Abstract
Transformation of a normal cell to a cancer cell is caused by mutations in genes that regulate proliferation, apoptosis, and invasion. Small GTPases such as Ras, Rho, Rac and Cdc42 orchestrate many of the signals that are required for malignant transformation. The p21-activated kinases (PAKs) are effectors of Rac and Cdc42. PAKs are a family of serine/threonine protein kinases comprised of six isoforms (PAK1–6), and they play important roles in cytoskeletal dynamics, cell survival and proliferation. They act as key signal transducers in several cancer signaling pathways, including Ras, Raf, NFκB, Akt, Bad and p53. Although PAKs are not mutated in cancers, they are overexpressed, hyperactivated or amplified in several human tumors and their role in cell transformation make them attractive therapeutic targets. This review discusses the evidence that PAK is important for cell transformation and some key signaling pathways it regulates. This review primarily discusses Group I PAKs (PAK1, PAK2 and PAK3) as Group II PAKs (PAK4, PAK5 and PAK6) are discussed elsewhere in this issue (by „Minden).
Introduction
Douglas Hanahan and Robert Weinberg developed a set of “hallmarks of cancer,” which serve as defining principles for understanding the complex series of changes in tissues that give rise to malignant tumors. The hallmarks include sustaining proliferative signaling, evading growth suppressors, resisting cell death, enabling replicative immortality, inducing angiogenesis and activating invasion and metastasis. Two emerging hallmarks from the last decade of research are working their way into general acceptance, reprogramming of energy metabolism and evading immune destruction.Citation1 Cancer cells acquire their hallmarks through mutations in oncogenes, some 200 or so, which have been identified. Despite this large number of genes, the mutations cluster in only about a dozen processes and cell signaling pathways in each tumor.Citation2 The dissection of these processes and signaling pathways has identified a wealth of targets for therapeutic intervention and several drugs are already on the market to treat tumors. Protein kinases are often mutated themselves and even when not mutated, often regulate key steps in hallmark processes. Biological studies suggest that PAKs play a key role in some of these hallmarks, including proliferative signaling, resisting cell death, activating invasion, metastasis and inducing angiogenesis.
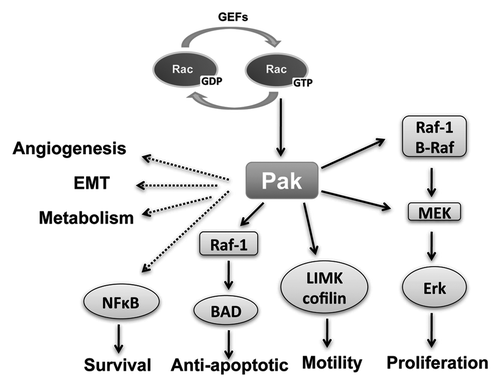
The small GTPases Ras, Rho, Rac and Cdc42 orchestrate many of the hallmarks of cancer. These proteins act as molecular switches existing in two conformational states, GDP and GTP bound. The exchange of GDP for GTP is accelerated by the association of guanine nucleotide exchange factors (GEFs). Mutations in Ras that disrupt the subsequent hydrolysis of GTP and cause Ras to remain its activated GTP-bound state, are found in about 20% of tumors. Upon activation, small GTPases interact with downstream effectors to elicit their responses. The p21-activated kinases (PAKs) are among the best characterized effectors of Rac and Cdc42. They are a family of serine/threonine protein kinases comprised of six isoforms (PAK1-6). PAKs are overexpressed and/or hyperactivated in several human tumors such as breast cancer, neurofibromatosis, colon cancer and lung cancer. They maintain cell transformation by promoting a number of hallmark processes including cell proliferation, survival, motility and angiogenesis ().
Figure 1. PAKs and cancer hallmarks. PAKs are effectors of Rac/Cdc42 and play a key role in some of cancer hallmarks, including proliferative signaling, resisting cell death, activating invasion and metastasis and inducing angiogenesis. PAKs can regulate cell proliferation through the Raf/Mek pathway. Cell motility can be affected by PAKs phosphorylation of cytoskeletal targets, such as LIMK, which phosphorylates cofilin. PAK1 also phosphorylates Bad directly and indirectly via Raf-1, thus promoting cell survival by anti-apoptosis. NFκB is regulated by PAK indirectly to promote cell survival. Other cancer hallmarks are also affected indirectly by PAKs.
PAK Activation and Amplification in Cancer
There is little evidence for cancer cells having activating mutations in PAK genes although a mutation was found in the kinase domain of PAK4 (E329K) in a colorectal tumor sample. It is not known if the mutation affects kinase activity.Citation3 However, PAK family members are amplified, overexpressed or hyperactivated in a number of human tumors. PAK1 is the isoform most commonly overexpressed but other family members, most often PAK4 is overexpressed in specific cancers (). PAK4, for example, is overexpressed in 75% of the NCI 60 cell line panel and a dominant negative mutant will block cell transformation of a colon cancer cell line.Citation4
Table 1. Cancers with amplified, overexpressed or activated PAK family members
Several distinct molecular mechanisms cause aberrant PAK signaling in cancer, including gene amplification and alteration of upstream regulators. Both PAK1 and PAK4 are localized to genomic regions, which are frequently amplified in cancer cells. The PAK1 gene is localized within the 11q13 region, and 11q13.5-q14 amplifications involving the PAK1 locus are found in bladder, ovary and breast cancer.Citation5-Citation8 PAK4 localizes to another amplicon, 19q13.2, and PAK4 gene amplification has been found in colorectal and pancreatic cancers.Citation3,Citation9
PAK gene amplifications are not frequent enough to be the only molecular mechanism leading to PAK overexpression in cancer. A 2008 report identified a novel mechanism for the overexpression of PAK1 through microRNA downregulation. Reddy et al. found that the levels of endogenous microRNA miR-7 inversely correlated with PAK1 expression in a variety of cancer cell lines.Citation10 Moreover, transfection of miR-7 downregulated PAK1 expression in breast cancer cells, and suppressed motility and invasiveness of these cells.Citation10 PAKs are also overexpressed in lung cancer but the mechanism is not known, although gene amplification is not likely.Citation8
PAK Target Recognition
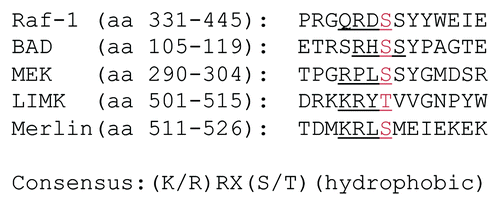
To date, over 40 proteins have been identified as substrates for PAKs (see ). As for most protein kinases, there is some flexibility in the recognition sequences phosphorylated by PAK. Shown in are the examples of phosphorylation sites for several PAK substrates. One study used PAK2 and compared a limited number of peptides derived from the substrate KKRKSGL. This yielded a recognition sequence for PAK2 that is characterized by two basic amino acids in the -2 and -3 positions. For example, the peptide (K/R)RXS, in which the -2 position is an arginine and the -3 position is an arginine or a lysine, is efficiently phosphorylated at the serine residue (X can be an acidic, basic or neutral amino acid).Citation11 A more comprehensive study used a wider array of peptides and found that PAK1 and PAK2 preferred large hydrophobic residues in positions from +1 to +3, in addition to their preference for basic amino acids at the -2 and -3 positions.Citation12 PAK1 and PAK2 have nearly identical substrate specificities, but the substrate specificity of PAK4 is significantly different. PAK4 has strong preference and for alanine at the +2 and serine at the +3 position. It should be noted that although there are differences in the preferred consensus sequences for Group I (PAK1, PAK2 and PAK3) and Group II PAKs (PAK4, PAK5 and PAK6), most known substrates are phosphorylated by both groups. Additionally, both groups strongly prefer serine over threonine as a phospho-acceptor site and do not phosphorylate tyrosine at all. Although the Rennefahrt study was able to identify a new PAK substrate by scanning databases, there are limitations to identifying substrates by sequence searches. The study found that none of the known PAK substrates fell into the top 2% of the predicted substrates, suggesting that other factors such as protein-protein interactions facilitate phosphorylation of what are otherwise less-ideal substrates.Citation12
Table 2. Reported PAK substrates
Figure 2. PAK phosphorylation sites. Activated PAK proteins phosphorylate a variety of substrates on serine/threonine residues, preferably in the context of basic residues such as K/R, R/X, X and S/T, to bring about cell survival and migration, cytoskeleton remodeling and gene regulation. Shown here are the sequences of phosphorylation sites of several PAK substrates. Consensus sequence is also shown. X can be acidic, basic or neutral amino acid.
PAK Regulation of Cancer Cell Hallmarks
The primary hallmark of cancer is the ability to form tumors. There are several ways to measure tumor cell growth. The most common way is to inject tumor cells into immune compromised or nude mice, where they will grow into tumors. A simpler assay is to grow cells suspended in soft agar. Tumor cells will grow into colonies, a property called anchorage independence, while untransformed cells will not grow. PAKs were first shown to be important for transformation in experiments where a kinase dead mutant of PAK was expressed in fibroblasts together with an oncogenic Ras mutant. The mutant behaved as a dominant negative mutant and prevented Ras from inducing anchorage independent growth in soft agar assays.Citation13 Kinase dead mutants of PAK4 also inhibit cell transformation.Citation4,Citation14 The kinase dead mutants do not act by sequestering Rac and Cdc42 because the p21 binding sites can be deleted and the inactive kinase domain by itself will inhibit transformation.Citation13 Although the use of these dominant negative mutants, and other technologies based on expressing fragments of PAK have since been replaced with siRNAs and small molecule drugs, they were invaluable in establishing the function of PAK in cancer.
To establish if PAK activation could cause tumors, studies were performed expressing activated mutants. Since PAK is not mutated in tumors, activated mutants were constructed. In most studies, activation of only PAK4 caused anchorage independent growth, although some studies found that activated PAK1 induced tumors when expressed with a weakly activated Raf-1 mutant.Citation14-Citation17 Additionally, transgenic mice that overexpresses a constitutively active PAK1 under a β-lactoglobulin promoter develops malignant mammary gland tumors, although with a relatively long latency period and low penetrance.Citation18 These studies established that activation primarily of PAK4 is sufficient for tumorigenesis, although in many tumors, PAK1 and PAK4 are necessary for transformation. The precise relationship between PAK1 and PAK4, and indeed other PAK isoforms are not understood. This may be important clinically if isoform specific inhibitors are eventually used therapeutically.
