Abstract
Oral isotretinoin (13-cis retinoic acid) is the most effective drug in the treatment of acne and restores all major pathogenetic factors of acne vulgaris. Isotretinoin is regarded as a prodrug which after isomerizisation to all-trans-retinoic acid (ATRA) induces apoptosis in cells cultured from human sebaceous glands, meibomian glands, neuroblastoma cells, hypothalamic cells, hippocampus cells, Dalton´s lymphoma ascites cells, B16F-10 melanoma cells, and neuronal crest cells and others. By means of translational research this paper provides substantial indirect evidence for isotretinoin´s mode of action by upregulation of forkhead box class O (FoxO) transcription factors. FoxOs play a pivotal role in the regulation of androgen receptor transactivation, insulin/insulin like growth factor-1 (IGF-1)-signaling, peroxisome proliferator-activated receptor-γ (PPARγ)- and liver X receptor-α (LXRα)-mediated lipogenesis, β-catenin signaling, cell proliferation, apoptosis, reactive oxygene homeostasis, innate and acquired immunity, stem cell homeostasis, as well as anti-cancer effects. An accumulating body of evidence suggests that the therapeutic, adverse, teratogenic and chemopreventive effecs of isotretinoin are all mediated by upregulation of FoxO-mediated gene transcription. These FoxO-driven transcriptional changes of the second response of retinoic acid receptor (RAR)-mediated signaling counterbalance gene expression of acne due to increased growth factor signaling with downregulated nuclear FoxO proteins. The proposed isotretinoin→ATRA→RAR→FoxO interaction offers intriguing new insights into the mode of isotretinoin action and explains most therapeutic, adverse and teratogenic effects of isotretinoin in the treatment of acne by a common mode of FoxO-mediated transcriptional regulation.
Introduction
With the observation of Peck et al. in 1979 that isotretinoin (13-cis-retinoic acid) produced marked clearing in patients with nodulocystic acne, a new era in acne treatment began.Citation1 Since its approval by the US FDA in 1982, isotretinoin has been considered a breakthrough treatment against severe nodulocystic acne.Citation2,Citation3 Isotretinoin is the most potent known inhibitor of sebum production. Multiple modes of action of isotretinoin, including suppression of sebaceous gland activity, normalization of the pattern of keratinization within the sebaceous gland follicle, inhibition of inflammation, reduction of growth of Propionibacterium acnes and normalization of the expression of tissue matrix metalloproteinases make isotretinoin the single most effective drug in the treatment of acne. Isotretinoin not only affects the sebaceous follicle but exerts adverse effects on various tissues in the body.Citation4 Isotretinoin undergoes significant and selective all-trans-isomerization to all-trans-retinoic acid (ATRA) in cultured sebocytes.Citation5 Isotretinoin has been considered as a prodrug mediating its activity through isomerization to ATRA.Citation5–Citation7 Despite its multiple actions on proliferation, metabolism, reactive oxygen homeostasis, inflammation, matrix remodeling and sebum suppression, the underlying mode of action and especially its unique sebostatic activity has not been unraveled despite more than 30 years of clinical use. The effectiveness of isotretinoin on all major pathogenetic aspects of acne implies that there is however a fundamental mechanism of action at the regulatory level of gene transcription which cannot be explained by primary transcriptional responses of ATRA to retinoic acid receptor (RAR).Citation2
Binding of ATRA initiates changes in interactions of RARs/retinoid X receptors (RXRs) with corepressor and coactivator proteins, activating transcription of primary target genes. Importantly, ATRA/RAR-signaling induces secondary responses in gene expression encoding transcription factors and signaling proteins that further augment a whole cascade of gene expression.Citation8 These transcription factors of the secondary response, especially FoxO proteins, then transcriptionally activate their target genes to generate the whole spectrum of retinoid-mediated transcriptional regulation. It has been speculated that the addition of ATRA leads to major intra- and interchromosomal transcription “interactomes” so that active ATRA-coregulated genes and their regulatory factors cooperate to generate specialized nuclear areas for coordinated transcriptional control.Citation8 These secondary responses and the full orchestration of transcription factors and coregulators of the second response to ATRA are less well characterized but appear to be crucial for isotretinoin's mode of action. It has recently been recognized that ATRA increased the expression of transcription factor FoxO3a in neuroblastoma cells.Citation9 FoxO3a has also been identified as a key regulator for ATRA-induced granulocytic differentiation and apoptosis in acute promyelocytic leukemia.Citation10 Treating acute promyelocytic leukemia cells with ATRA, FoxO3a phosphorylation was reduced and FoxO3a trans-located into the nucleus. Intriguingly, FoxO3a is a strong inducer of the transcription factor FoxO1.Citation11 FoxO1 expression is stimulated by activated FoxO3a at the promoter of FoxO1 in a positive feed back loop.Citation11 The transcription of FoxO genes is stimulated by FoxO3 and repressed by growth factors like insulin and insulin-like growth factor-1 (IGF-1) which are increased in puberty and acne-associated syndromes with insulin resistance.Citation11,Citation12 Thus, there is evidence from translational research for a relationship between retinoid signaling and FoxO-mediated gene regulation. This relationship and the fact, that a multitude of cellular events in acne pathophysiology, isotretinon action and isotretinoin-induced adverse effects can be related to FoxO regulation resulted in the formulation of a recent hypothesis for the role of FoxO1 in acne pathogenesis and isotretinoin's mode of action.Citation13
Indirect evidence will be provided in this paper which strongly suggests that acne may be explained by a growth factor-induced nuclear deficiency of FoxO1, whereas isotretinoin increases nuclear FoxO1 levels and thus reverses acne-related imbalances of FoxO homeostasis.Citation13 In fact, all adverse and teratogenic effects of isotretinoin can be explained by FoxO-mediated proapoptotic signaling. To understand the pluripotent and multifunctional roles of FoxO transcription factors, a brief introduction in the extending network of FoxO transcription factors is helpful.
FoxO-Transcription Factors
Forkhead box O (FoxO) transcription factors FoxO1, FoxO3a, FoxO4 and FoxO6 are important regulatory proteins that modulate the expression of genes involved in cell cycle control, DNA damage repair, apoptosis, oxidative stress, cell differentiation, glucose metabolism, inflammation, immune functions and regulation of stem cell homeostasis.Citation14–Citation19 FoxO1 represents the predominant FoxO isoform. FoxO1 and FoxO3a are proteins with a length of about 650 amino acids. FoxOs contain a conserved DNA binding domain and either activate or inhibit the transcription of target genes containing a consensus DNA binding sequence TTG TTT AC.Citation14,Citation19 Furthermore, FoxO proteins can interact with several other transcription factors like androgen receptor (AR) or β-catenin, therby modifying gene regulation. Central to the regulation of FoxO transcription factors is a shuttling system, which confines FoxO factors to either the nucleus or the cytosol ().Citation14,Citation15 Among other involved and less important kinases, shuttling of FoxOs requires protein phosphorylation of nuclear FoxOs by phosphoinositol-3-kinase (PI3K)-mediated activation of the serine/threonine kinase Akt (also known as protein kinase B, PKB).Citation11–Citation14 Activated (phosphorylated) Akt translocates into the nucleus for FoxO phosphorylation. Phosphorylated FoxOs leave the nucleus, thereby changing gene regulation (). Dysregulation of FoxO1 and its nuclear export by insulin, IGF-1, fibroblast growth factors (FGFs) or other growth factors modifying the activation of PI3K/Akt affect the transcriptional activity of key target genes and nuclear receptors involved in acne pathogenesis. Increased growth factor signaling is an endocrinological hallmark of puberty as well as insulinotropic western nutrition with increased consumption of milk and other insulinotropic dairy products and carbohydrates with high glycemic index.Citation20–Citation23
Isotretinoin, FoxO1 and Suppression of Androgen Receptor Transactivation
Androgen receptor (AR)-mediated signal transduction plays an essential role for the stimulation of the size of sebocytes and sebum production as well as keratinocyte proliferation in the ductus seboglandularis and the acroinfundibulum. ARs are expressed in basal and differentiating sebocytes and pilosebaceous duct keratinocytes.Citation24–Citation25 Androgens induce the expression of sterol regulatory element binding protein (SREBP), the most important transcription factor of lipogenesis.Citation26 Androgen-insensitive subjects who lack functional ARs do not produce sebum and do not develop acne.Citation27 Increased AR protein levels have been determined in skin of acne patients.Citation28
AR is a modular protein organized into functional domains, consisting of an N-terminal transcription activation domain (TAD), a DNA-binding domain and a C-terminal ligand-binding domain for androgens.Citation29 Ligand-activated ARs induce the transcription of androgen-responsive target genes. The TAD of AR mediates the majority of AR transcriptional activity and provides the most active coregulator interaction surface.Citation30 The AR integrates a multitude of regulatory signals and its final transcriptional activity is integrated by the action of more than 150 known coregulators which are either coactivators or corepressors.Citation31 FoxO1 is an important metabolically regulated AR corepressor and binds to the TAD, where it disrupts p160 coactivator binding and suppresses N-terminal/C-terminal-interaction, which is most important for AR transcriptional activity ().Citation32 The AR repressive function of FoxO1 is attenuated by increased growth factor signaling with activation of the PI3K/Akt cascade.Citation33,Citation34 On the other hand, the expression of several growth factors like IGF-1 and regulatory proteins of cell cycle control and lipogenesis are dependent on androgen signal transduction,Citation35 pointing to the hierarchical control of AR-mediated gene expression for downstream AR-dependent growth factor signaling. Nuclear FoxO1 extrusion by increased growth factor signaling and upregulation of AR transcriptional activity will thus augment the expression of a substantial set of AR-responsive target genes involved in acne pathogenesis. FoxO1 regulation of AR activity at the genomic level is the connecting piece explaining the functional interaction of insulin/IGF-1 and androgens in the pathogenesis of acne.
