dx.doi.org/10.1111/j.1600-0854.2012.01374.x; PMID: 22540229
- McLauchlan H, Newell J, Morrice N, Osborne A, West M, Smythe E. A novel role for Rab5-GDI in ligand sequestration into clathrin-coated pits. Curr Biol 1998; 8:34 - 45; http://dx.doi.org/10.1016/S0960-9822(98)70018-1; PMID: 9427626
- Echard A, Jollivet F, Martinez O, Lacapère JJ, Rousselet A, Janoueix-Lerosey I, et al. Interaction of a Golgi-associated kinesin-like protein with Rab6. Science 1998; 279:580 - 5; http://dx.doi.org/10.1126/science.279.5350.580; PMID: 9438855
- Roland JT, Kenworthy AK, Peranen J, Caplan S, Goldenring JR. Myosin Vb interacts with Rab8a on a tubular network containing EHD1 and EHD3. Mol Biol Cell 2007; 18:2828 - 37; http://dx.doi.org/10.1091/mbc.E07-02-0169; PMID: 17507647
- Wu XS, Rao K, Zhang H, Wang F, Sellers JR, Matesic LE, et al. Identification of an organelle receptor for myosin-Va. Nat Cell Biol 2002; 4:271 - 8; http://dx.doi.org/10.1038/ncb760; PMID: 11887186
- Nielsen E, Christoforidis S, Uttenweiler-Joseph S, Miaczynska M, Dewitte F, Wilm M, et al. Rabenosyn-5, a novel Rab5 effector, is complexed with hVPS45 and recruited to endosomes through a FYVE finger domain. J Cell Biol 2000; 151:601 - 12; http://dx.doi.org/10.1083/jcb.151.3.601; PMID: 11062261
- Simonsen A, Lippé R, Christoforidis S, Gaullier JM, Brech A, Callaghan J, et al. EEA1 links PI(3)K function to Rab5 regulation of endosome fusion. Nature 1998; 394:494 - 8; http://dx.doi.org/10.1038/28879; PMID: 9697774
- McBride HM, Rybin V, Murphy C, Giner A, Teasdale R, Zerial M. Oligomeric complexes link Rab5 effectors with NSF and drive membrane fusion via interactions between EEA1 and syntaxin 13. Cell 1999; 98:377 - 86; http://dx.doi.org/10.1016/S0092-8674(00)81966-2; PMID: 10458612
- Simonsen A, Gaullier JM, D’Arrigo A, Stenmark H. The Rab5 effector EEA1 interacts directly with syntaxin-6. J Biol Chem 1999; 274:28857 - 60; http://dx.doi.org/10.1074/jbc.274.41.28857; PMID: 10506127
- Zhang J, Naslavsky N, Caplan S. Rabs and EHDs: alternate modes for traffic control. Biosci Rep 2012; 32:17 - 23; http://dx.doi.org/10.1042/BSR20110009; PMID: 21981138
- Daumke O, Lundmark R, Vallis Y, Martens S, Butler PJ, McMahon HT. Architectural and mechanistic insights into an EHD ATPase involved in membrane remodelling. Nature 2007; 449:923 - 7; http://dx.doi.org/10.1038/nature06173; PMID: 17914359
- Jakobsson J, Ackermann F, Andersson F, Larhammar D, Löw P, Brodin L. Regulation of synaptic vesicle budding and dynamin function by an EHD ATPase. J Neurosci 2011; 31:13972 - 80; http://dx.doi.org/10.1523/JNEUROSCI.1289-11.2011; PMID: 21957258
- Pant S, Sharma M, Patel K, Caplan S, Carr CM, Grant BD. AMPH-1/Amphiphysin/Bin1 functions with RME-1/Ehd1 in endocytic recycling. Nat Cell Biol 2009; 11:1399 - 410; http://dx.doi.org/10.1038/ncb1986; PMID: 19915558
- Bonifacino JS, Rojas R. Retrograde transport from endosomes to the trans-Golgi network. Nat Rev Mol Cell Biol 2006; 7:568 - 79; http://dx.doi.org/10.1038/nrm1985; PMID: 16936697
- Johannes L, Popoff V. Tracing the retrograde route in protein trafficking. Cell 2008; 135:1175 - 87; http://dx.doi.org/10.1016/j.cell.2008.12.009; PMID: 19109890
- Arighi CN, Hartnell LM, Aguilar RC, Haft CR, Bonifacino JS. Role of the mammalian retromer in sorting of the cation-independent mannose 6-phosphate receptor. J Cell Biol 2004; 165:123 - 33; http://dx.doi.org/10.1083/jcb.200312055; PMID: 15078903
- Naslavsky N, McKenzie J, Altan-Bonnet N, Sheff D, Caplan S. EHD3 regulates early-endosome-to-Golgi transport and preserves Golgi morphology. J Cell Sci 2009; 122:389 - 400; http://dx.doi.org/10.1242/jcs.037051; PMID: 19139087