The most prominent hallmarks of cancer for which a role of PAK has been established are stimulation of cell proliferation (including anchorage-independent growth), stimulation of cell survival (e.g., inhibition of apoptosis), and stimulation of cell motility. PAK activation will stimulate each of these hallmarks, while PAK inhibition inhibits the hallmark. Each of these three hallmarks has at least one known target in a well-established signaling pathway, which is a direct PAK target (). For cell proliferation, PAK contributes to the canonical MAP kinase cascade of Ras/Raf/MeK/ERK. In anti-apoptotic signaling PAK contributes to the BAD/Bcl-2 pathway. To regulate cell motility, PAK targets LIM kinase, which phosphorylates cofilin. PAKs have also been implicated in other cellular processes that are relevant in tumorigenesis, including angiogenesis,Citation19 epithelial-mesenchymal transitionCitation20 and metabolism,Citation21,Citation22 although the signaling pathways are not as well established as for other processes. The molecular targets of PAK and the effects on their signaling pathways will be discussed later, but first we will address PAK in several specific tumors for which a role has been established including breast, neurofibromatosis 1, neurofibromatosis 2, colon and lung.
Breast cancer
The cancer for which PAK is most extensively documented is breast cancer. More than 50% of human breast cancers display overexpression and/or hyperactivation of PAK1 and PAK1 is found on a chromosomal region amplified in 17% of breast cancers.Citation8,Citation23 In addition, transgenic expression of an activated PAK1 mutant in mouse mammary tissue causes tumors.Citation18 PAK1 also promotes mammary epithelial cell transformation in 3-dimensional culture model systems. Furthermore, PAK1 expression and its nuclear accumulation increased progressively during the transition from ductal hyperplasia to ductal carcinoma in situ to adenocarcinoma in widely used multistep polyoma-middle T-antigen transgenic mice.Citation18 PAK4 also promotes tumorigenesis in breast cancer cells.Citation17 Together, these studies make a strong case for an important role of PAK in breast cancer, suggesting PAK expression in the transformation process progresses with increasing stages of tumors. Several signaling pathways such as MAPK and MET, NFκB, BAD and estrogen receptor α (ERα) are activated by PAK1 during the progression of breast cancer and these pathways will discussed below.Citation18,Citation23-Citation28
Numerous studies have found that expression of PAK1 promotes mammary cell growth. For example, activated PAK1 causes human mammary epithelial (HMLE) cells to form anchorage-independent colonies, and its kinase activity is necessary for PAK1-induced transformation. These effects are due to PAK1 simultaneously activating of MAPK and MET signaling.Citation24 PAK1 overexpression in mammary tissue also increases the activation of MEK1/2 and p38-MAPK in mammary tumor epithelial cells.Citation18
PAK is activated through pathways that are important for breast cancer growth. Growth factors such as prolactin and the oncogene human epidermal growth factor receptor 2 (HER2 or ErbB2) can activate MAPK signaling pathway through PAK1. The prolactin receptor (PRL-R) can initiate and sustain Erk1/2 signaling via the PI3K-dependent Rac/PAK pathway rather than the canonical ErbB2/Shc/Grb2/SOS/Ras route.Citation29 PRL-R signaling pathway also activates PAK1 through JAK/STAT5, leading to the induction of cyclin D1.Citation30 ErbB2 gene overexpression, amplification, or mutation occurs in about 25% of human breast cancer.Citation31 ErbB2 signaling activates a Rac-PAK signaling pathway that contributes to ErbB2 mediated transformation through the MAPK/Erk and Akt pathways.Citation32,Citation33 ErbB2 expression correlates with PAK levels and enzymatic activity in ER-positive human breast cancer. ErbB2 activates Rac and PAK in a 3D breast epithelial cell culture system, and loss of Rac or PAK activity blocks the morphologic effects of ErbB2 in these cells, accompanied by loss of Erk and Akt activation.Citation32 Moreover, PAK is required for ErbB2 transformation in a xenograft model of breast cancer.Citation32
PAK regulates survival signals in breast cancer. A study examined PAK1 activity in a pre-malignant progression series of MCF10A mammary epithelial cell variants. PAK1 expression levels increased in correlation with the progression stages in this series, indicating a role for PAK1 in the early stages of cell transformation.Citation34 Activation of the transcription factor NFκB appears to be a prominent mechanism by which PAK1 regulates survival of breast cancer cells. Friedland et al. showed a functional link between the resistance of mammary epithelial cells to apoptosis in 3-dimensional cultures and PAK1-mediated activation of NFκB.Citation28 Notably, NFκB also promoted cell proliferation via cyclin D1 transcription in breast cancer cells.Citation23 Phosphorylation of the pro-apoptotic proteins BAD and FKHR, and phosphorylation of DLC1 are other mechanisms by which PAK1 may promote breast cancer cell survival.Citation35,Citation36
PAK promotes cell motility signals in breast cancer. PAK substrates that control different aspects of cytoskeletal dynamics, such as LIM kinase, p41-ARC, filamin A, Op18/stathmin and TCoB, are likely to promote the invasiveness of breast cancer cells.Citation37 In addition, the multimodular protein Scrib positively regulates activation of PAK1 and participates in lamellipodia formation at the leading edge of migratory breast cancer cells.Citation38 Moreover, PAK-phosphorylated alternate-spliced isoform of the steroid receptor coactivator-3 (SRC-3Delta4) bridges EGFR and focal adhesion kinase (FAK), enhancing breast carcinoma cell migration and metastasis.Citation39
PAK is also involved in estrogen receptor signals in breast cancer. Approximately 70% of all breast cancers express the estrogen receptor (ERα), and tamoxifen, a selective anti-estrogen, is widely used to treat this group of breast cancers. PAK1 is one of many kinases that phosphorylate ERα.Citation25,Citation40 Deregulated activation of PAK1 produces multiple or inappropriate phosphorylation of ERα, creating a promiscuous receptor that is resistant to tamoxifen and stimulates cell growth in the absence of estrogen.Citation25,Citation40 The nuclear levels of active PAK1 increased in breast cancer patients with tamoxifen resistance.Citation25,Citation41 Moreover, ER activation by PAK1 induces upregulation of cyclin D1 in breast cancer cells, as well as in the mammary epithelium.Citation23 Patients who were negative for PAK1 obtained more benefit from tamoxifen treatment.Citation41 The link between PAK1 and ERα raises the possibility that tamoxifen resistance might be prevented or reversed by PAK1 inhibition.
Neurofibromatosis
Neurofibromatosis types 1 and 2 (NF1 and NF2) are dominantly inherited autosomal diseases caused by loss-of-function mutations in the tumor suppressor genes NF1 and NF2, respectively. NF1 is a common disease, having a birth incidence of about 1 in 3,000, while NF2 is a relatively rare disorder with an incidence of about 1 in 25,000. Neurofibromatosis patients are predisposed to the development of multiple tumors of the central and peripheral nervous system. Schwann cells, the cells that comprise the myelin sheath around nerves, are predominantly affected in both tumors. Patients carry heterozygous mutations in either the NF1 or NF2 gene but their tumors typically display loss of the residual wild-type allele, conforming to the classic two hit Knudsen paradigm seen with most tumor suppressors. Although NF1 and NF2 are genetically and clinically distinct diseases, loss of each gene product leads to abnormal activation of PAK1, albeit through different mechanisms. Experimental results suggest that PAK1 is important for the malignant growth in both types of neurofibromatosis.
The mechanism of PAK1 activation through NF1 proceeds through the Ras pathway. The product of the NF1 gene is a cytoplasmic protein called neurofibromin. Neurofibromin is widely expressed across a range of tissues but with high concentrations in the nervous system. Neurofibromin is a GTPase activating protein (GAP) and acts by accelerating the intrinsic GTPase activity of Ras. Consequently, loss of neurofibromin is associated with increased levels of activated GTP-bound Ras, which activates oncogenic pathways, including the MAPK cascade and PI3K. Downstream signals of PI3K activate PAK via Rac and Cdc42. Dominant negative PAK mutants are potent inhibitors of Ras transformation in both rat Schwann cells and a malignant peripheral nerve sheet tumor (MPNST or neurofibrosarcoma) cell line from an NF1 patient.Citation42
While NF1 activates PAK through effector pathways, NF2 interacts directly with PAK1. The NF2 gene product is a cytoskeleton-associated tumor suppressor named Merlin (also called Schwannomin). Merlin is structurally related to the moesin/ezrin/radixin proteins, which link the actin cytoskeleton to cell surface glycoproteins that control growth and cellular remodeling. Merlin is widely expressed in Schwann cells, meningeal cells, peripheral nerves and the lens. In non-neoplastic cells, Merlin mediates contact-dependent growth inhibition. The growth suppressive function of Merlin depends on its phosphorylation status at Ser518.Citation43 Under growth restrictive conditions, Merlin is unphosphorylated and inhibits cell proliferation, while under growth permissive conditions, Merlin is phosphorylated. Both cAMP-dependent protein kinase A (PKA) and PAK1 are able to phosphorylate Merlin at Ser518 and thereby inhibit its growth suppressive activity.Citation44-Citation46 Phosphorylation of Merlin at Ser518 was also demonstrated by PAK2 and PAK6.Citation45
While PAK phosphorylates and inhibits Merlin, there is also an important inhibitory feedback mechanism from Merlin to PAK. Group I PAKs are downstream targets of Merlin. Merlin associates with inactive PAK and prevents its activation, perhaps by competing with Rac.Citation47,Citation48 Phosphorylation at Ser518 induces a conformation change in Merlin and consequently disrupts interaction with PAK1, allowing PAK1 to be activated. Thus, in NF2 patients, loss of Merlin is associated with abnormal PAK1 activity, which also leads to elevated levels of Rac as well as pronounced cell ruffling.Citation49,Citation50 In cell culture experiments, the PAK1 inhibitors CEP-1347 and WR-PAK18 were able to inhibit the growth of Merlin-deficient tumor cells, but not Merlin-positive cells.Citation47 The loss of PAK activity restored normal cell growthCitation51 and movement to cells lacking Merlin function.Citation52
Recently, PAK2 has been shown to be essential for the activation of proliferation signals Wnt/β-catenin signaling in schwannoma cells, and depletion of PAK2 suppressed active β-catenin, c-myc and cyclin D1.Citation53 In NF2 tumors, loss of PAK activity, however, did not reduce Erk or Akt activity, two signaling proteins that are thought to mediate PAK function in NF1.Citation52 Together, these studies suggest that PAK is a major player underlying Schwann cell transformation and an attractive target for therapeutics in both NF1 and NF2. There are multiple signaling pathways that PAK regulates in Schwann cells and the signals may differ between NF1 and NF2.