Moreover, like an amplification loop, AR receptor signaling increased IGF-1-expression and IGF-1/IGF-1 receptor (IGF1R)-signaling in the ventral prostate gland.Citation35 Oral isotretinoin treatment has recently been shown to decrease serum IGF-1 levels,Citation36 which may decrease AR-mediated gene expression. Furthermore, decreased AR protein levels have been observed in skin of male acne patients after oral isotretinoin treatment.Citation37 These data imply that isotretinoin treatment may downregulate the transcriptional activity of AR by increasing the nuclear concentration of the AR cosuppressor FoxO1. Furthermore, the isotretinoin-induced decrease of IGF-1 serum levels may impair IGF-1/PI3K/Akt-mediated nuclear export of FoxO1. Moreover, IGF-1 is regarded as an androgen-dependent stimulator of 5α-reductase activity.Citation38 In fact, experimental evidence has been provided for decreased androgen 5α-reduction in skin and liver of men with severe acne after oral isotretnoin treatment.Citation39 The isotretinoin-induced decrease of IGF-1 may reduce the conversion of less potent testosterone to the more potent dihydrotestosterone (DHT), thereby decreasing the activity status of the AR ligand binding domain, which binds DHT 10 times stronger than testosterone. Free bioactive IGF-1 is controlled by IGF binding proteins (IGFBPs). In human dermal papilla cells, ATRA induced a significant increase of IGFBP-3,Citation40 which reduced the bioavailability of free IGF-1 for IGF-1/IGF1R-signaling with potential impact on nuclear FoxO1 import. Thus, at least four mechanisms of isotretinoin treatment may explain reduced AR transcriptional activity affecting both the FoxO1 regulated N-terminal TAD and the androgen-regulated C-terminal AR ligand binding domain ().
Androgen Receptor CAG Repeat Polymorphism and Acne Relapse after Isotretinoin
The high prevalence of acne (>80%) in adolescents of industrialized countries with western life style as well as the increasing persistence of acne into adulthood clearly points to the predominace of environmental and nutrional factors in acne.Citation41,Citation42 However, there is also clear evidence for a genetic disposition for acne from various twin studies. Sebum excretion exhibited higher correlations in monozygotic vs. dizygotic twins.Citation43 The proportion of branched fatty acids in the fraction of sebaceous wax esters highly correlated in monozygotic compared with dizygotic twins.Citation44 Apolipoprotein A1 serum levels were significantly lower in acne twins and a family history of acne was also significantly associated with an increased risk of developing acne.Citation45 A clinical study evaluating the role of heredity confirmed the importance of heredity as a prognostic factor for the development of acne and showed that a family history of acne is associated with earlier occurrence of the disease, increased number of retentional lesions and therapeutic difficulties.Citation46 Especially, the risk for a relapse after oral isotretinoin treatment was significantly higher in the population of patients with a positive family history of acne.Citation46
AR polymorphism with shortened CAG repeats (<20) encoding the polyglutamine tract of the N-terminal TAD domain of the AR has been associated with increased genetic disopistion for acne and other androgen-driven diseases like hirsutism and androgenetic alopecia.Citation47–Citation49 On the other hand, AR polymorphism with extended CAG repeats results in androgen insensitivity as observed in Kenendy syndrome.Citation27,Citation50 The N-terminal TAD domain of the AR is the interacting site for AR corregulators,Citation29,Citation31 which modify N-terminal-C-terminal interaction of the AR protein most important in the regulation of AR transcriptional activity.Citation29 Intriguingly, FoxO1 binds to the N-terminal domain of AR and inhibits N-terminal/C-terminal interaction of the AR.Citation32 It is conceivable that a shorter polyglutamine tract of the AR (CAG repeats <20) decreases the ability and affinity for FoxO1 binding, thus increasing coactivator binding and raising the basal state of AR transcriptional activity. Impaired FoxO1 binding to ARs with TAD with shortened polyglutamine tracts could thus explain the increased susceptibility for acne of individuals with AR polymorphisms with shortened CAG repeats (<20) in comparsion with individuals with normal (>20) or extended CAG repeats (>30). In acne patients with shortened CAG repeats, isotretinoin-induced upregulation of nuclear FoxO1 would thus have less inhibitory effects on AR transcriptional activity. Impaired FoxO1-AR-TAD interactions may explain the necessity for higher isotretinoin doses to reach therapeutical effects. Taken together, dimished FoxO1 interaction with ARs with shortened polyglutamine tracts (CAG repeats <20) may explain increased relapse rates of isotretinoin treatment in patients with a high genetic disposition for acne due to AR polymorphism with reduced CAG repeat numbers.
The Potential Role of FoxO1 for Isotretinoin's Sebum Suppressive Effect
Isotretinoin is the strongest known sebum suppressive drug for the treatment of acne. The sebaceous gland is actively involved in lipid metabolism. Isotretinoin is the most effective retinoid in reducing sebaceous gland size (up to 90%), by decreasing proliferation, disturbing the differentiation of basal sebocytes and suppressing sebum production in vivo.Citation51 During isotretinoin treatment a marked decrease of wax esters, a limited decrease of squalene and a relative increase of cholesterol concentration has been detected in skin surface lipids.Citation52 Oral isotretinoin was also shown to decrease glyceride fraction, whereas the relative composition of free sterols and total ceramides were increased in comedonal lipids.Citation53 Isotretinoin exerts pronounced, direct inhibitory effects on proliferation, lipid synthesis and differentiation of human sebocytes in vitro.Citation54 Inhibition of sebocyte proliferation and lipid synthesis were found to be independent mechanisms of isotretinoin activity.Citation55 Eight weeks of isotretinoin treatment downregulated numerous genes encoding lipid-metabolizing enzymes involved in the synthesis of cholesterol, steroids and fatty acids and increased the expression of genes encoding extracellular matrix proteins like collagen and fibrobectin.Citation56,Citation57 To understand the sebum suppressive effect of isotretinoin, the inbibtory effects of isotretinoin (1) on sebocyte lipid synthesis, (2) the inhibition of sebocyte proliferation, (3) isotretinoin's effect on sebocyte apoptosis and (4) isotretinoin's effects on sebocyte stem cell homeostasis have to be elucidated. Evidence from translational research points to the involvement of the FoxO transcription factors FoxO1 and and FoxO3a in all four aspects of isotretinoin action.