- Seaman MN. Cargo-selective endosomal sorting for retrieval to the Golgi requires retromer. J Cell Biol 2004; 165:111 - 22; http://dx.doi.org/10.1083/jcb.200312034; PMID: 15078902
- Belenkaya TY, Wu Y, Tang X, Zhou B, Cheng L, Sharma YV, et al. The retromer complex influences Wnt secretion by recycling wntless from endosomes to the trans-Golgi network. Dev Cell 2008; 14:120 - 31; http://dx.doi.org/10.1016/j.devcel.2007.12.003; PMID: 18160348
- Franch-Marro X, Wendler F, Guidato S, Griffith J, Baena-Lopez A, Itasaki N, et al. Wingless secretion requires endosome-to-Golgi retrieval of Wntless/Evi/Sprinter by the retromer complex. Nat Cell Biol 2008; 10:170 - 7; http://dx.doi.org/10.1038/ncb1678; PMID: 18193037
- Harterink M, Port F, Lorenowicz MJ, McGough IJ, Silhankova M, Betist MC, et al. A SNX3-dependent retromer pathway mediates retrograde transport of the Wnt sorting receptor Wntless and is required for Wnt secretion. Nat Cell Biol 2011; 13:914 - 23; http://dx.doi.org/10.1038/ncb2281; PMID: 21725319
- Pan CL, Baum PD, Gu M, Jorgensen EM, Clark SG, Garriga G. C. elegans AP-2 and retromer control Wnt signaling by regulating mig-14/Wntless. Dev Cell 2008; 14:132 - 9; http://dx.doi.org/10.1016/j.devcel.2007.12.001; PMID: 18160346
- Port F, Kuster M, Herr P, Furger E, Bänziger C, Hausmann G, et al. Wingless secretion promotes and requires retromer-dependent cycling of Wntless. Nat Cell Biol 2008; 10:178 - 85; http://dx.doi.org/10.1038/ncb1687; PMID: 18193032
- Yang PT, Lorenowicz MJ, Silhankova M, Coudreuse DY, Betist MC, Korswagen HC. Wnt signaling requires retromer-dependent recycling of MIG-14/Wntless in Wnt-producing cells. Dev Cell 2008; 14:140 - 7; http://dx.doi.org/10.1016/j.devcel.2007.12.004; PMID: 18160347
- Burd CG. Physiology and pathology of endosome-to-Golgi retrograde sorting. Traffic 2011; 12:948 - 55; http://dx.doi.org/10.1111/j.1600-0854.2011.01188.x; PMID: 21382144
- Saint-Pol A, Yélamos B, Amessou M, Mills IG, Dugast M, Tenza D, et al. Clathrin adaptor epsinR is required for retrograde sorting on early endosomal membranes. Dev Cell 2004; 6:525 - 38; http://dx.doi.org/10.1016/S1534-5807(04)00100-5; PMID: 15068792
- Meyer C, Zizioli D, Lausmann S, Eskelinen EL, Hamann J, Saftig P, et al. mu1A-adaptin-deficient mice: lethality, loss of AP-1 binding and rerouting of mannose 6-phosphate receptors. EMBO J 2000; 19:2193 - 203; http://dx.doi.org/10.1093/emboj/19.10.2193; PMID: 10811610
- Choudhury R, Diao A, Zhang F, Eisenberg E, Saint-Pol A, Williams C, et al. Lowe syndrome protein OCRL1 interacts with clathrin and regulates protein trafficking between endosomes and the trans-Golgi network. Mol Biol Cell 2005; 16:3467 - 79; http://dx.doi.org/10.1091/mbc.E05-02-0120; PMID: 15917292
- Scott GK, Fei H, Thomas L, Medigeshi GR, Thomas GA. A PACS-1, GGA3 and CK2 complex regulates CI-MPR trafficking. EMBO J 2006; 25:4423 - 35; http://dx.doi.org/10.1038/sj.emboj.7601336; PMID: 16977309
- Carroll KS, Hanna J, Simon I, Krise J, Barbero P, Pfeffer SR. Role of Rab9 GTPase in facilitating receptor recruitment by TIP47. Science 2001; 292:1373 - 6; http://dx.doi.org/10.1126/science.1056791; PMID: 11359012
- Díaz E, Pfeffer SR. TIP47: a cargo selection device for mannose 6-phosphate receptor trafficking. Cell 1998; 93:433 - 43; http://dx.doi.org/10.1016/S0092-8674(00)81171-X; PMID: 9590177
- Haft CR, de la Luz Sierra M, Barr VA, Haft DH, Taylor SI. Identification of a family of sorting nexin molecules and characterization of their association with receptors. Mol Cell Biol 1998; 18:7278 - 87; PMID: 9819414