Colon cancer
PAK1, PAK4 and PAK5 have been implicated in colon cancer cell transformation through expression studies as well as functional studies where they regulate cell adhesion and migration.Citation54-Citation56
Overexpression of PAK1 is observed in 70% of colon cancer samples and is correlated with several signaling pathways including, Wnt, Erk and Akt pathways. Reduction of PAK1 expression decreased cell proliferation, migration/invasion, and survival. Rac1/PAK1 cascade controls β-catenin S675 phosphorylation and its activation in colon cancer cells. Downregulation of PAK1 in colon cancer cells reduces the β-catenin levels and cell proliferation. PAK1 also directly phosphorylated β-catenin at Ser675, leading to more stable and transcriptional active β-catenin.Citation57 Erk and Akt, downstream targets of PAK1 are involved in colon cancer progression. PAK inhibition alone is equivalent to the dual inhibition of Erk and Akt, whereas inactivation of either the Erk or Akt pathway alone partially inhibited cell migration/invasion and survival and had no effect on proliferation. Thus, in at least this one case, instead of simultaneously inhibiting both Erk and Akt, PAK1 may be a convergence point for therapy.Citation58
Lung cancer
Lung cancer, although not as well established as other cancers, is emerging as a tumor depends on PAK1 signaling. A mouse model for Ras-induced lung cancers is highly sensitive to Rac inhibition, suggesting that lung cancers may be dependent on PAK.Citation59 PAK1 is expressed strongly in the nucleus and cytoplasm of squamous nonsmall cell lung carcinomas (NSCLCs).Citation8 Finally, selective inhibition of PAK1 but not PAK2 delayed cell-cycle progression in vitro and in vivo.Citation8
Melanoma and other cancers
There are several cancers in which a role for PAK is implied but has not been documented as rigorously. In melanomas, two large scale melanoma sequencing projects found a novel mutation in Rac1, P29S in about 10% of the tumor samples. The mutation caused an increase in GTP-bound Rac1 and furthermore, expression of the mutant in melanocytes increased proliferation and phosphor-ERK levels (see below for a discussion of PAK regulation of ERK).Citation60,Citation61 Though neither study directly addressed PAK, it is likely that PAKs are required for some melanomas to progress.
In some cases such as pancreatic tumors and ovarian cancers, PAKs are amplified, but functional data are not available. In other cases, the reagents used to test the involvement of PAK were not that specific. For example, a new PAK inhibitor OSU-03012 inhibited migration in thyroid tumor cells, but since this compound also inhibits PDK1, albeit at higher doses, it is premature to conclude that PAK is required in thyroid tumors.Citation62
PAK Regulation of Cell Signals
PAKs regulate several cell signaling pathways controlling tumor cell growth and survival including MAPK/Erks,Citation13 p53,Citation63 NFκB,Citation64 SmadCitation65 and STAT3.Citation66 In some cases the relationship between PAK with these signaling pathways has been established, while in other cases the direct connection with PAK has yet to be determined. The Erk, NFκB and more recently p53 pathways are the best documented examples of PAK regulation of cancer signaling pathways, and they will be discussed in this section.
MAPK
The canonical MAPK cascade is widely associated with cell proliferation and consists of Ras/Raf/MEK/(MAPK)Erk. Historically, this was the first cancer relevant signal shown to be regulated by PAK pathway. PAK phosphorylates two mediators of the MAP kinase pathway, MEK1 and Raf1, at Ser298 and at Ser338, respectively.Citation13,Citation67-Citation70 While phosphorylation of these sites by PAK is not sufficient to activate Raf1 or MEK1, it significantly facilitates the activation of these kinases by their upstream activators Ras and Raf1, respectively. The ability of PAK to regulate the MAP kinase pathway is likely to contribute to cell proliferation.
Akt and BAD
Apoptosis, or programmed cell death, is a fundamental process in the development of multicellular organisms. Apoptosis enables an organism to eliminate unwanted or defective cells through an organized process of cellular disintegration. It is a prominent tumor-suppression mechanism and cancer cells require inactivation of pro-apoptotic pathways for tumor formation and progression. PAK activity has been shown to downregulate several important pro-apoptotic pathways.
PAK1 protects cells from intrinsic apoptotic signals via a PAK-Raf1-BAD pathway. PAK1 and PAK5 phosphorylate Raf1 at Ser338 and stimulate translocation of a subpopulation of Raf1 to the mitochondria.Citation71-Citation74 At the mitochondria, Raf-1 forms a protective complex with Bcl-2 and phosphorylates the pro-apoptotic protein BAD at Ser112. Bcl-2 is a proto-oncogene that maintains the integrity of the mitochondrial barrier if bound in protective complexes, whereas binding of Bcl-2 to the pro-apoptotic protein BAD induces release of pro-apoptotic factors from the mitochondria and leads to apoptosis. Phosphorylation of BAD at specific sites, including Ser112, renders it unable to bind Bcl-2. The phenotype of Raf-1 knock out cells supports a protective role of Raf-1 in apoptosis, as these cells have high rates of apoptosis while exhibiting normal proliferative rates and Erk activation.Citation75
NFκB
PAK activates nuclear factor-κB (NFκB) a transcription factor, which is important for cell transformation through its effects on cell survival and proliferation, and it is essential for oncogenes such as Ras and Raf to transform cells. Inactive NFκB is retained in the cytoplasm due to a heterodimeric interaction with its inhibitory protein known as the inhibitor of κB (IκB). Phosphorylation and degradation of IκB is required for the activation and nuclear translocation of NFκB and the subsequent transactivation of NFκB target genes. Phosphorylation of IκB on serine 32 and serine 36 by the IκB kinases inhibitor of IκB kinase IKKα and IKKβ is an important initiation signal for IκB degradation and NFκB release.
Several studies showed that PAK1 can activate NFκB.Citation64,Citation76-Citation81 It has been shown that PAK1 activates NFκB through the phosphorylation and degradation of IκB,Citation64,Citation76 however, there is no evidence that PAK1 phosphorylates IκB. Moreover, PAK1 stimulation of the nuclear translocation of the p65 subunit of NFκB is independent of the phosphorylation of IKKα/β.Citation64,Citation76 In a report of Helicobacter pylori-induced NFκB activation, the PAK1 autoregulatory domain was shown to be required for interaction with NFκB-interacting kinase (NIK), which controls the activities of IKKα/β.Citation82 Therefore, PAK1 may affect the association of NIK, the IKKs, IκB, or NFκB with the scaffolding proteins IKK complex-associated protein or IKKγ. Indeed, it has been shown that the expression of active PAK1 reduces the coprecipitation of IKKβ with NIK from cells and dominant negative forms of IKKα/β block the PAK1 activation of NFκB.Citation64 However, despite numerous studies showing that PAK regulates NFκB, the direct target of PAK in this pathway has not been determined.
LIMK
There are several established PAK substrates that control cytoskeletal dynamics. The most well established target is LIM kinase. PAK1 and PAK4 both phosphorylate LIM-kinase at threonine residue 508 within LIM-kinase's activation loop, which stimulates LIM-kinase activity. LIM-kinase phosphorylates and inhibits the actin-regulatory protein cofilin. Cofilin depolymerizes actin filaments, thus by phosphorylating cofilin, PAK1 stimulates filaments accumulation by preventing their depolymerization.Citation83
p53
The tumor suppressor p53 is mutated in over 50% of human tumors, where it cooperates with Ras to transform cells and acts as a DNA damage checkpoint in the cell cycle.Citation84 p53 was identified in screen of 113 cell based reporter assays with the pan PAK inhibitor PF-3758309, an ATP-competitive inhibitor.Citation63 Induction of p53 by a DNA-damaging agent is reduced in cells treated with PF-3758309.Citation63 Conversely, activating p53 with the p53 degradation inhibitor, Nutlin-3, has no effect on PAK4 activation, consistent with PAK acting upstream of p53.Citation63 Moreover, other reports also showed activated PAK4 induces p53 and p21,Citation85 and PAK-family kinases and p53 expression have been reported to be co-regulated.Citation86,Citation87 Together these studies suggest that PAK is upstream of p53, although the mechanisms by which PAK regulates p53 are not well understood. The physiological significance of PAK regulation in cancer cells remains to be worked out. In one study, there was no correlation between p53 status and cancer cell sensitivity to PAK inhibition,Citation63 while another study found that loss of p53 was a synthetic lethal with PAK3. That is, loss of either p53 or PAK3 did not affect cells, but loss of both p53 and PAK3 together prevented cell growth.Citation88
Therapeutic Prospects
Because of their central position in cancer hallmarks, protein kinases currently constitute a major focus for drug discovery and most major pharmaceutical companies have kinase programs to develop inhibitors. Small molecular weight inhibitors typically target the highly conserved ATP-binding pockets of the kinase domain and compete with ATP binding. Because of similarities in the active sites of many kinases, specificity issues are common for inhibitors targeting the ATP-binding pocket, and cross-reactivity may cause unwanted toxicities. However, this approach has been successful and in recent years a number of protein kinase inhibitors have successfully been taken through clinical trials to enter clinical practice. Sorafenib (Nexavar®), imatinib mesylate (Gleevec®), temsirolimus (Torisel®), erlotinib (Tarceva®), sunitinib (Sutent®) and gefitinib (Iressa®) are examples of such small molecule kinase inhibitors. The targets for these drugs include Raf-1, Abl, mTOR and the receptor tyrosine kinases EGFR and VEGFR. PAKs have roles in several cellular processes, including cell cycle, cell motility, angiogenesis and evasion from apoptosis. PAK has been shown to be upregulated or hyperactive in several cancers such as breast, glioma, colorectal, prostate, lung (NSCLC) and MPNST. The importance of PAK in cell and animal models of tumorigenesis and metastasis provides the rationale for developing PAK inhibitors as anti-cancer therapeutics. The current status of inhibitor development is discussed in this issue by Coleman and Kissil.