Isotretinoin, FoxO1 and Inhibition of Lipid Metabolism
The sebaceous gland belongs to the type of glands and organs with most active lipid biosynthesis. FoxO transcription factors play a critical role in metabolism and especially in lipid metabolism.Citation19,Citation58 FoxOs have been implicated in regulating cellular proliferation, stress resistance, apoptosis and longevity. Through the insulin receptor substrate/PI3K/Akt signal cascade, FoxO1 integrates insulin action with the systemic nutrient and energy homeostasis. FoxOs are expressed ubiquitously in mammalian tissues, especially adipose, brain, heart, liver, lung, ovary, pancreas, prostate, skin, skeletal muscle, spleen, thymus and testis.Citation19 Recently, FoxO1 protein has been detected in human sebaceous glands by immune histochemistry (Liakou A, Zouboulis CC, personal communication).
FoxO1 and PPARγ.
In human sebocytes, testosterone alone is not able to induce the full program of sebaceous lipogenesis.Citation59,Citation60 Peroxisome proliferator-activated receptors (PPARs) and their ligands have been identified as important coregulators for sebaceous lipogenesis.Citation61 Specific agonists of each PPAR isoform (α, δ and γ) stimulate sebocyte differentiation. Fatty acids of n-3- and n-6 origin and their eicosanoid derivatives play an important role as natural PPAR ligands that modulate PPAR function. PPARγ and its natural ligand prostaglandin J2 are most important in the regulation of lipid metabolism, sebaceous gland development and function. PPARγ plays a significant role in mediating insulin sensitivity, glucose and lipid homeostasis, and is expressed on sebocytes increasing human sebum production.Citation62,Citation63 PPARγ is transrepressed by FoxO1 like AR (). FoxO1 directly binds and represses the PPARγ2 promoter as well as PPARγ function.Citation64,Citation65 It has been shown in adipocytes that growth factor signaling with reduced nuclear FoxO1 concentrations augments PPARγ activity required for terminal differentiation and prevents FoxO1-PPARγ interaction which rescues transrepression of genes involved in lipogenesis.Citation66 In fact, serum IGF-1 levels correlate with facial sebum excretion.Citation67 PPARγ heterodimerizes with RXR and binds to PPAR response elements (PPRE) in promoters of target genes. One mechanism by which FoxO1 antagonizes PPARγ activity is through disruption of DNA binding as FoxO1 inhibits the DNA binding activity of the PPARγ/RXRα heterodimeric complexes which have recently been detected in sebocytes (). Thus, growth factor signaling inhibits the transrepressive effect of FoxO1 on AR and PPARγ/RXRα heterodimers, thus amplifying the complete program of sebaceous lipogenesis.
FoxO1, LXR and SREBP1.
Liver X receptors (LXRs) like PPARs play a critical role in lipid metabolism. Expression of LXRα and LXRβ has been detected in SZ95 sebocytes and LXR ligands enhance the expression of LXRα stimulating lipid synthesis.Citation68 LXRs directly control the expression of sterol response element binding protein-1 (SREBP-1).Citation69 A LXRE motif is present in the PPARγ promoter, on which LXRα/RXRα heterodimer is bound and activated by a LXR ligand.Citation70 () In SZ95 sebocytes activation of LXRα induced lipid synthesis that was accompanied with the induction of SREBP-1 and PPARs.Citation68,Citation71 In SEB-1 sebocytes, IGF-1 induced SREBP-1 expression and increased lipogenesis via activation of the PI3K/Akt signaling pathway.Citation72 FoxO1 plays an important role in the regulation of the SREBP-1c promoter activity. In skeletal muscle, SREBP-1c expression is regulated by LXRα/RXRα heterodimer and RXRγ or RXRα, together with LXRα have been shown to activate the SREBP-1c promoter,Citation73 whereas the expression of FoxO1 negatively correlated with SREBP-1c expression (). Thus, evidence from translational research corroborates the fundamental impact of nuclear FoxO1 on the regulation and SREBP-1c expression, the key transcription factor of multiple lipogenic target genes expressed in adipocyte, hepatocyte, skeletal muscle and sebocyte. Taken together, research data from various cell types with prominent lipid synthesis exhibit the suppressive regulatory effect of nuclear FoxO1 in direct transcriptional regulation of AR and PPARγ as well as coregulation of PPARγ/RXRα and LXR/RXRα heterodimers ().
Isotretinoin, FoxO1 and the Regulation of Cell Proliferation and Apoptosis
Isotretinoin has been found to be superior to other non-aromatic retinoids, such as tretinoin and alitretinoin, in reducing sebocyte proliferation and suppressing sebum production.Citation74 This superior effect of isotretinoin has been attributed to the delayed initiation of retinoid inactivation under incubation of sebocytes with isotretinoin, a fact that leads to high intracellular ATRA concentrations. In contrast, incubation with ATRA leads to rapid enhancement of cellular retinoic acid binding protein-2 (CRABP-2) expression, which reduces the free intracellular concentration of ATRA through promotion of its metabolism by cytochrome P450 enzymes, and by induction of CYP1A1 expression, a major xenobiotic metabolizing enzyme, in cultured sebocytes.Citation5 The antiproliferative activity of retinoids on human sebocytes and rat preputial sebocyte-like cells in vitro was found to be mediated by RAR.Citation5,Citation75
Isotretinoin exerts a dose- and time-dependent antiproliferative effect on SEB-1 sebocytes and immortalized SZ95 sebocytes.Citation5,Citation55,Citation76,Citation77 A portion of this decrease was attributed to cell cycle arrest at the G1/S phase of the cell cycle, as evidenced by decreased DNA synthesis, increased p21 protein and decreased cyclin D1 protein.Citation76 Isotretinoin-induced apoptosis was not apparent within the first 24-hour treatment period.Citation78 Marginal induction of apoptosis in SEB-1 sebocytes by isotretinoin was detected after 48 and 72 hours of treatment which already points to delayed secondary responses of transcriptional regulation. The ability of isotretinoin to induce sebocyte apoptosis was not recapitulated by alitretinoin (9-cis-retinoic acid) or ATRA. The induction of cell cycle arrest and apoptosis by isotretinoin was specific to sebocytes, as the compound failed to induce apoptosis in HaCaT keratinocytes or normal human epidermal keratinocytes.Citation76 Furthermore, the RAR pan-antagonist AGN 193109 did not inhibit the apoptosis induced by isotretinoin which suggested an RAR-independent apoptotic mechanism. These observations have been interpreted in a way that, in sebocytes, isotretinoin causes inhibition of cell proliferation after intracellular metabolism to ATRA by an RAR-mediated pathway and cell cycle arrest and apoptosis by an RAR-independent mechanism, which contributes to its sebosuppressive effect. Induction of cell cycle arrest and apoptosis by isotretinoin is likely to contribute to the overall effect on suppression of sebum, but isotretinoin also inhibits sebaceous lipid synthesis by an RAR- and RXR-mediated pathway.Citation2,Citation7,Citation76
FoxOs and cell cycle arrest.