- Xu Y, Hortsman H, Seet L, Wong SH, Hong W. SNX3 regulates endosomal function through its PX-domain-mediated interaction with PtdIns(3)P. Nat Cell Biol 2001; 3:658 - 66; http://dx.doi.org/10.1038/35083051; PMID: 11433298
- Zhang P, Wu Y, Belenkaya TY, Lin X. SNX3 controls Wingless/Wnt secretion through regulating retromer-dependent recycling of Wntless. Cell Res 2011; 21:1677 - 90; http://dx.doi.org/10.1038/cr.2011.167; PMID: 22041890
- Zhou CZ, Li de La Sierra-Gallay I, Quevillon-Cheruel S, Collinet B, Minard P, Blondeau K, et al. Crystal structure of the yeast Phox homology (PX) domain protein Grd19p complexed to phosphatidylinositol-3-phosphate. J Biol Chem 2003; 278:50371 - 6; http://dx.doi.org/10.1074/jbc.M304392200; PMID: 14514667
- Bonifacino JS, Hurley JH. Retromer. Curr Opin Cell Biol 2008; 20:427 - 36; http://dx.doi.org/10.1016/j.ceb.2008.03.009; PMID: 18472259
- Seaman MN. Recycle your receptors with retromer. Trends Cell Biol 2005; 15:68 - 75; http://dx.doi.org/10.1016/j.tcb.2004.12.004; PMID: 15695093
- Griffin CT, Trejo J, Magnuson T. Genetic evidence for a mammalian retromer complex containing sorting nexins 1 and 2. Proc Natl Acad Sci U S A 2005; 102:15173 - 7; http://dx.doi.org/10.1073/pnas.0409558102; PMID: 16214895
- Rojas R, Kametaka S, Haft CR, Bonifacino JS. Interchangeable but essential functions of SNX1 and SNX2 in the association of retromer with endosomes and the trafficking of mannose 6-phosphate receptors. Mol Cell Biol 2007; 27:1112 - 24; http://dx.doi.org/10.1128/MCB.00156-06; PMID: 17101778
- Cullen PJ, Korswagen HC. Sorting nexins provide diversity for retromer-dependent trafficking events. Nat Cell Biol 2012; 14:29 - 37; http://dx.doi.org/10.1038/ncb2374; PMID: 22193161
- Wassmer T, Attar N, Bujny MV, Oakley J, Traer CJ, Cullen PJ. A loss-of-function screen reveals SNX5 and SNX6 as potential components of the mammalian retromer. J Cell Sci 2007; 120:45 - 54; http://dx.doi.org/10.1242/jcs.03302; PMID: 17148574
- Haft CR, de la Luz Sierra M, Bafford R, Lesniak MA, Barr VA, Taylor SI. Human orthologs of yeast vacuolar protein sorting proteins Vps26, 29, and 35: assembly into multimeric complexes. Mol Biol Cell 2000; 11:4105 - 16; PMID: 11102511
- Carlton J, Bujny M, Peter BJ, Oorschot VM, Rutherford A, Mellor H, et al. Sorting nexin-1 mediates tubular endosome-to-TGN transport through coincidence sensing of high- curvature membranes and 3-phosphoinositides. Curr Biol 2004; 14:1791 - 800; http://dx.doi.org/10.1016/j.cub.2004.09.077; PMID: 15498486
- Cozier GE, Carlton J, McGregor AH, Gleeson PA, Teasdale RD, Mellor H, et al. The phox homology (PX) domain-dependent, 3-phosphoinositide-mediated association of sorting nexin-1 with an early sorting endosomal compartment is required for its ability to regulate epidermal growth factor receptor degradation. J Biol Chem 2002; 277:48730 - 6; http://dx.doi.org/10.1074/jbc.M206986200; PMID: 12198132
- Frost A, Perera R, Roux A, Spasov K, Destaing O, Egelman EH, et al. Structural basis of membrane invagination by F-BAR domains. Cell 2008; 132:807 - 17; http://dx.doi.org/10.1016/j.cell.2007.12.041; PMID: 18329367
- Peter BJ, Kent HM, Mills IG, Vallis Y, Butler PJ, Evans PR, et al. BAR domains as sensors of membrane curvature: the amphiphysin BAR structure. Science 2004; 303:495 - 9; http://dx.doi.org/10.1126/science.1092586; PMID: 14645856
- Pylypenko O, Lundmark R, Rasmuson E, Carlsson SR, Rak A. The PX-BAR membrane-remodeling unit of sorting nexin 9. EMBO J 2007; 26:4788 - 800; http://dx.doi.org/10.1038/sj.emboj.7601889; PMID: 17948057