One of the pressing issues with the use of drugs is identifying the tumors and subpopulations of patients who will respond to a given treatment. With most kinase inhibitors, the patients who respond the best have mutations in the targeted kinase. Patients with mutations in Ras fail to respond to any kinase inhibitor, which is unfortunate because Ras is mutated in about 20% of tumors, far more frequently than any of the kinases. Since many of the signals that are regulated by PAK are intrinsic to the Ras pathway, tumors with mutations in Ras may respond to PAK inhibitors in addition to those in which PAK itself is amplified. A survey with the PAK inhibitor PF-3758309 of 92 tumor cell lines derived from colorectal, non-small-cell lung cancer, pancreatic, and breast tumors, found that 46% exhibited IC50 values less than 10 nM.Citation63 In another study, a strong synergy was found with inhibiting PAK and drugs that act in cell signaling pathways that have been discussed in this review. Among the tested compounds, antagonists of inhibitor of apoptosis proteins (IAP; 12- and 57-fold), epidermal growth factor receptor (EGFR; 2.9-, 7.4-, 12.8- and 15-fold), MEK1/2 (8.5-fold), and Src family kinases (5.4-fold) displayed dramatically enhanced efficacy when tested in cells with PAK1 knocked down.Citation8 It is encouraging that so many tumors respond to PAK inhibitors. However, the mutations and amplifications in tumors that respond to PAK inhibitors have yet to be determined. Additionally, the synergies observed with PAK inhibitors and other drugs suggest that PAK inhibitors are likely to be most effective in combination with other treatments.
| Abbreviations: | ||
| Abl1 | = | Abelson murine leukemia viral oncogene homolog 1 |
| BAD | = | Bcl-2 antagonist of cell death |
| CPI17 | = | 17-kDa PKC-potentiated inhibitory protein of PP1 |
| CtBP1 | = | C-terminal-binding protein 1 |
| DLC1 | = | dynein light chain 1 |
| ER | = | estrogen receptor |
| ESE1 | = | epithelium-specific Ets transcription factor 1 |
| FKHR | = | Forkhead box protein O1 |
| G α z | = | guanine nucleotide binding protein (G protein), alpha z |
| GEF-H1 | = | guanine nucleotide exchange factor H1 |
| GIT1 | = | G protein-coupled receptor kinase-interactor 1 |
| MBS | = | myosin binding subunit of type 1 protein phosphatase |
| MEK1 | = | mitogen-activated protein kinase kinase 1 |
| MEKK1 | = | mitogen-activated protein kinase kinase kinase 1 |
| MLCK | = | myosin light chain kinase |
| MNK1 | = | MAP kinase interacting kinase 1 |
| NET1 | = | neuroepithelial cell transforming gene 1 (RhoA-specific guanine nucleotide exchange factor) |
| P41-ARC | = | actin-related protein 2/3 complex 41kDa subunit |
| p47 phox | = | neutrophil NADPH oxidase activator 1 |
| p67 phox | = | neutrophil NADPH oxidase factor 2 |
| PGAM-B | = | phosphoglyceratemutase-B |
| PGM | = | phosphoglucomutase |
| PIX | = | PAK-interacting exchange factor |
| Plk1 | = | Polo-like kinase 1 |
| Rho GDI | = | Rho GDP dissociation inhibitor |
| R-MLC | = | regulatory myosin light chain |
| SHARP | = | SMART/HDAC1 associated repressor protein |
| SNAIL1 | = | snail 1 zinc finger protein |
| STAT5A | = | signal transducer and activator of transcription 5A |
| Syk | = | spleen tyrosine kinase |
| TCoB | = | tubulin cofactor B |
Acknowledgments
J.F. is supported by a grant from the NIH (GM48241).
References
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell 2011; 144:646 - 74; http://dx.doi.org/10.1016/j.cell.2011.02.013; PMID: 21376230
- Jones S, Zhang X, Parsons DW, Lin JC-H, Leary RJ, Angenendt P, et al. Core signaling pathways in human pancreatic cancers revealed by global genomic analyses. Science 2008; 321:1801 - 6; http://dx.doi.org/10.1126/science.1164368; PMID: 18772397
- Parsons DW, Wang TL, Samuels Y, Bardelli A, Cummins JM, DeLong L, et al. Colorectal cancer: mutations in a signalling pathway. Nature 2005; 436:792; http://dx.doi.org/10.1038/436792a; PMID: 16094359
- Callow MG, Clairvoyant F, Zhu S, Schryver B, Whyte DB, Bischoff JR, et al. Requirement for PAK4 in the anchorage-independent growth of human cancer cell lines. J Biol Chem 2002; 277:550 - 8; http://dx.doi.org/10.1074/jbc.M105732200; PMID: 11668177
- Bekri S, Adeálaïde J, Merscher S, Grosgeorge J, Caroli-Bosc F, Perucca-Lostanlen D, et al. Detailed map of a region commonly amplified at 11q13-->q14 in human breast carcinoma. Cytogenet Cell Genet 1997; 79:125 - 31; http://dx.doi.org/10.1159/000134699; PMID: 9533029
- Brown LA, Kalloger SE, Miller MA, Shih IeM, McKinney SE, Santos JL, et al. Amplification of 11q13 in ovarian carcinoma. Genes Chromosomes Cancer 2008; 47:481 - 9; http://dx.doi.org/10.1002/gcc.20549; PMID: 18314909
- Bostner J, Ahnström Waltersson M, Fornander T, Skoog L, Nordenskjöld B, Stål O. Amplification of CCND1 and PAK1 as predictors of recurrence and tamoxifen resistance in postmenopausal breast cancer. Oncogene 2007; 26:6997 - 7005; http://dx.doi.org/10.1038/sj.onc.1210506; PMID: 17486065
- Ong CC, Jubb AM, Haverty PM, Zhou W, Tran V, Truong T, et al. Targeting p21-activated kinase 1 (PAK1) to induce apoptosis of tumor cells. Proc Natl Acad Sci U S A 2011; 108:7177 - 82; http://dx.doi.org/10.1073/pnas.1103350108; PMID: 21482786
- Chen S, Auletta T, Dovirak O, Hutter C, Kuntz K, El-ftesi S, et al. Copy number alterations in pancreatic cancer identify recurrent PAK4 amplification. Cancer Biol Ther 2008; 7:1793 - 802; http://dx.doi.org/10.4161/cbt.7.11.6840; PMID: 18836286
- Reddy SD, Ohshiro K, Rayala SK, Kumar R. MicroRNA-7, a homeobox D10 target, inhibits p21-activated kinase 1 and regulates its functions. Cancer Res 2008; 68:8195 - 200; http://dx.doi.org/10.1158/0008-5472.CAN-08-2103; PMID: 18922890
- Tuazon PT, Spanos WC, Gump EL, Monnig CA, Traugh JA. Determinants for substrate phosphorylation by p21-activated protein kinase (γ-PAK). Biochemistry 1997; 36:16059 - 64; http://dx.doi.org/10.1021/bi9717845; PMID: 9405039
- Rennefahrt UEE, Deacon SW, Parker SA, Devarajan K, Beeser A, Chernoff J, et al. Specificity profiling of Pak kinases allows identification of novel phosphorylation sites. J Biol Chem 2007; 282:15667 - 78; http://dx.doi.org/10.1074/jbc.M700253200; PMID: 17392278
- Tang Y, Chen Z, Ambrose D, Liu J, Gibbs JB, Chernoff J, et al. Kinase-deficient Pak1 mutants inhibit Ras transformation of Rat-1 fibroblasts. Mol Cell Biol 1997; 17:4454 - 64; PMID: 9234703
- Qu J, Cammarano MS, Shi Q, Ha KC, de Lanerolle P, Minden A. Activated PAK4 regulates cell adhesion and anchorage-independent growth. Mol Cell Biol 2001; 21:3523 - 33; http://dx.doi.org/10.1128/MCB.21.10.3523-3533.2001; PMID: 11313478
- Tang Y, Yu J, Field J. Signals from the Ras, Rac, and Rho GTPases converge on the Pak protein kinase in Rat-1 fibroblasts. Mol Cell Biol 1999; 19:1881 - 91; PMID: 10022875
- Vadlamudi RK, Adam L, Wang R-A, Mandal M, Nguyen D, Sahin A, et al. Regulatable expression of p21-activated kinase-1 promotes anchorage-independent growth and abnormal organization of mitotic spindles in human epithelial breast cancer cells. J Biol Chem 2000; 275:36238 - 44; http://dx.doi.org/10.1074/jbc.M002138200; PMID: 10945974
- Liu Y, Chen N, Cui X, Zheng X, Deng L, Price S, et al. The protein kinase Pak4 disrupts mammary acinar architecture and promotes mammary tumorigenesis. Oncogene 2010; 29:5883 - 94; http://dx.doi.org/10.1038/onc.2010.329; PMID: 20697354
- Wang RA, Zhang H, Balasenthil S, Medina D, Kumar R. PAK1 hyperactivation is sufficient for mammary gland tumor formation. Oncogene 2006; 25:2931 - 6; http://dx.doi.org/10.1038/sj.onc.1209309; PMID: 16331248
- Kiosses WB, Daniels RH, Otey C, Bokoch GM, Schwartz MA. A role for p21-activated kinase in endothelial cell migration. J Cell Biol 1999; 147:831 - 44; http://dx.doi.org/10.1083/jcb.147.4.831; PMID: 10562284
- Yang Z, Rayala S, Nguyen D, Vadlamudi RK, Chen S, Kumar R. Pak1 phosphorylation of snail, a master regulator of epithelial-to-mesenchyme transition, modulates snail's subcellular localization and functions. Cancer Res 2005; 65:3179 - 84; PMID: 15833848
- Shalom-Barak T, Knaus UG. A p21-activated kinase-controlled metabolic switch up-regulates phagocyte NADPH oxidase. J Biol Chem 2002; 277:40659 - 65; http://dx.doi.org/10.1074/jbc.M206650200; PMID: 12189148
- Gururaj A, Barnes CJ, Vadlamudi RK, Kumar R. Regulation of phosphoglucomutase 1 phosphorylation and activity by a signaling kinase. Oncogene 2004; 23:8118 - 27; http://dx.doi.org/10.1038/sj.onc.1207969; PMID: 15378030