There is compelling evidence that retinoids alter the expression of FoxO transcription factors.Citation8–Citation10 It could be shown in neuroblastoma cells that ATRA induced increased expression of FoxO3a.Citation9 ATRA treatment of acute promyelocytic leukemia cells increased nuclear levels of FoxO3a which was associated with granulocytic differentiation and apoptosis.Citation10 FoxO3a is the strongest activator of the FoxO1 promoter, thus increasing the transcription of FoxO1.Citation11 Upregulation of FoxO3a correlated with the expression of FoxO target genes p27, p130 and manganese superoxide dismutase (MnSOD).Citation9 FoxO expression induces a cell cycle exit into quiescence. Increased expression of p130 protein is often associated with cell cycle exit and an entry into quiescence or senescence ().Citation79,Citation80 Intriguingly, the pattern of ATRA-activated FoxO target genes of cell cycle arrest just resembles the observed changes of cell cycle proteins in isotretinoin-treated SEB-1 sebocytes like upregulation of p21 and downregulation of cyclin D1 ().Citation76 Recent studies on isotretinoin-induced changes in gene expression and apoptosis focused primarily on the regulatory role of RAR and RXR.Citation78 However, it appears that not the primary ATRA-RAR/RXR interactions are responsible for the proapoptotic effect of isotretinoin but secondary responses due to upregulation of FoxO-transcription factors. Upregulated nuclear FoxO transcription factors are pivotal inducers of apoptosis in various cell systems.Citation8,Citation10,Citation11,Citation14,Citation15 Increased CRABP-2 expression has been detected in suprabasal sebocytes of sebaceous follicles of isotretinoin-treated acne patients.Citation81 CRABP-2 was strongly expressed in sebocytes compared to epidermis of isotretinoin-treated patients, pointing to a preferential transport of ATRA to RARs in sebocytes. Proapoptotic actitivies of ATRA are mediated predominantly by RAR and CRABP-2, its cognate intracellular lipid binding protein which delivers ATRA to RAR, whereas fatty acid binding protein 5 (FABP-5) shuttles the hormone to PPARβ/δ which exert pro-proliferative responses like those observed in keratinocytes.Citation82
The ability of ATRA to mediate proapoptotic signaling is thus cell specific and is associated with a high CRABP-2/FABP-5 ration which results in partitioning of ATRA to RAR signaling.Citation82 ATRA-induced G1/G0 growth arrest of HL-60 cells is known to require the activation of the RARα and RXR.Citation83 Interestingly, FoxO3 has been identified as a key regulator for ATRA-induced apoptosis in acute promyelocytic leukemia.Citation10 These data show that beside the sebocyte various other cell types are susceptible for isotretinoin/ATRA-induced apoptosis.Citation84
There is substantiated evidence that several transcription factors including FoxOs act downstream of ATRA.Citation8 The high correlation of gene-regulatory effects between known apoptotic mechanisms of FoxO-transcription factors and isotretinoin-induced apoptosis in SEB-1 sebocytes corroborates the suggestion that isotretinoin mediates its antiproliferative and apoptotic effects by upregulation of FoxO transcription factors, especially FoxO1 and FoxO3a ().Citation13
NGAL and IGFBP-3.
Isotretinoin treatment of acne patients significantly upregulated the expression of neutrophil gelatinase-associated lipocalin (NGAL), which has been identified as an inducer of isotretinoin-mediated sebocyte apoptosis.Citation85 However, other NGAL-independent mediators of apoptosis could not be excluded. Both isotretinoin and ATRA increased the expression of NGAL in SEB-1 sebocytes ten-fold and seven-fold, respectively.Citation85 This similar range of NGAL expression allows the conclusion that NGAL-mediated apoptosis is not a specific mechanism of isotretinoin-induced sebocyte apoptosis. Remarkably, a 3.43-fold increased expression of IGF binding protein-3 (IGFBP-3) during isotretinoin treatment was exclusively observed in sebocytes but not in whole skin.Citation85 The expression of IGFBP-3 has been shown to be retinoid responsive. For instance, IGFBP-3 is upregulated by ATRA in human dermal papilla cells.Citation40 IGFBP-3 is a peculiar IGF-1 binding protein, which translocates into the nucleus and interferes with RAR/RXR leading to changes of receptor transactivation.Citation86,Citation87 Nuclear IGFBP-3 is a potent inducer of apoptosis.Citation86 Intriguingly, IGFBP-3 is a known FoxO target gene.Citation15 In prostate cancer cells IGFBP-3 enhanced RXR response element and inhibited RARE signaling. Thus, RXRα-IGFBP-3 interaction leads to modulation of the transcriptional activity of RXRα which is essential for mediating the effects of IGFBP-3 on apoptosis.Citation86 There might be a common unifying mechanisms of NGAL- and IGFBP-3-mediated sebocyte apoptosis. The promoter region of the LCN2 gene contains consensus sequences for binding both RAR- and RXR.Citation85 FoxO-mediated upregulation of IGFBP-3 may interact with RXR on the LCN2 promoter thus activating the expression of NGAL. This proposed FoxO/IGFBP-3-mediated gene regulatory mechanism of apoptosis would perfectly fit into FoxOs' biological role as inducers of apoptosis, metabolic rest (transcription factor of starvation) and activator of innate immunity associated with increased expression of antimicrobial peptides like defensin-β1.Citation88 Both antimicrobial peptides and NGAL function as effectors of innate immunity against microbial pathogens.Citation85,Citation88 Is is thus not surprising that the expression of defensin-β1 is upregulated by FoxO as well as isotretinoin treatment.Citation85,Citation88 Isotretinoin-induced FoxO-activation of the IGFBP-3 promoter might be the underlying cause of isotretinoin-induced sebocyte apoptosis by nuclear IGFBP-3 overexpression. IGFBP-3/RXRα-mediated apoptosis as well as FoxO1-mediated downregulation of the AR transcriptional activity, PPARγ function and SREBP-1c promoter activity all together could thus contribute to the sebum-suppressive and apoptotic effect of isotretinoin treatment.
Isotretinoin-induced nuclear overexpression of FoxO1 and IGFBP-3 might also mediate the anti-comedogenic effects of isotretinoin as upregulated IGFBP-3 suppresses proliferation of transient amplifying keratinocytes.Citation89 Comedo formation results from increased proliferation and retention of infundibular keratinocytes.Citation90 The antiproliferative activity of nuclear IGFBP-3 has also been confirmed in myeloid leukemia cells, while IGFBP-3 enhances signaling through RXR/RXR homodimers, it blunts signaling by activated RAR/RXR heterodimers.Citation91 In human breast cancer, ATRA mediated IGFBP-3-promoted apoptosis by enhancing the activity of RXRα.Citation92 Thus, FoxO-mediated antiproliferative and apoptosis-inducing effects may explain the chemopreventive activity of isotretinoin in certain types of cancers.
Does Isotretinoin Induce FoxO-Mediated Sebocyte and Sebocyte Stem Cell Arrest?
Fascinating research of the last years has elucidated various signals controlling sebocyte differentiation in vivo and major signaling pathways regulating differentiation of the sebaceous gland, recently reviewed in this journal.Citation93 Activation of c-myc and hedgehog signaling cascades and repression of β-catenin signaling are important for the differentiation and maturation process experienced by sebocytes. They are essential inductive events responsible for the morphogenesis of the sebaceous gland during embryonal and neonatal development.Citation93 There is good evidence that activation of c-myc in mouse skin results in enhanced sebaceous gland morphogenesis,Citation94,Citation95 and induction of sebocyte cell fate even within the interfollicular epidermis.Citation96 The effect of c-myc is somewhat surprising because c-myc is reported to act downstream of β-catenin and is a direct target gene of canonical Wingless (Wnt) signaling.Citation97,Citation98 In skin, c-myc and β-catenin exert opposing effects on sebocyte differentiation. Analysis of transgenic mice with simultaneous activation of c-myc and β-catenin revealed mutual antagonism: c-myc blocked β-catenin-mediated formation of ectopic hair follicles and β-catenin reduced c-myc-stimulated sebocyte differentiation.Citation99 Pulse-chase experiments in mouse skin suggested the existence of slow-cycling cells in the gland and a small cluster of cells at the base of the sebaceous glands expressed the transcriptional repressor Blimp1.Citation100,Citation101 Blimp1-expressing cells were suggested to be progenitors that give rise to all cells within the sebaceous gland. However, the functional signaling relationship between Blimp1 and c-myc is currently contradictory. On one hand, Blimp1 is not selectively expressed in sebaceous gland progenitor cells, but is also expressed by terminally differentiating cells in the interfollicular epidermis, sebaceous gland and hair follicle.Citation99,Citation102 A recent study implies that Blimp-1 is expressed late in embryonic development and is restricted to the evolving sebaceous gland and Blimp-1 labels only the most mature cellular constituents.Citation102 More confusing is the fact that despite Blimp1's known negative regulation of the c-myc promoter,Citation101 no correlation between Blimp1 and c-myc levels has been found in individual human sebaceous cells.Citation99 This contradiction suggested that additional factors regulate levels of c-myc protein in sebocytes.Citation99 Do FoxO transcription factors represent the missing link to understand these controversies in c-myc regulation?
FoxOs and c-myc.