- Mari M, Bujny MV, Zeuschner D, Geerts WJ, Griffith J, Petersen CM, et al. SNX1 defines an early endosomal recycling exit for sortilin and mannose 6-phosphate receptors. Traffic 2008; 9:380 - 93; http://dx.doi.org/10.1111/j.1600-0854.2007.00686.x; PMID: 18088323
- van Weering JR, Verkade P, Cullen PJ. SNX-BAR-mediated endosome tubulation is co-ordinated with endosome maturation. Traffic 2012; 13:94 - 107; http://dx.doi.org/10.1111/j.1600-0854.2011.01297.x; PMID: 21973056
- Gomez TS, Billadeau DDA. A FAM21-containing WASH complex regulates retromer-dependent sorting. Dev Cell 2009; 17:699 - 711; http://dx.doi.org/10.1016/j.devcel.2009.09.009; PMID: 19922874
- Harbour ME, Breusegem SY, Antrobus R, Freeman C, Reid E, Seaman MN. The cargo-selective retromer complex is a recruiting hub for protein complexes that regulate endosomal tubule dynamics. J Cell Sci 2010; 123:3703 - 17; http://dx.doi.org/10.1242/jcs.071472; PMID: 20923837
- Temkin P, Lauffer B, Jäger S, Cimermancic P, Krogan NJ, von Zastrow M. SNX27 mediates retromer tubule entry and endosome-to-plasma membrane trafficking of signalling receptors. Nat Cell Biol 2011; 13:715 - 21; http://dx.doi.org/10.1038/ncb2252; PMID: 21602791
- Seaman MN. Identification of a novel conserved sorting motif required for retromer-mediated endosome-to-TGN retrieval. J Cell Sci 2007; 120:2378 - 89; http://dx.doi.org/10.1242/jcs.009654; PMID: 17606993
- Hierro A, Rojas AL, Rojas R, Murthy N, Effantin G, Kajava AV, et al. Functional architecture of the retromer cargo-recognition complex. Nature 2007; 449:1063 - 7; http://dx.doi.org/10.1038/nature06216; PMID: 17891154
- Rojas R, van Vlijmen T, Mardones GA, Prabhu Y, Rojas AL, Mohammed S, et al. Regulation of retromer recruitment to endosomes by sequential action of Rab5 and Rab7. J Cell Biol 2008; 183:513 - 26; http://dx.doi.org/10.1083/jcb.200804048; PMID: 18981234
- Seaman MN, Harbour ME, Tattersall D, Read E, Bright N. Membrane recruitment of the cargo-selective retromer subcomplex is catalysed by the small GTPase Rab7 and inhibited by the Rab-GAP TBC1D5. J Cell Sci 2009; 122:2371 - 82; http://dx.doi.org/10.1242/jcs.048686; PMID: 19531583
- Gokool S, Tattersall D, Seaman MN. EHD1 interacts with retromer to stabilize SNX1 tubules and facilitate endosome-to-Golgi retrieval. Traffic 2007; 8:1873 - 86; http://dx.doi.org/10.1111/j.1600-0854.2007.00652.x; PMID: 17868075
- Naslavsky N, Rahajeng J, Sharma M, Jovic M, Caplan S. Interactions between EHD proteins and Rab11-FIP2: a role for EHD3 in early endosomal transport. Mol Biol Cell 2006; 17:163 - 77; http://dx.doi.org/10.1091/mbc.E05-05-0466; PMID: 16251358
- Henry GD, Corrigan DJ, Dineen JV, Baleja JD. Charge effects in the selection of NPF motifs by the EH domain of EHD1. Biochemistry 2010; 49:3381 - 92; http://dx.doi.org/10.1021/bi100065r; PMID: 20329706
- Kieken F, Sharma M, Jovic M, Giridharan SS, Naslavsky N, Caplan S, et al. Mechanism for the selective interaction of C-terminal Eps15 homology domain proteins with specific Asn-Pro-Phe-containing partners. J Biol Chem 2010; 285:8687 - 94; http://dx.doi.org/10.1074/jbc.M109.045666; PMID: 20106972
- Zhang J, Reiling C, Reinecke JB, Prislan I, Marky LA, Sorgen PL, et al. Rabankyrin-5 interacts with EHD1 and Vps26 to regulate endocytic trafficking and retromer function. Traffic 2012; 13:745 - 57; http://dx.doi.org/10.1111/j.1600-0854.2012.01334.x; PMID: 22284051
- Schnatwinkel C, Christoforidis S, Lindsay MR, Uttenweiler-Joseph S, Wilm M, Parton RG, et al. The Rab5 effector Rabankyrin-5 regulates and coordinates different endocytic mechanisms. PLoS Biol 2004; 2:E261; http://dx.doi.org/10.1371/journal.pbio.0020261; PMID: 15328530