- Balasenthil S, Sahin AA, Barnes CJ, Wang RA, Pestell RG, Vadlamudi RK, et al. P21-activated kinase-1 signaling mediates cyclin D1 expression in mammary epithelial and cancer cells. J Biol Chem 2004; 279:1422 - 8; http://dx.doi.org/10.1074/jbc.M309937200; PMID: 14530270
- Shrestha Y, Schafer EJ, Boehm JS, Thomas SR, He F, Du J, et al. PAK1 is a breast cancer oncogene that coordinately activates MAPK and MET signaling. Oncogene 2012; 31:3397 - 408; http://dx.doi.org/10.1038/onc.2011.515; PMID: 22105362
- Rayala SK, Talukder AH, Balasenthil S, Tharakan R, Barnes CJ, Wang RA, et al. P21-activated kinase 1 regulation of estrogen receptor-alpha activation involves serine 305 activation linked with serine 118 phosphorylation. Cancer Res 2006; 66:1694 - 701; http://dx.doi.org/10.1158/0008-5472.CAN-05-2922; PMID: 16452229
- Wang RA, Mazumdar A, Vadlamudi RK, Kumar R. P21-activated kinase-1 phosphorylates and transactivates estrogen receptor-alpha and promotes hyperplasia in mammary epithelium. EMBO J 2002; 21:5437 - 47; http://dx.doi.org/10.1093/emboj/cdf543; PMID: 12374744
- Du J, Sun C, Hu Z, Yang Y, Zhu Y, Zheng D, et al. Lysophosphatidic acid induces MDA-MB-231 breast cancer cells migration through activation of PI3K/PAK1/ERK signaling. PLoS One 2010; 5:e15940; http://dx.doi.org/10.1371/journal.pone.0015940; PMID: 21209852
- Friedland JC, Lakins JN, Kazanietz MG, Chernoff J, Boettiger D, Weaver VM. alpha6beta4 integrin activates Rac-dependent p21-activated kinase 1 to drive NF-kappaB-dependent resistance to apoptosis in 3D mammary acini. J Cell Sci 2007; 120:3700 - 12; http://dx.doi.org/10.1242/jcs.03484; PMID: 17911169
- Aksamitiene E, Achanta S, Kolch W, Kholodenko BN, Hoek JB, Kiyatkin A. Prolactin-stimulated activation of ERK1/2 mitogen-activated protein kinases is controlled by PI3-kinase/Rac/PAK signaling pathway in breast cancer cells. Cell Signal 2011; 23:1794 - 805; http://dx.doi.org/10.1016/j.cellsig.2011.06.014; PMID: 21726627
- Tao J, Oladimeji P, Rider L, Diakonova M. PAK1-Nck regulates cyclin D1 promoter activity in response to prolactin. Mol Endocrinol 2011; 25:1565 - 78; http://dx.doi.org/10.1210/me.2011-0062; PMID: 21719533
- Shalaby MR, Shepard HM, Presta L, Rodrigues ML, Beverley PC, Feldmann M, et al. Development of humanized bispecific antibodies reactive with cytotoxic lymphocytes and tumor cells overexpressing the HER2 protooncogene. J Exp Med 1992; 175:217 - 25; http://dx.doi.org/10.1084/jem.175.1.217; PMID: 1346155
- Arias-Romero LE, Villamar-Cruz O, Pacheco A, Kosoff R, Huang M, Muthuswamy SK, et al. A Rac-Pak signaling pathway is essential for ErbB2-mediated transformation of human breast epithelial cancer cells. Oncogene 2010; 29:5839 - 49; http://dx.doi.org/10.1038/onc.2010.318; PMID: 20711231
- Pickl M, Ries CH. Comparison of 3D and 2D tumor models reveals enhanced HER2 activation in 3D associated with an increased response to trastuzumab. Oncogene 2009; 28:461 - 8; http://dx.doi.org/10.1038/onc.2008.394; PMID: 18978815
- Li Q, Mullins SR, Sloane BF, Mattingly RR. p21-Activated kinase 1 coordinates aberrant cell survival and pericellular proteolysis in a three-dimensional culture model for premalignant progression of human breast cancer. Neoplasia 2008; 10:314 - 29; PMID: 18392133
- Vadlamudi RK, Bagheri-Yarmand R, Yang Z, Balasenthil S, Nguyen D, Sahin AA, et al. Dynein light chain 1, a p21-activated kinase 1-interacting substrate, promotes cancerous phenotypes. Cancer Cell 2004; 5:575 - 85; http://dx.doi.org/10.1016/j.ccr.2004.05.022; PMID: 15193260
- Mazumdar A, Kumar R. Estrogen regulation of Pak1 and FKHR pathways in breast cancer cells. FEBS Lett 2003; 535:6 - 10; http://dx.doi.org/10.1016/S0014-5793(02)03846-2; PMID: 12560069
- Vadlamudi RK, Li F, Adam L, Nguyen D, Ohta Y, Stossel TP, et al. Filamin is essential in actin cytoskeletal assembly mediated by p21-activated kinase 1. Nat Cell Biol 2002; 4:681 - 90; http://dx.doi.org/10.1038/ncb838; PMID: 12198493
- Nola S, Sebbagh M, Marchetto S, Osmani N, Nourry C, Audebert S, et al. Scrib regulates PAK activity during the cell migration process. Hum Mol Genet 2008; 17:3552 - 65; http://dx.doi.org/10.1093/hmg/ddn248; PMID: 18716323
- Long W, Yi P, Amazit L, LaMarca HL, Ashcroft F, Kumar R, et al. SRC-3-4 mediates the interaction of EGFR with FAK to promote cell migration. Mol Cell 2010; 37:321 - 32; http://dx.doi.org/10.1016/j.molcel.2010.01.004; PMID: 20159552
- Bostner J, Skoog L, Fornander T, Nordenskjöld B, Stål O. Estrogen receptor-alpha phosphorylation at serine 305, nuclear p21-activated kinase 1 expression, and response to tamoxifen in postmenopausal breast cancer. Clin Cancer Res 2010; 16:1624 - 33; http://dx.doi.org/10.1158/1078-0432.CCR-09-1733; PMID: 20179234
- Kok M, Zwart W, Holm C, Fles R, Hauptmann M, Van't Veer LJ, et al. PKA-induced phosphorylation of ERα at serine 305 and high PAK1 levels is associated with sensitivity to tamoxifen in ER-positive breast cancer. Breast Cancer Res Treat 2011; 125:1 - 12; http://dx.doi.org/10.1007/s10549-010-0798-y; PMID: 20213082
- Tang Y, Marwaha S, Rutkowski JL, Tennekoon GI, Phillips PC, Field J. A role for Pak protein kinases in Schwann cell transformation. Proc Natl Acad Sci U S A 1998; 95:5139 - 44; http://dx.doi.org/10.1073/pnas.95.9.5139; PMID: 9560242
- Surace EI, Haipek CA, Gutmann DH. Effect of merlin phosphorylation on neurofibromatosis 2 (NF2) gene function. Oncogene 2004; 23:580 - 7; http://dx.doi.org/10.1038/sj.onc.1207142; PMID: 14724586
- Kissil JL, Johnson KC, Eckman MS, Jacks T. Merlin phosphorylation by p21-activated kinase 2 and effects of phosphorylation on merlin localization. J Biol Chem 2002; 277:10394 - 9; http://dx.doi.org/10.1074/jbc.M200083200; PMID: 11782491
- Xiao G-H, Beeser A, Chernoff J, Testa JR. p21-activated kinase links Rac/Cdc42 signaling to merlin. J Biol Chem 2002; 277:883 - 6; http://dx.doi.org/10.1074/jbc.C100553200; PMID: 11719502
- Alfthan K, Heiska L, Grönholm M, Renkema GH, Carpeán O. Cyclic AMP-dependent protein kinase phosphorylates merlin at serine 518 independently of p21-activated kinase and promotes merlin-ezrin heterodimerization. J Biol Chem 2004; 279:18559 - 66; http://dx.doi.org/10.1074/jbc.M313916200; PMID: 14981079
- Hirokawa Y, Tikoo A, Huynh J, Utermark T, Hanemann CO, Giovannini M, et al. A clue to the therapy of neurofibromatosis type 2: NF2/merlin is a PAK1 inhibitor. Cancer J 2004; 10:20 - 6; http://dx.doi.org/10.1097/00130404-200401000-00006; PMID: 15000491
- Kissil JL, Wilker EW, Johnson KC, Eckman MS, Yaffe MB, Jacks T. Merlin, the product of the Nf2 tumor suppressor gene, is an inhibitor of the p21-activated kinase, Pak1. Mol Cell 2003; 12:841 - 9; http://dx.doi.org/10.1016/S1097-2765(03)00382-4; PMID: 14580336
- Pelton PD, Sherman LS, Rizvi TA, Marchionni MA, Wood P, Friedman RA, et al. Ruffling membrane, stress fiber, cell spreading and proliferation abnormalities in human Schwannoma cells. Oncogene 1998; 17:2195 - 209; http://dx.doi.org/10.1038/sj.onc.1202141; PMID: 9811451
- Shaw RJ, Paez JG, Curto M, Yaktine A, Pruitt WM, Saotome I, et al. The Nf2 tumor suppressor, merlin, functions in Rac-dependent signaling. Dev Cell 2001; 1:63 - 72; http://dx.doi.org/10.1016/S1534-5807(01)00009-0; PMID: 11703924
- Yi C, Wilker EW, Yaffe MB, Stemmer-Rachamimov A, Kissil JL. Validation of the p21-activated kinases as targets for inhibition in neurofibromatosis type 2. Cancer Res 2008; 68:7932 - 7; http://dx.doi.org/10.1158/0008-5472.CAN-08-0866; PMID: 18829550
- Chow HY, Stepanova D, Koch J, Chernoff J. p21-Activated kinases are required for transformation in a cell-based model of neurofibromatosis type 2. PLoS One 2010; 5:e13791; http://dx.doi.org/10.1371/journal.pone.0013791; PMID: 21072183
- Zhou L, Ercolano E, Ammoun S, Schmid MC, Barczyk MA, Hanemann CO. Merlin-deficient human tumors show loss of contact inhibition and activation of Wnt/β-catenin signaling linked to the PDGFR/Src and Rac/PAK pathways. Neoplasia 2011; 13:1101 - 12; PMID: 22247700
- Carter JH, Douglass LE, Deddens JA, Colligan BM, Bhatt TR, Pemberton JO, et al. Pak-1 expression increases with progression of colorectal carcinomas to metastasis. Clin Cancer Res 2004; 10:3448 - 56; http://dx.doi.org/10.1158/1078-0432.CCR-03-0210; PMID: 15161701
- Gong W, An Z, Wang Y, Pan X, Fang W, Jiang B, et al. P21-activated kinase 5 is overexpressed during colorectal cancer progression and regulates colorectal carcinoma cell adhesion and migration. Int J Cancer 2009; 125:548 - 55; http://dx.doi.org/10.1002/ijc.24428; PMID: 19415746