FoxO transcription factors have been identified as important regulators of stem cell homeostasis.Citation103 FoxOs play an increasing physiological role in the maintenance and integrity of stem cell compartments in a broad spectrum of tissues.Citation103 For instance, FoxOs cooperate to affect quiescence of hematopoietic stem stells by regulation of mediators of the G0/G1 and G1/S arrest including Rb/p130, cyclin G2, p27, p57, p21 and cyclin D2.Citation103 FoxO-mediated stem cell regulation of stem cell quiescence resembles isotretinoin-mediated effects on sebocyte cell cycle arrest. Thus, the question arises whether isotretinoin's sebumsuppressive effects are related to FoxO-induced quiescence of sebocyte stem cells? Recent evidence points to a substantial molecular cross talk between FoxO and c-myc dependent signal transduction.Citation104,Citation105 In colon cancer cells, induction of the transcriptional repressor protein Mxi1-SRα of the Mad/Mxd family of proteins by FoxO3a repressed myc-dependent gene expression.Citation104 FoxO3a activation induced a switch in promoter occupancy from myc to Mxi1 on the E-box containing promoter regions of two studied myc target genes. siRNA-mediated transient silencing of Mxi1 or all Mad/Mxd proteins reduced exit from S phase in response to FoxO3a activation and stable silencing of Mxi1 or Mad1 reduced the growth inhibitory effect of FoxO3a. Thus, the induction of Mad/Mxd proteins contributes to the inhibition of proliferation in response to FoxO3a activation. Direct regulation of Mxi1 by FoxO3a appears to be an additional mechanism through which the PI3K/Akt/FoxO pathway can modulate c-myc function.Citation104
There is another important connection bewteen FoxO and c-myc regulation of the p27 cyclin dependent kinase inhibitor. It has been shown in murine WEHI 231 immature B lymphoma cells that inhibition of PI3K/Akt signaling decreased the levels of NFκB and c-myc, which has been shown to repress p27 promoter activity.Citation105 p27 is coordinately regulated via two arms of a signaling pathway that are inversely controlled upon inhibition of PI3K: induction of the activator FoxO3a and downregulation of the repressor c-myc.Citation105 FoxO1a, FoxO3a and FoxO4 transactivate the p27 promoter.Citation105 FoxO3a induced p27 transcription and apoptosis of Ba/F3 cells.Citation106 The p27 cyclin-dependent kinase (CDK) inhibitor plays an essential role in transition through the G1 phase, in particular the restriction point, via binding to and inhibiting such complexes as cyclin E-CDK2 and cyclin-A CDK2.Citation107 There is strong evidence that FoxOs induce G1 arrest through expression of p27, p21 and p130 and increase the duration of the G2 phase of the cell cycle by inducing cyclin G2 ().Citation80,Citation108–Citation112
Assuming that this regulatory mechanism operates in sebocytes and sebocyte stem cells as well, the reciprocal control of FoxO3a and c-myc via the PI3K pathway could modify sebcaous gland proliferation via p27 regulation. High levels of growth factors, insulin and IGF-1 in puberty, hyperinsulinemic western diet (hyperglycemic carbohydrates and insulinotropic milk) or acne-associated syndromes with insulin resistance would trans-locate FoxOs from the nucleus by increased PI3K/Akt singaling, whereas isotretinoin treatment with proposed upregulation of FoxOs counterbalances the effect of increased growth factor signaling in acne and downregualtes increased sebocyte proliferation and induces sebocyte apoptosis, the main regulatory features of FoxO transcription factors ().
Sox9, FoxO and β-catenin.
The earliest known signal necessary for sebaceous gland development is the transcription factor Sox9.Citation113 The Sox family of transcription factors has emerged as modulators of canonical Wnt/β-catenin signaling in development and diverse disease contexts, recently reviewed elsewhere.Citation114 Sox physically interact with β-catenin and modulate the transcription of Wnt-target genes.Citation114 On the other hand, Wnt signaling also regulates Sox expression resulting in feedback regulatory loops that fine tune cellular responses to β-catenin/Tcf activity.Citation114 Sox9 in mouse intestinal epithelium requires Wnt signaling, but Sox9 then locally attenuates Wnt-target gene expression.Citation115,Citation116 These observations clearly demonstrate, that β-catenin maintains a molecular cross-talk with other transcription factors, especially in early steps of stem cell regulation.
FoxO transcription factors not only interact with c-myc signaling but also interact with β-catenin signaling and may be a modulating element between c-myc-driven sebocyte proliferation and Wnt/β-catenin-regulated sebaceous gland morphogenesis. Blocking canonical Wnt signalling during skin development by expression of a dominant negative mutant transcription factor Lef1 (ΔNLef1) results in transdifferentiation of hair follicle keratinocytes into mature sebocytes.Citation117,Citation118 A high proportion of human sebaceous adenomas and sebaceomas exhibit double nucleotide mutations within the β-catenin binding domain of the lef1 gene. These mutations within the NH2 terminus of Lef1 prevent β-catenin binding and inhibit expression of β-catenin target genes.Citation119 Transgenic mice expressing N-terminally deleted ΔNLef1 in the skin develop spontaneous sebaceous tumours.Citation118 Suppression in Wnt/β-catenin signaling activity by overexpression of Smad7 with accelerated cytoplasmic β-catenin degradation resulted in increased sebaceous gland morphogenesis and increased sebocyte differentiation.Citation120 Sebaceous gland hyperplasia observed in aged UV-exposed skin exhibits upregulation of Smad7 expression,Citation121 which is associated with reduced β-catenin levels.Citation120
Thus, there is good evidence that suppression of Wnt/β-catenin signaling promotes sebocyte differention. c-myk is reported to be a direct target gene of canonical Wnt/β-catenin signaling and to act downstram of β-catenin.Citation97,Citation98 Therefore, it should be expected that downregulation of β-catenin would suppress c-myc as well. However, it is surprising that activation of c-myc in mouse skin enhanced sebaceous gland morphogenesis,Citation94,Citation95 and induced a sebocyte cell fate even within the interfollicular epidermis.Citation96 c-myc and β-catenin exert thus opposing effects on sebocyte differentiation. Analysis of transgenic mice with simultaneous activation of c-myc and β-catenin revealed this mutual antagonism: c-myc blocked β-catenin-mediated formation of ectopic hair follicles and β-catenin reduced c-mycstimulated sebocyte differentiation.Citation99
Does Isotretinoin Inhibit AR-Mediated Suppression of Wnt/β-Catenin Signaling?
Wingless proteins (Wnts) are secreted lipid-modified proteins that bind to a receptor complex comprising frizzled and the low-density lipoprotein receptor-related proteins 5 or 6 (LRP5 or LRP6).Citation122 Activation of this receptor complex by Wnts leads to inactivation of glycogen synthase kinase 3β (GSK-3β), which prevents the proteosomal degradation of the transcriptional coactivator β-catenin and, thereby, promotes its accumulation in the cytoplasm. β-Catenin translocates into the nucleus where it associates with the T-cell factor (Tcf)/lymphoid-enhancer binding factor (Lef) family of transcription factors and regulates the expression of Wnt target genes.Citation122
There is recent evidence for a cross-regulation of signaling pathways of nuclear hormone receptors with the canonical Wnt pathway.Citation123 The best characterized interaction between nuclear hormone receptors and the canonical Wnt pathway stems from the discovery that RAR binds directly to β-catenin in breast cancer cells.Citation124 ATRA decreased the activity of the β-catenin-Lef/Tcf signaling pathway. β-catenin interacted directly with the RAR in a retinoid-dependent manner, but not with RXR and RAR competed with Tcf for β-catenin binding.Citation124 Similar interactions have been discovered for vitamin D receptor (VDR), PPARγ, RXR, LXRα and β, estrogen receptor (ER) and AR.Citation123
AR and β-catenin interact by direct binding and complexing, AR/β-catenin interactions are ligand sensitive, whereby complexing occurs in the presence of dihydrotestosterone (DHT).Citation125 Intriguingly, AR has an inhibitory effect on Tcf/Lef-mediated transcription and can compete with Tcf/Lef molecules for β-catenin binding.Citation126–Citation128 Repression of the β-catenin/Tcf signaling is mediated by ligand-occupied AR that is in competition with Tcf for nuclear β-catenin.Citation128 As outlined above, inhibition of Wnt/β-catenin/Tcf/Lef-signaling is a requirement for sebocyte differentiation. The reciprocal relationship between AR and β-catenin on Tcf/Lef-mediated transcription allows the conclusion that a decrease in liganded AR would increase β-catenin-mediated Tcf/Lef-signaling, thus inhibiting sebocyte differentiation. Remarkably, a significant reduction in AR protein expression in skin of acne patients has been observed during oral isotretinoin treatment.Citation37 However, the time course of reduced AR expression in skin after a usual 3 to 4 month lasting isotretinoin treatment is not known. Moreover, the role of AR and FoxOs in sebocyte stem cell homeostasis has not been studied but may contribute to a prolonged downregulation of Wnt signaling in sebaceous stem cells. It is conceivable that an impairment of sebaceous stem cells would contribute to insufficient epidermal regeneration after epithelial injury. The clinical observation of impaired wound healing after systemic isotretinoin treatment might find here a plausible explanation. Thus, further studies with cultured sebocytes, human sebaceous glands and stem cells should address the possible FoxO1/AR and AR/β-catenin interaction in the presence or absence of isotretinion.