- Liu Y, Xiao H, Tian Y, Nekrasova T, Hao X, Lee HJ, et al. The pak4 protein kinase plays a key role in cell survival and tumorigenesis in athymic mice. Mol Cancer Res 2008; 6:1215 - 24; http://dx.doi.org/10.1158/1541-7786.MCR-08-0087; PMID: 18644984
- Zhu G, Wang Y, Huang B, Liang J, Ding Y, Xu A, et al. A Rac1/PAK1 cascade controls beta-catenin activation in colon cancer cells. Oncogene 2012; 31:1001 - 12; http://dx.doi.org/10.1038/onc.2011.294; PMID: 21822311
- Huynh N, Liu KH, Baldwin GS, He H. P21-activated kinase 1 stimulates colon cancer cell growth and migration/invasion via ERK- and AKT-dependent pathways. Biochim Biophys Acta 2010; 1803:1106 - 13; http://dx.doi.org/10.1016/j.bbamcr.2010.05.007; PMID: 20595063
- Kissil JL, Walmsley MJ, Hanlon L, Haigis KM, Bender Kim CF, Sweet-Cordero A, et al. Requirement for Rac1 in a K-ras induced lung cancer in the mouse. Cancer Res 2007; 67:8089 - 94; http://dx.doi.org/10.1158/0008-5472.CAN-07-2300; PMID: 17804720
- Hodis E, Watson IR, Kryukov GV, Arold ST, Imielinski M, Theurillat JP, et al. A landscape of driver mutations in melanoma. Cell 2012; 150:251 - 63; http://dx.doi.org/10.1016/j.cell.2012.06.024; PMID: 22817889
- Krauthammer M, Kong Y, Ha BH, Evans P, Bacchiocchi A, McCusker JP, et al. Exome sequencing identifies recurrent somatic RAC1 mutations in melanoma. Nat Genet 2012; 44:1006 - 14; http://dx.doi.org/10.1038/ng.2359; PMID: 22842228
- Porchia LM, Guerra M, Wang YC, Zhang Y, Espinosa AV, Shinohara M, et al. 2-amino-N-4-[5-(2-phenanthrenyl)-3-(trifluoromethyl)-1H-pyrazol-1-yl]-phenyl acetamide (OSU-03012), a celecoxib derivative, directly targets p21-activated kinase. Mol Pharmacol 2007; 72:1124 - 31; http://dx.doi.org/10.1124/mol.107.037556; PMID: 17673571
- Murray BW, Guo C, Piraino J, Westwick JK, Zhang C, Lamerdin J, et al. Small-molecule p21-activated kinase inhibitor PF-3758309 is a potent inhibitor of oncogenic signaling and tumor growth. Proc Natl Acad Sci U S A 2010; 107:9446 - 51; http://dx.doi.org/10.1073/pnas.0911863107; PMID: 20439741
- Frost JA, Swantek JL, Stippec S, Yin MJ, Gaynor R, Cobb MH. Stimulation of NFkappa B activity by multiple signaling pathways requires PAK1. J Biol Chem 2000; 275:19693 - 9; http://dx.doi.org/10.1074/jbc.M909860199; PMID: 10779525
- Lee SH, Jung YS, Chung JY, Oh AY, Lee SJ, Choi DH, et al. Novel tumor suppressive function of Smad4 in serum starvation-induced cell death through PAK1-PUMA pathway. Cell Death Dis 2011; 2:e235; http://dx.doi.org/10.1038/cddis.2011.116; PMID: 22130069
- Teng TS, Lin B, Manser E, Ng DCH, Cao X. Stat3 promotes directional cell migration by regulating Rac1 activity via its activator betaPIX. J Cell Sci 2009; 122:4150 - 9; http://dx.doi.org/10.1242/jcs.057109; PMID: 19861492
- Beeser A, Jaffer ZM, Hofmann C, Chernoff J. Role of group A p21-activated kinases in activation of extracellular-regulated kinase by growth factors. J Biol Chem 2005; 280:36609 - 15; http://dx.doi.org/10.1074/jbc.M502306200; PMID: 16129686
- Frost JA, Steen H, Shapiro P, Lewis T, Ahn N, Shaw PE, et al. Cross-cascade activation of ERKs and ternary complex factors by Rho family proteins. EMBO J 1997; 16:6426 - 38; http://dx.doi.org/10.1093/emboj/16.21.6426; PMID: 9351825
- Tran NH, Frost JA. Phosphorylation of Raf-1 by p21-activated kinase 1 and Src regulates Raf-1 autoinhibition. J Biol Chem 2003; 278:11221 - 6; http://dx.doi.org/10.1074/jbc.M210318200; PMID: 12551923
- King AJ, Sun H, Diaz B, Barnard D, Miao W, Bagrodia S, et al. The protein kinase Pak3 positively regulates Raf-1 activity through phosphorylation of serine 338. Nature 1998; 396:180 - 3; http://dx.doi.org/10.1038/24184; PMID: 9823899
- Jin S, Zhuo Y, Guo W, Field J. p21-activated Kinase 1 (Pak1)-dependent phosphorylation of Raf-1 regulates its mitochondrial localization, phosphorylation of BAD, and Bcl-2 association. J Biol Chem 2005; 280:24698 - 705; http://dx.doi.org/10.1074/jbc.M413374200; PMID: 15849194
- Wu X, Carr HS, Dan I, Ruvolo PP, Frost JA. p21 activated kinase 5 activates Raf-1 and targets it to mitochondria. J Cell Biochem 2008; 105:167 - 75; http://dx.doi.org/10.1002/jcb.21809; PMID: 18465753
- Cotteret S, Jaffer ZM, Beeser A, Chernoff J. p21-Activated kinase 5 (Pak5) localizes to mitochondria and inhibits apoptosis by phosphorylating BAD. Mol Cell Biol 2003; 23:5526 - 39; http://dx.doi.org/10.1128/MCB.23.16.5526-5539.2003; PMID: 12897128
- Ye DZ, Jin S, Zhuo Y, Field J. p21-Activated kinase 1 (Pak1) phosphorylates BAD directly at serine 111 in vitro and indirectly through Raf-1 at serine 112. PLoS One 2011; 6:e27637; http://dx.doi.org/10.1371/journal.pone.0027637; PMID: 22096607
- Hüser M, Luckett J, Chiloeches A, Mercer K, Iwobi M, Giblett S, et al. MEK kinase activity is not necessary for Raf-1 function. EMBO J 2001; 20:1940 - 51; http://dx.doi.org/10.1093/emboj/20.8.1940; PMID: 11296227
- Dadke D, Fryer BH, Golemis EA, Field J. Activation of p21-activated kinase 1-nuclear factor kappaB signaling by Kaposi's sarcoma-associated herpes virus G protein-coupled receptor during cellular transformation. Cancer Res 2003; 63:8837 - 47; PMID: 14695200
- Orr AW, Hahn C, Blackman BR, Schwartz MA. p21-activated kinase signaling regulates oxidant-dependent NF-kappa B activation by flow. Circ Res 2008; 103:671 - 9; http://dx.doi.org/10.1161/CIRCRESAHA.108.182097; PMID: 18669917
- Foryst-Ludwig A, Naumann M. p21-activated kinase 1 activates the nuclear factor kappa B (NF-kappa B)-inducing kinase-Ikappa B kinases NF-kappa B pathway and proinflammatory cytokines in Helicobacter pylori infection. J Biol Chem 2000; 275:39779 - 85; http://dx.doi.org/10.1074/jbc.M007617200; PMID: 11016939
- Adams LS, Teegarden D. 1,25-dihydroxycholecalciferol inhibits apoptosis in C3H10T1/2 murine fibroblast cells through activation of nuclear factor kappaB. J Nutr 2004; 134:2948 - 52; PMID: 15514257
- Fan S, Gao M, Meng Q, Laterra JJ, Symons MH, Coniglio S, et al. Role of NF-kappaB signaling in hepatocyte growth factor/scatter factor-mediated cell protection. Oncogene 2005; 24:1749 - 66; http://dx.doi.org/10.1038/sj.onc.1208327; PMID: 15688034
- Wu R, Abramson AL, Symons MH, Steinberg BM. Pak1 and Pak2 are activated in recurrent respiratory papillomas, contributing to one pathway of Rac1-mediated COX-2 expression. Int J Cancer 2010; 127:2230 - 7; http://dx.doi.org/10.1002/ijc.25226; PMID: 20131316
- Neumann M, Foryst-Ludwig A, Klar S, Schweitzer K, Naumann M. The PAK1 autoregulatory domain is required for interaction with NIK in Helicobacter pylori-induced NF-kappaB activation. Biol Chem 2006; 387:79 - 86; http://dx.doi.org/10.1515/BC.2006.011; PMID: 16497167
- Edwards DC, Sanders LC, Bokoch GM, Gill GN. Activation of LIM-kinase by Pak1 couples Rac/Cdc42 GTPase signalling to actin cytoskeletal dynamics. Nat Cell Biol 1999; 1:253 - 9; http://dx.doi.org/10.1038/12963; PMID: 10559936
- Roger L, Gadea G, Roux P. Control of cell migration: a tumour suppressor function for p53?. Biol Cell 2006; 98:141 - 52; http://dx.doi.org/10.1042/BC20050058; PMID: 16480340
- Cammarano MS, Nekrasova T, Noel B, Minden A. Pak4 induces premature senescence via a pathway requiring p16INK4/p19ARF and mitogen-activated protein kinase signaling. Mol Cell Biol 2005; 25:9532 - 42; http://dx.doi.org/10.1128/MCB.25.21.9532-9542.2005; PMID: 16227603
- Park SY, Lee JH, Ha M, Nam JW, Kim VN. miR-29 miRNAs activate p53 by targeting p85 alpha and CDC42. Nat Struct Mol Biol 2009; 16:23 - 9; http://dx.doi.org/10.1038/nsmb.1533; PMID: 19079265
- Swami S, Raghavachari N, Muller UR, Bao YP, Feldman D. Vitamin D growth inhibition of breast cancer cells: gene expression patterns assessed by cDNA microarray. Breast Cancer Res Treat 2003; 80:49 - 62; http://dx.doi.org/10.1023/A:1024487118457; PMID: 12889598
- Baldwin A, Grueneberg DA, Hellner K, Sawyer J, Grace M, Li W, et al. Kinase requirements in human cells: V. Synthetic lethal interactions between p53 and the protein kinases SGK2 and PAK3. Proc Natl Acad Sci U S A 2010; 107:12463 - 8; http://dx.doi.org/10.1073/pnas.1007462107; PMID: 20616055
- Aoki H, Yokoyama T, Fujiwara K, Tari AM, Sawaya R, Suki D, et al. Phosphorylated Pak1 level in the cytoplasm correlates with shorter survival time in patients with glioblastoma. Clin Cancer Res 2007; 13:6603 - 9; http://dx.doi.org/10.1158/1078-0432.CCR-07-0145; PMID: 18006760
- Holm C, Rayala S, Jirström K, Stål O, Kumar R, Landberg G. Association between Pak1 expression and subcellular localization and tamoxifen resistance in breast cancer patients. J Natl Cancer Inst 2006; 98:671 - 80; http://dx.doi.org/10.1093/jnci/djj185; PMID: 16705121
- Ching YP, Leong VY, Lee MF, Xu HT, Jin DY, Ng IO. P21-activated protein kinase is overexpressed in hepatocellular carcinoma and enhances cancer metastasis involving c-Jun NH2-terminal kinase activation and paxillin phosphorylation. Cancer Res 2007; 67:3601 - 8; http://dx.doi.org/10.1158/0008-5472.CAN-06-3994; PMID: 17440071
- O'Sullivan GC, Tangney M, Casey G, Ambrose M, Houston A, Barry OP. Modulation of p21-activated kinase 1 alters the behavior of renal cell carcinoma. Int J Cancer 2007; 121:1930 - 40; http://dx.doi.org/10.1002/ijc.22893; PMID: 17621631
- Mahlamäki EH, Kauraniemi P, Monni O, Wolf M, Hautaniemi S, Kallioniemi A. High-resolution genomic and expression profiling reveals 105 putative amplification target genes in pancreatic cancer. Neoplasia 2004; 6:432 - 9; http://dx.doi.org/10.1593/neo.04130; PMID: 15548351
- Ito M, Nishiyama H, Kawanishi H, Matsui S, Guilford P, Reeve A, et al. P21-activated kinase 1: a new molecular marker for intravesical recurrence after transurethral resection of bladder cancer. J Urol 2007; 178:1073 - 9; http://dx.doi.org/10.1016/j.juro.2007.05.012; PMID: 17644138
- Schraml P, Schwerdtfeger G, Burkhalter F, Raggi A, Schmidt D, Ruffalo T, et al. Combined array comparative genomic hybridization and tissue microarray analysis suggest PAK1 at 11q13.5-q14 as a critical oncogene target in ovarian carcinoma. Am J Pathol 2003; 163:985 - 92; http://dx.doi.org/10.1016/S0002-9440(10)63458-X; PMID: 12937139
- Davidson B, Shih IeM, Wang TL. Different clinical roles for p21-activated kinase-1 in primary and recurrent ovarian carcinoma. Hum Pathol 2008; 39:1630 - 6; http://dx.doi.org/10.1016/j.humpath.2008.03.009; PMID: 18656238
- Kaur R, Yuan X, Lu ML, Balk SP. Increased PAK6 expression in prostate cancer and identification of PAK6 associated proteins. Prostate 2008; 68:1510 - 6; http://dx.doi.org/10.1002/pros.20787; PMID: 18642328
- Mao X, Onadim Z, Price EA, Child F, Lillington DM, Russell-Jones R, et al. Genomic alterations in blastic natural killer/extranodal natural killer-like T cell lymphoma with cutaneous involvement. J Invest Dermatol 2003; 121:618 - 27; http://dx.doi.org/10.1046/j.1523-1747.2003.12406.x; PMID: 12925224
- Liu RX, Wang WQ, Ye L, Bi YF, Fang H, Cui B, et al. p21-activated kinase 3 is overexpressed in thymic neuroendocrine tumors (carcinoids) with ectopic ACTH syndrome and participates in cell migration. Endocrine 2010; 38:38 - 47; http://dx.doi.org/10.1007/s12020-010-9324-6; PMID: 20960100
- Dummler B, Ohshiro K, Kumar R, Field J. Pak protein kinases and their role in cancer. Cancer Metastasis Rev 2009; 28:51 - 63; http://dx.doi.org/10.1007/s10555-008-9168-1; PMID: 19165420
- Kumar R, Gururaj AE, Barnes CJ. p21-activated kinases in cancer. Nat Rev Cancer 2006; 6:459 - 71; http://dx.doi.org/10.1038/nrc1892; PMID: 16723992
- Shin EY, Shin KS, Lee CS, Woo KN, Quan SH, Soung NK, et al. Phosphorylation of p85 beta PIX, a Rac/Cdc42-specific guanine nucleotide exchange factor, via the Ras/ERK/PAK2 pathway is required for basic fibroblast growth factor-induced neurite outgrowth. J Biol Chem 2002; 277:44417 - 30; http://dx.doi.org/10.1074/jbc.M203754200; PMID: 12226077
- Foster DB, Shen LH, Kelly J, Thibault P, Van Eyk JE, Mak AS. Phosphorylation of caldesmon by p21-activated kinase. Implications for the Ca(2+) sensitivity of smooth muscle contraction. J Biol Chem 2000; 275:1959 - 65; http://dx.doi.org/10.1074/jbc.275.3.1959; PMID: 10636898
- McFawn PK, Shen L, Vincent SG, Mak A, Van Eyk JE, Fisher JT. Calcium-independent contraction and sensitization of airway smooth muscle by p21-activated protein kinase. Am J Physiol Lung Cell Mol Physiol 2003; 284:L863 - 70; PMID: 12513968
- Van Eyk JE, Arrell DK, Foster DB, Strauss JD, Heinonen TY, Furmaniak-Kazmierczak E, et al. Different molecular mechanisms for Rho family GTPase-dependent, Ca2+-independent contraction of smooth muscle. J Biol Chem 1998; 273:23433 - 9; http://dx.doi.org/10.1074/jbc.273.36.23433; PMID: 9722579
- Takizawa N, Koga Y, Ikebe M. Phosphorylation of CPI17 and myosin binding subunit of type 1 protein phosphatase by p21-activated kinase. Biochem Biophys Res Commun 2002; 297:773 - 8; http://dx.doi.org/10.1016/S0006-291X(02)02302-1; PMID: 12359219
- Ohtakara K, Inada H, Goto H, Taki W, Manser E, Lim L, et al. p21-activated kinase PAK phosphorylates desmin at sites different from those for Rho-associated kinase. Biochem Biophys Res Commun 2000; 272:712 - 6; http://dx.doi.org/10.1006/bbrc.2000.2854; PMID: 10860820
- Zenke FT, Krendel M, DerMardirossian C, King CC, Bohl BP, Bokoch GM. p21-activated kinase 1 phosphorylates and regulates 14-3-3 binding to GEF-H1, a microtubule-localized Rho exchange factor. J Biol Chem 2004; 279:18392 - 400; http://dx.doi.org/10.1074/jbc.M400084200; PMID: 14970201
- Zhao ZS, Lim JP, Ng YW, Lim L, Manser E. The GIT-associated kinase PAK targets to the centrosome and regulates Aurora-A. Mol Cell 2005; 20:237 - 49; http://dx.doi.org/10.1016/j.molcel.2005.08.035; PMID: 16246726
- Dan C, Kelly A, Bernard O, Minden A. Cytoskeletal changes regulated by the PAK4 serine/threonine kinase are mediated by LIM kinase 1 and cofilin. J Biol Chem 2001; 276:32115 - 21; http://dx.doi.org/10.1074/jbc.M100871200; PMID: 11413130
- Sanders LC, Matsumura F, Bokoch GM, de Lanerolle P. Inhibition of myosin light chain kinase by p21-activated kinase. Science 1999; 283:2083 - 5; http://dx.doi.org/10.1126/science.283.5410.2083; PMID: 10092231
- Goeckeler ZM, Masaracchia RA, Zeng Q, Chew TL, Gallagher P, Wysolmerski RB. Phosphorylation of myosin light chain kinase by p21-activated kinase PAK2. J Biol Chem 2000; 275:18366 - 74; http://dx.doi.org/10.1074/jbc.M001339200; PMID: 10748018
- Alberts AS, Qin H, Carr HS, Frost JA. PAK1 negatively regulates the activity of the Rho exchange factor NET1. J Biol Chem 2005; 280:12152 - 61; http://dx.doi.org/10.1074/jbc.M405073200; PMID: 15684429
- Daub H, Gevaert K, Vandekerckhove J, Sobel A, Hall A. Rac/Cdc42 and p65PAK regulate the microtubule-destabilizing protein stathmin through phosphorylation at serine 16. J Biol Chem 2001; 276:1677 - 80; http://dx.doi.org/10.1074/jbc.C000635200; PMID: 11058583
- Vadlamudi RK, Li F, Barnes CJ, Bagheri-Yarmand R, Kumar R. p41-Arc subunit of human Arp2/3 complex is a p21-activated kinase-1-interacting substrate. EMBO Rep 2004; 5:154 - 60; http://dx.doi.org/10.1038/sj.embor.7400079; PMID: 14749719
- DerMardirossian C, Schnelzer A, Bokoch GM. Phosphorylation of RhoGDI by Pak1 mediates dissociation of Rac GTPase. Mol Cell 2004; 15:117 - 27; http://dx.doi.org/10.1016/j.molcel.2004.05.019; PMID: 15225553
- Chew TL, Masaracchia RA, Goeckeler ZM, Wysolmerski RB. Phosphorylation of non-muscle myosin II regulatory light chain by p21-activated kinase (gamma-PAK). J Muscle Res Cell Motil 1998; 19:839 - 54; http://dx.doi.org/10.1023/A:1005417926585; PMID: 10047984
- Ramos E, Wysolmerski RB, Masaracchia RA. Myosin phosphorylation by human cdc42-dependent S6/H4 kinase/gammaPAK from placenta and lymphoid cells. Recept Signal Transduct 1997; 7:99 - 110; PMID: 9392438
- Vadlamudi RK, Barnes CJ, Rayala S, Li F, Balasenthil S, Marcus S, et al. p21-activated kinase 1 regulates microtubule dynamics by phosphorylating tubulin cofactor B. Mol Cell Biol 2005; 25:3726 - 36; http://dx.doi.org/10.1128/MCB.25.9.3726-3736.2005; PMID: 15831477
- Goto H, Tanabe K, Manser E, Lim L, Yasui Y, Inagaki M. Phosphorylation and reorganization of vimentin by p21-activated kinase (PAK). Genes Cells 2002; 7:91 - 7; http://dx.doi.org/10.1046/j.1356-9597.2001.00504.x; PMID: 11895474
- Li QF, Spinelli AM, Wang R, Anfinogenova Y, Singer HA, Tang DD. Critical role of vimentin phosphorylation at Ser-56 by p21-activated kinase in vimentin cytoskeleton signaling. J Biol Chem 2006; 281:34716 - 24; http://dx.doi.org/10.1074/jbc.M607715200; PMID: 16990256
- Tang DD, Bai Y, Gunst SJ. Silencing of p21-activated kinase attenuates vimentin phosphorylation on Ser-56 and reorientation of the vimentin network during stimulation of smooth muscle cells by 5-hydroxytryptamine. Biochem J 2005; 388:773 - 83; http://dx.doi.org/10.1042/BJ20050065; PMID: 15766329
- Wang R, Li QF, Anfinogenova Y, Tang DD. Dissociation of Crk-associated substrate from the vimentin network is regulated by p21-activated kinase on ACh activation of airway smooth muscle. Am J Physiol Lung Cell Mol Physiol 2007; 292:L240 - 8; http://dx.doi.org/10.1152/ajplung.00199.2006; PMID: 16997882