Parallels between Adipocyte and Sebocyte Differentiation
There are striking similarities in the regulation of Wnt signaling between sebocyte differentiation and adipogenesis. As already outlined, reduced Wnt signaling is required for sebocyte differentiation, whereas increased Wnt signaling inhibits sebocyte differentiation.Citation93 When Wnt signaling is off, adipogenesis is initiated, when it is on, adipogenesis is repressed.Citation129 Thus, Wnt signaling like FoxO1 functions as a lipogenic switch. Wnt signaling maintains preadipocytes in an undifferentiated state through inhibition of the adipogenic transcription factors CCAAT/enhancer binding protein α (C/EBPα) and PPARγ.Citation129 High expression of C/EBPα, C/EBPβ and PPARγ has been detected in immortalized SZ95 sebocytes which is important for sebocyte differentiation and sebaceous lipogenesis.Citation130 Intriguingly, the master transcription factors C/EBPα and PPARγ are under direct or indirect control by members of the FOX family.Citation131–Citation133 Expression of FoxO1, FoxO3a and FoxO4 is increased during adipogenesis coincident with expression of PPARγ and C/EBPα, but FoxO1 activation is delayed until the end of clonal expansion.Citation133 Remarkably, expression of constitutively active FoxO1 mutants prevent the differentiation of 3T3-L1 preadipocytes in adipocytes.Citation131,Citation132 Oral isotretinoin treatment is expected to force high expression of FoxO3a and FoxO1, which may inhibit sebocyte C/EBPα and PPARγ activity. Thus, evidence from translational research clearly demonstrates that FoxOs are involved in the regulation of AR, c-myc, C/EBPα, PPARγ; LXRα and SREBP-1c, all important regulatory transcription factors involved in differentiation of actively lipid synthesizing cells like sebocytes.
There is another regulatory metabolic relationship between Wnt signaling and ATRA. Wnt suppresses CYP26, an enzyme that is responsible for degrading ATRA into inactive metabolites.Citation134 Low Wnt signaling would result in less CYP26 suppression with low levels of ATRA, whereas high Wnt signaling would have a stronger inhibitory effect on CYP26 resulting in high ATRA levels. In isotretinoin-treated sebocytes, high intracellular ATRA levels due to isotretinoin isomerization resemble a constellation of high Wnt signaling, thus suppressing sebocyte differentiation.
FoxO Proteins Interact with β-Catenin
Recent evidence corroborated the important role of Wnt signaling for sebocyte differentiation and sebaceous gland morphogenesis.Citation93,Citation99 In 2005, Essers et al. reported an evolutionarily conserved interaction between β-catenin and FoxO proteins.Citation135 In mammalian cells, β-catenin interacts with FoxO1 and FoxO3a. This interaction requires armadillo repeats 1 to 8 of β-catenin and the C-terminal half of FoxO proteins.Citation135 Binding of β-catenin to FoxO enhances the transcriptional activity of FoxO.Citation135 Interestingly, high Wnt signaling with elevated levels of β-catenin are known to inhibit sebaceous gland morphogenesis and sebocyte differentiation. It is conceivable that high nuclear levels of β-catenin bind to FoxO3a and FoxO1 and augment their transcriptional proapoptotic effects.Citation14,Citation15,Citation19 It is well demonstrated that FoxOs and Tcf factors compete for the limited nuclear pool of β-catenin.Citation136,Citation137 These observations confirm the pivotal role of the evolutionarily conserved FoxO/β-catenin interaction and provide new insights into the complex signaling network of AR, FoxO, Wnt, Sox, β-catenin and c-myc in the development and homeostasis of the sebaceous gland.
Retinoids modify this regulatory network at multiple sites: ATRA induces upregulation FoxO3a.Citation9,Citation10 Moreover, the Wnt pathway can moduclate RAR signaling and vice versa.Citation123 ATRA decreases c-myc-dependent target genes.Citation124 Isotretinoin reduces IGF-1 serum levels.Citation36 The activated PI3K/Akt pathway promotes FoxO shuttling from the nucleus to the cytoplasm, inhibits GSK3β which prevents proteasomal degradation of β-catenin.Citation125 These data imply that a forced intracellular upregulation of ATRA by isotretinoin administration interferes with the activity of multiple important transcription factors involved in gene regulation orchestrated by FoxO transcription factors.
Isotretinoin and FoxO-Mediated Anti-Inflammatory Effects
Isotretinoin treatment in acne exerts various anti-inflammatory effects including modulation of metalloproteinase function, downregulation of reactive oxygen formation, inhibition of pro-inflammatory NFκB-mediated cytokine signaling and modulation of acquired and innate immunity. It will be shown that upregulated FoxO transcription factors are again most likely candidates which mediate all these anti-inflammatory effects.
FoxOs and metalloproteinases.
Isotretinoin is known to inhibit scarring in acne and affects dermal tissue remodeling. NFκB and activator protein-1 are activated in acne lesions with consequent elevated expression of inflammatory cytokines and matrix degrading metalloproteinases (MMPs). These elevated gene products have been shown to be molecular mediators of inflammation and collagen degradation in acne lesions in vivo.Citation138 Sebum contains proMMP-9, which was decreased following per os or topical treatment with isotretinoin in parallel to the clinical improvement of acne. Sebum also contains MMP-1, MMP-13, tissue inhibitor of metalloproteinase-1 (TIMP-1) and TIMP-2, but only MMP-13 was decreased following treatment with isotretinoin. The origin of MMPs and TIMPs in sebum is attributed to keratinocytes and sebocytes, since HaCaT keratinocytes in culture secrete proMMP-2, proMMP-9, MMP-1, MMP-13, TIMP-1 and TIMP-2. SZ95 sebocytes in culture secreted proMMP-2 and proMMP-9. Isotretinoin inhibited the arachidonic acid-induced secretion and mRNA expression of proMMP-2 and -9 in both cell types and of MMP-13 in HaCaT keratinocytes.Citation139
Thus, there is evidence for the influence of isotretinoin on the regulation of certain MMPs, however there is little information on its regulatory role at the level of gene transcription. The question arises whether FoxOs may regulate the promoter activity of certain MMPs? Interestingly, Tanaka et al. recently investigated the effect of UV-induced changes in FoxO1a expression and the roles of FoxO1a in the regulation of collagen synthesis and MMP expression in human dermal fibroblasts.Citation140 It should be emphasized that primarly the dermal compartment with its fibroblasts and not the keratinocytes and sebocytes is the primary target of tissue destruction and remodeling in acne. Interestingly, in UVA- or UVB-irradiated fibroblasts the expression of FoxO1a mRNA decreased significantly. The expression of type I collagen also decreased. On the other hand, MMP-1 and MMP-2 mRNA levels increased. FoxO1a small interfering RNA transfection induced the downregulation of FoxO1a expression, it also induced a decrease in type 1 collagen expression, and it increased MMP-1 and MMP-2 expression. In contrast, the addition of FoxO1a-peptide induced an increase in type 1 collagen expression and decreased in MMP-1 and MMP-2 expression.Citation140 Therefore it was concluded that FoxO1a plays a substantial role in skin photoaging, and control of FoxO1a may be a novel approach to prevent the collagen deficiency observed in photoaged skin. This is exactly the rationale of topical ATRA-treatment for aged, UV-damaged skin: to increase collagen synthesis and to reduce the activity of matrix degrading MMPs.Citation141,Citation142
There is even more evidence for the regulatory role of FoxOs in MMP expression. In endothelial cells certain vascular endothelial growth factor (VEGF)-responsive genes require FoxO1 activity for optimal expression like MMP-10.Citation143 Furthermore, resveratrol, a PI3K inhibitor, can enhance the apoptosis-inducing potential of TRAIL by activating FoxO3a and its target genes associated with an inhibition of MMP-2 and MMP-9 expression.Citation144 Astrocyte-elevated gene-1 (AEG-1) has been reported to be upregulated in several malignant cells and plays a critical role in Ha-ras-mediated oncogenesis through the PI3K/Akt signaling pathway. Interestingly, AEG-1 knockdown induced cell apoptosis through upregulation of FoxO3a activity. This alteration of FoxO3a activity was dependent on reduction of Akt activity in LNCaP and PC-3 cells. AEG-1 knockdown was associated with increased levels of FoxO3a and attenuated the expression of MMP-9.Citation145 In vascular smooth muscel cells, the C-terminal transactivation domain of FoxO4 is required for FoxO4-activated MMP-9 transcription. FoxO4 activates transcription of the MMP-9 gene in response to tumor necrosis factor-α (TNFα) signaling.Citation146 FoxO4 activates the MMP-9 promoter by binding to the transcription factor Sp1, whereas FoxO1 failed to activate the MMP-9 promoter.Citation146 These data show that distinct FoxO isoforms are able to regulate or coregulate MMP promoters thus linking MMP activity to FoxO signaling. Together, there is an overlap in the inhibitory acitivity of FoxO1 and FoxO3a and isotretinoin, respectively, regulating the expression of MMP-1, MMP-2 and MMP-3. In conclusion, isotretinoin's suppressive effect on MMP expression can be well explained by isotretinoin-induced upregulation of FoxO1 and FoxO3a modifying MMP promoter activity.
Isotretinoin, FoxOs, TLRs and NFκB signaling.
The growth factor-stimulated PI3K/Akt pathway activates NFκB signaling that inhibits apoptosis and triggers inflammatory responses and mediates just the opposite of FoxO-mediated gene transcription.Citation147 Acne in puberty and acne-associated syndromes are associated with increased insulin/IGF-1 signaling.Citation12,Citation20,Citation21 Excessive insulin/IGF-1 signaling activates the Akt/IKK/NFκB pathway.Citation147 The canonical pathway of NFκB activation transduces signals from Toll-like receptors (TLRs) and several cytokine receptors like interleukin-1 receptor (IL-1R) mainly to the IKKβ kinase.Citation148,Citation149
Activation of PI3K/Akt signaling by IGF-1 has been shown to increase SREBP-1 expression and sebaceous lipogenesis.Citation72 Sebaceous triglycerides are a preferred nutrient source of P. acnes, a critical milieu factor for P. acnes follicular hypercolonization and biofilm formation which trigger TLR-signaling of surrounding cells of the follicular environment. Indeed, TLR expression was found to be increased in the epidermis of acne lesions (TLR2, TLR4) and macrophages (TLR2) in which P. acnes induced cytokine production through a TLR2-dependent pathway.Citation150,Citation151 Distinct strains of P. acnes induced selective human β-defensin-2 and IL-8 expression in human keratinocytes through TLRs.Citation152 P. acnes, by acting on TLR2, activates NFκB and stimulates the secretion of IL-6 and IL-8 by follicular keratinocytes and IL-8 and IL-12 by macrophages, giving rise to inflammation. Thus, TLRs play an important role in the induction of innate immunity and inflammatory cytokine responses in acne.Citation153 Both, the insulin/IGF-1-mediated upregulation of Akt-mediated NFκB-signaling and P. acnes-TLR-mediated NFκB-signaling contribute to the upregulation of inflammatory cytokines in acne. Intriguingly, TLR2 contains a PI3K binding motif and activation of PI3K is particularly important for TLR2 signaling.Citation154 In response to bacterial ligands, Src family kinases initiate TLR2-associated signaling, followed by recruitment of PI3K and phospholipase Cγ necessary for the downstream activation of proinflammatory gene transcription.Citation155 PI3K activation is not only associated with TLR signaling but as well as with IL-1/IL-1R signaling, which both converge in increased activation of NFκB.Citation147 Furthermore, a direct interaction between PI3K and TLRs or their adaptor proteins, such as MyD88, has been reported.Citation154,Citation156 Thus, growth factor-signaling via PI3K/Akt/NFκB as well as TLR2/PI3K/Akt/NFκB signal transduction are integrated at the level of Akt activation most likely resulting in a nuclear deficiency of FoxOs. Isotretinoin treatment with upregulation of FoxOs will counterbalance the nuclear FoxO deficiency of growth factor-activated PI3K/Akt and will thereby attenuate PI3K/Akt-mediated proinflammatory NFκB signaling.
In a vicious cycle, P. acnes might stimulate TLR2 on sebocytes which further increase PI3K/Akt-mediated sebaceous lipogenesis. TLR2 and TLR4 are constitutively expressed on SZ95 sebocytes.Citation157 Interestingly, P. acnes exposure to hamster sebaceous glands has been shown to augment lipogenesis in vivo and in vitro.Citation158 This observation implicates that TLR2-mediated PI3K/Akt activation might not only be involved in the stimulation of inflammatory responses to P. acnes but also to P. acnes-triggered TLR2/PI3K/Akt-stimulated sebaceous lipogenesis. Downregulation of PI3K/Akt-mediated sebaceous lipogenesis by isotretinoin-induced upregulation of nuclear FoxOs would just impair lipogenesis and reduce the lipophilic follicular milieu for P. acnes overgrowth and P. acnes-mediated proinflammatory TLR2/PI3K/Akt/NFκB signal transduction.
Isotretinoin, FoxOs and Acquired Immunity
It is well known that isotretinoin exerts anti-inflammatory activity.Citation2,Citation3 Recent studies have highlighted a fundamental role for FoxO transcription factors in immune system homeostasis.Citation159 In vitro overexpression studies suggested that FoxO1 and FoxO3a are important for growth factor withdrawal-induced lymphocyte cell death. Moreover, FoxO factors importantly regulate cell cycle progression of lymphocytes. FoxOs are of pivotal importance for the control of lymphocyte homeostasis including critical functions in the termination and resolution of an immune response.
There is a functional link between upregulated TLR2-signaling in acne with increased interleukin-1α (IL-1α) production and T-cell mediated acquired immunity because selected IL-1 receptor associated kinases (IRAK-1, 2, M and 4) are bifunctional and can be recruited either to the TLR complex and thus mediate TLR-signaling or can associate with adapter proteins involved in T- and B-cell receptor-mediated signaling pathways linking TLR/IRAK signaling to adaptive immune responses.Citation160 ATRA has been shown to downregulate TLR2 expression and function.Citation161 TLR2/PI3K-signaling appears to be the connecting element between upregulated innate and adaptive immune responses in acne. Increased CD4+ T cell infiltration and IL-1 activity has been detected in acne-prone skin areas prior to follicular hyperkeratinization and comedo formation.Citation162
Intriguingly, FoxO family members play critical roles in the suppression of T cell activation and T cell homing.Citation16–Citation18 FoxO1 deficiency in vivo resulted in spontaneous T cell activation and effector differentiation.Citation17,Citation18 Functional studies validated interleukin 7 receptor-α (IL-7Rα) as a FoxO1 target gene essential for FoxO1 maintenance of naïve T cells. These findings reveal crucial functions of FoxO1-dependent transcription in control of T cell homeostasis and tolerance. FoxO1 links homing and survival of naive T cells by regulating L-selectin, CCR7 and IL-7Rα.Citation163
Isotretinoin, FoxOs and Innate Immunity
There is new evidence that metabolism and growth factor status determine the activity of genes involved in innate immunity which may play a role in P. acnes hypercolonization. A recent study underlined the pivotal role of FoxOs in the regulation of innate immunity.Citation88 In Drosophila flies FoxO transcription factor control the expression of several antimicrobial peptides (AMPs) in various tissues including skin.Citation88 AMP induction is lost in foxo null mutants but enhanced when FoxO is overexpressed. In Drosophila, AMP activation can be achieved independently of immunoregulatory pathogen-dependent pathways by FoxO, indicating the existence of cross-regulation of metabolism and innate immunity at the promoter level of FoxO-activated AMP genes.Citation88 In contrast, insulin and IGF-1 dependent signaling with Akt-mediated translocation of FoxO from the nucleus into the cytosol reduces the expression of AMPs. It is thus conceivable that insulinotropic western diet (milk, dairy and hyperglycemic carbohydrates) affects the balance and activity of AMPs. We have to ask whether insulinotropic western diet impairs innate immunity of the pilosebaceous follicle in such a way that P. acnes hypercolonization is promoted. Both isotretinoin-induced upregulation of FoxO1 with reduced sebaceous lipogenesis and isotretinoin-induced stimulation of the AMP response are synergistic mechanisms which could explain isotretinoin's suppressive effects on sebaceous lipogenesis, P. acnes growth and bacterial follicular colonization.