- Chan W, Kozma R, Yasui Y, Inagaki M, Leung T, Manser E, et al. Vimentin intermediate filament reorganization by Cdc42: involvement of PAK and p70 S6 kinase. Eur J Cell Biol 2002; 81:692 - 701; http://dx.doi.org/10.1078/0171-9335-00281; PMID: 12553669
- Jung JH, Pendergast AM, Zipfel PA, Traugh JA. Phosphorylation of c-Abl by protein kinase Pak2 regulates differential binding of ABI2 and CRK. Biochemistry 2008; 47:1094 - 104; http://dx.doi.org/10.1021/bi701533j; PMID: 18161990
- Roig J, Tuazon PT, Zipfel PA, Pendergast AM, Traugh JA. Functional interaction between c-Abl and the p21-activated protein kinase γ-PAK. Proc Natl Acad Sci U S A 2000; 97:14346 - 51; http://dx.doi.org/10.1073/pnas.97.26.14346; PMID: 11121037
- Tran NH, Wu X, Frost JA. B-Raf and Raf-1 are regulated by distinct autoregulatory mechanisms. J Biol Chem 2005; 280:16244 - 53; http://dx.doi.org/10.1074/jbc.M501185200; PMID: 15710605
- Huang Z, Traugh JA, Bishop JM. Negative control of the Myc protein by the stress-responsive kinase Pak2. Mol Cell Biol 2004; 24:1582 - 94; http://dx.doi.org/10.1128/MCB.24.4.1582-1594.2004; PMID: 14749374
- Edin ML, Juliano RL. Raf-1 serine 338 phosphorylation plays a key role in adhesion-dependent activation of extracellular signal-regulated kinase by epidermal growth factor. Mol Cell Biol 2005; 25:4466 - 75; http://dx.doi.org/10.1128/MCB.25.11.4466-4475.2005; PMID: 15899852
- Chaudhary A, King WG, Mattaliano MD, Frost JA, Diaz B, Morrison DK, et al. Phosphatidylinositol 3-kinase regulates Raf1 through Pak phosphorylation of serine 338. Curr Biol 2000; 10:551 - 4; http://dx.doi.org/10.1016/S0960-9822(00)00475-9; PMID: 10801448
- Zang M, Hayne C, Luo Z. Interaction between active Pak1 and Raf-1 is necessary for phosphorylation and activation of Raf-1. J Biol Chem 2002; 277:4395 - 405; http://dx.doi.org/10.1074/jbc.M110000200; PMID: 11733498
- De la Mota-Peynado A, Chernoff J, Beeser A. Identification of the atypical MAPK Erk3 as a novel substrate for p21-activated kinase (Pak) activity. J Biol Chem 2011; 286:13603 - 11; http://dx.doi.org/10.1074/jbc.M110.181743; PMID: 21317288
- Li F, Adam L, Vadlamudi RK, Zhou H, Sen S, Chernoff J, et al. p21-activated kinase 1 interacts with and phosphorylates histone H3 in breast cancer cells. EMBO Rep 2002; 3:767 - 73; http://dx.doi.org/10.1093/embo-reports/kvf157; PMID: 12151336
- Frost JA, Xu S, Hutchison MR, Marcus S, Cobb MH. Actions of Rho family small G proteins and p21-activated protein kinases on mitogen-activated protein kinase family members. Mol Cell Biol 1996; 16:3707 - 13; PMID: 8668187
- Slack-Davis JK, Eblen ST, Zecevic M, Boerner SA, Tarcsafalvi A, Diaz HB, et al. PAK1 phosphorylation of MEK1 regulates fibronectin-stimulated MAPK activation. J Cell Biol 2003; 162:281 - 91; http://dx.doi.org/10.1083/jcb.200212141; PMID: 12876277
- Eblen ST, Slack-Davis JK, Tarcsafalvi A, Parsons JT, Weber MJ, Catling AD. Mitogen-activated protein kinase feedback phosphorylation regulates MEK1 complex formation and activation during cellular adhesion. Mol Cell Biol 2004; 24:2308 - 17; http://dx.doi.org/10.1128/MCB.24.6.2308-2317.2004; PMID: 14993270
- Coles LC, Shaw PE. PAK1 primes MEK1 for phosphorylation by Raf-1 kinase during cross-cascade activation of the ERK pathway. Oncogene 2002; 21:2236 - 44; http://dx.doi.org/10.1038/sj.onc.1205302; PMID: 11948406
- Gallagher ED, Xu S, Moomaw C, Slaughter CA, Cobb MH. Binding of JNK/SAPK to MEKK1 is regulated by phosphorylation. J Biol Chem 2002; 277:45785 - 92; http://dx.doi.org/10.1074/jbc.M207702200; PMID: 12228228
- Orton KC, Ling J, Waskiewicz AJ, Cooper JA, Merrick WC, Korneeva NL, et al. Phosphorylation of Mnk1 by caspase-activated Pak2/gamma-PAK inhibits phosphorylation and interaction of eIF4G with Mnk. J Biol Chem 2004; 279:38649 - 57; http://dx.doi.org/10.1074/jbc.M407337200; PMID: 15234964
- Maroto B, Ye MB, von Lohneysen K, Schnelzer A, Knaus UG. P21-activated kinase is required for mitotic progression and regulates Plk1. Oncogene 2008; 27:4900 - 8; http://dx.doi.org/10.1038/onc.2008.131; PMID: 18427546
- Tuazon PT, Lorenson MY, Walker AM, Traugh JA. p21-activated protein kinase gamma-PAK in pituitary secretory granules phosphorylates prolactin. FEBS Lett 2002; 515:84 - 8; http://dx.doi.org/10.1016/S0014-5793(02)02444-4; PMID: 11943200
- Jakobi R, Moertl E, Koeppel MA. p21-activated protein kinase γ-PAK suppresses programmed cell death of BALB3T3 fibroblasts. J Biol Chem 2001; 276:16624 - 34; http://dx.doi.org/10.1074/jbc.M007753200; PMID: 11278362
- Tang Y, Zhou H, Chen A, Pittman RN, Field J. The Akt proto-oncogene links Ras to Pak and cell survival signals. J Biol Chem 2000; 275:9106 - 9; http://dx.doi.org/10.1074/jbc.275.13.9106; PMID: 10734042
- Schürmann A, Mooney AF, Sanders LC, Sells MA, Wang H-G, Reed J-C, et al. p21-activated kinase 1 phosphorylates the death agonist bad and protects cells from apoptosis. Mol Cell Biol 2000; 20:453 - 61; http://dx.doi.org/10.1128/MCB.20.2.453-461.2000; PMID: 10611223
- Liberali P, Kakkonen E, Turacchio G, Valente C, Spaar A, Perinetti G, et al. The closure of Pak1-dependent macropinosomes requires the phosphorylation of CtBP1/BARS. EMBO J 2008; 27:970 - 81; http://dx.doi.org/10.1038/emboj.2008.59; PMID: 18354494
- Manavathi B, Rayala SK, Kumar R. Phosphorylation-dependent regulation of stability and transforming potential of ETS transcriptional factor ESE-1 by p21-activated kinase 1. J Biol Chem 2007; 282:19820 - 30; http://dx.doi.org/10.1074/jbc.M702309200; PMID: 17491012
- Wang J, Frost JA, Cobb MH, Ross EM. Reciprocal signaling between heterotrimeric G proteins and the p21-stimulated protein kinase. J Biol Chem 1999; 274:31641 - 7; http://dx.doi.org/10.1074/jbc.274.44.31641; PMID: 10531372
- Knaus UG, Morris S, Dong HJ, Chernoff J, Bokoch GM. Regulation of human leukocyte p21-activated kinases through G protein--coupled receptors. Science 1995; 269:221 - 3; http://dx.doi.org/10.1126/science.7618083; PMID: 7618083
- Martyn KD, Kim MJ, Quinn MT, Dinauer MC, Knaus UG. p21-activated kinase (Pak) regulates NADPH oxidase activation in human neutrophils. Blood 2005; 106:3962 - 9; http://dx.doi.org/10.1182/blood-2005-03-0859; PMID: 16099876
- Ahmed S, Prigmore E, Govind S, Veryard C, Kozma R, Wientjes FB, et al. Cryptic Rac-binding and p21(Cdc42Hs/Rac)-activated kinase phosphorylation sites of NADPH oxidase component p67(phox). J Biol Chem 1998; 273:15693 - 701; http://dx.doi.org/10.1074/jbc.273.25.15693; PMID: 9624165
- Vadlamudi RK, Manavathi B, Singh RR, Nguyen D, Li F, Kumar R. An essential role of Pak1 phosphorylation of SHARP in Notch signaling. Oncogene 2005; 24:4591 - 6; http://dx.doi.org/10.1038/sj.onc.1208672; PMID: 15824732
- Wang RA, Vadlamudi RK, Bagheri-Yarmand R, Beuvink I, Hynes NE, Kumar R. Essential functions of p21-activated kinase 1 in morphogenesis and differentiation of mammary glands. J Cell Biol 2003; 161:583 - 92; http://dx.doi.org/10.1083/jcb.200212066; PMID: 12732616
- Miah SM, Sada K, Tuazon PT, Ling J, Maeno K, Kyo S, et al. Activation of Syk protein tyrosine kinase in response to osmotic stress requires interaction with p21-activated protein kinase Pak2/gamma-PAK. Mol Cell Biol 2004; 24:71 - 83; http://dx.doi.org/10.1128/MCB.24.1.71-83.2004; PMID: 14673144
- Sakurada K, Kato H, Nagumo H, Hiraoka H, Furuya K, Ikuhara T, et al. Synapsin I is phosphorylated at Ser603 by p21-activated kinases (PAKs) in vitro and in PC12 cells stimulated with bradykinin. J Biol Chem 2002; 277:45473 - 9; http://dx.doi.org/10.1074/jbc.M206673200; PMID: 12237306
- Buscemi N, Foster DB, Neverova I, Van Eyk JE. p21-activated kinase increases the calcium sensitivity of rat triton-skinned cardiac muscle fiber bundles via a mechanism potentially involving novel phosphorylation of troponin I. Circ Res 2002; 91:509 - 16; http://dx.doi.org/10.1161/01.RES.0000035246.27856.53; PMID: 12242269
- Chong C, Tan L, Lim L, Manser E. The mechanism of PAK activation. Autophosphorylation events in both regulatory and kinase domains control activity. J Biol Chem 2001; 276:17347 - 53; http://dx.doi.org/10.1074/jbc.M009316200; PMID: 11278486
- Gatti A, Huang Z, Tuazon PT, Traugh JA. Multisite autophosphorylation of p21-activated protein kinase γ-PAK as a function of activation. J Biol Chem 1999; 274:8022 - 8; http://dx.doi.org/10.1074/jbc.274.12.8022; PMID: 10075701