Taken together, isotretinoin-mediated upregulation of FoxO1 may exert anti-inflammatory effects by downregualtion of T-cell responses and upregulation of innate immunity.Citation16–Citation18 In contrast, growth factor (insulin, IGF-1, FGF)-induced nuclear deficiency of FoxOs would activate T-cell proliferation and decreases expression of AMPs. A decrease of AMPs would increase the number of pathogens (P. acnes) stimulating TLR-induced proinflammatory genes. These pro-inflammatory changes of acquired and innate immunity in acne may be counterbalanced by isotretinoin-mediated upregulation of FoxO1.
FoxO1 and Isotretinoin-Mediated Suppression of Oxidative Stress
Isotretinoin treatment in acne and rosacea has beneficial effects due to its ability to suppress the formation of reactive oxygen species (ROS). The ability of neutrophils to produce ROS was significantly increased in patients with inflammatory acne.Citation164 The involvement of ROS generated by neutrophils appears to play an important role in the disruption of the integrity of the follicular epithelium promoting inflammatory processes of acne. Patients with inflammatory acne showed a significantly increased level of hydrogen peroxide produced by neutrophils compared to patients with comedonal acne and healthy controls.Citation165 In acne patients, lower levels of superoxide dismutase (SOD) and catalase have been measured in polymorphonuclear neutrophils (PMN) in comparison to controls, which may be responsible for the increased levels of superoxide anion radicals in the epidermis.Citation166–Citation168 The effect of isotretinoin on the generation of ROS by stimulated human neutrophils showed that isotretinoin exerted an antioxidant activity against the superoxide anion.Citation169
One of the most important functions of FoxOs is the protection of cells from oxidative damage by increasing transcription of multiple genes regulating scavenging of ROS.Citation14,Citation18 Activated FoxO proteins promote stress resistance by binding to the promoters of the genes encoding manganese superoxide dismutase (MnSOD) and catalase, two scavenger enzymes that play essential roles in oxidative detoxification in mammals.Citation11,Citation135,Citation170–Citation172 FoxO-mediated oxidative-stress resistance is influenced by multiple other pathways like β-catenin which binds directly to FoxO proteins and enhances their transcriptional activity in mammalian cells.Citation135
Moreover, FoxO1 conrols the promoter activity of the key enzyme of cytochrome synthesis, heme oxigenase.Citation173 Upregulated FoxO1 downregulates the synthesis of heme, the prothetic group of hemoglobin and various cytochromes of the mitochondrial respiratory chain involved in ROS formation.Citation173 Thus, isotretinoin-induced upregulation of FoxO1 explains the suppression of mitochondrial ROS generation and increased ROS catabolism thereby normalizing increased ROS generation in acne.
FoxO-Upregulation Explains all Adverse Effects of Isotretinoin Therapy
All patients treated with isotretinoin suffer from multiple side effects. This already shows that isotretinoin affects other organ systems. The side-effect profiles qualitatively resemble toxic effects of vitamin A or hypervitaminosis A syndrome.Citation174
FoxO1 and Isotretinoin-Induced Hepatotoxicity
In approximately 15–20% of patients treated with isotretinoin mild-to-moderate transitory elevations of mitochondrial liver enzymes (aspartate aminotransferase and alanine aminotransferase) have been observed.Citation175 Circulating levels of alkaline phosphatase, lactic dehydrogenase and bilirubin may also become elevated during retinoid therapy.Citation2,Citation175 Again, we have to ask whether upregulated hepatic FoxO1 is the common cause of liver toxicity resulting in mitochondrial dysfunction with increased release of mitochondrial enzymes and increase in bilirubin?
The critical role of FoxO1 in hepatic glucose and lipid metabolism is well established and reviewed extensively elsewhere in reference 19. Under ordinary conditions, feeding stimulates insulin secretion from pancreatic β-cells, and FoxO1 in the liver is inhibited by insulin signal via IRS/PI3K/Akt cascade (). In fasting state, insulin signal is weak and FoxO1 is activated by translocation into the nuclei to trigger gluconeogenesis for glucose supply. Under insulin resistance conditions, however, hyperactive FoxO1 promotes gluconeogenesis in such an uncontrolled way that it leads to hyperglycemia. It is well known that isotretinoin impairs insulin resistance.Citation176,Citation177 This fact can be well explained by FoxO1-mediated upregulation of phosphoenolpyruvate carboxykinase (PEPCK), the key enzyme of gluconeogenesis.Citation19 Thus, hyperactive FoxO1 explains impaired insulin sensitivity and an increased disposition for hyperglycemia observed under isotretinoin treatment ().
Furthermore, recent observations indicate that activated FoxO1 impairs fatty acid oxidation. A hepatic increase in fatty acids may promote dyslipidemia which may arise at least in part from mitochondrial dysfunction ().Citation178–Citation180 By directly binding the promoter, FoxO1 induces heme oxigenase-1 (Hmox1) that reduces the heme content required for expression, stability and function of electron transport chain (ETC) components.Citation178,Citation181 Heme is the functional prosthetic group of all cytochromes in the liver which drive the mitochondrial ETC. FoxO1-mediated induction of Hmox1 disrupts the ETC and impairs mitochondrial metabolism including fatty acid β-oxidation. Hyperactivated FoxO during severe insulin resistance contributes to the accumulation of hepatic lipids.Citation178–Citation180 Adenoviral delivery of constitutively nuclear FoxO1 to mouse liver promotes hepatic triglyceride accumulation that can progress to steatosis as seen in hypervitaminosis A syndrome.Citation180 The lipid accumulation is associated with decreased fatty acid oxidation. In rats, administration of isotretinoin (100 mg/kg diet) increased the total hepatic lipid and triglyceride content as well as serum triglyceride concentrations.Citation182
FoxO1 mediated increase in heme oxigenase-1 with resultant mitochondrial dysfunction is of fundamental biological importance and explains the isotretinoin-mediated increase of mitochondrial liver enzymes. ATRA, alitretinoin and isotretinoin are able to induce membrane permeability transition observed as swelling and decrease in membrane potential in isolated rat liver cells ().Citation183 Isotretinoin appeared to be the most effective and stimulated the release of cytochrome c from mitochondria, suggesting a potential target of retinoids in the induction of cell apoptosis.Citation183 Isotretinoin's effect on mitochondrial permeability via FoxO1-mediated inhibition of the ETC not only explains the increased release of mitochondrial liver enzymens but also increased bilirubin levels following increased heme catabolism.
Remarkably, FoxO1-upregulated heme oxigenase-1 and disturbance of mitochondrial function and integrity may be an important trigger for the intrinsic pathway of apoptosis. In this regard, isotretinoin mimics growth factor withdrawal with upregulation of FoxO1/heme oxigenase-induced intrinsic pathway of apoptosis mediated through mitochondrial instability ().
Togehter, the effects of isotretinoin on hepatic glucose, lipid and heme metabolism as well as mitochondria-dependend apoptosis may be well explained by isotretinoin-induced overexpression of hepatic FoxO1.
Isotretinoin-Induced Hypertriglyceridemia
Isotretinoin at pharmacological doses elevates plasma triglycerides and induces overt hypertriglyceridemia.Citation184–Citation186 There are at least three mechanisms involved in the generation of isotretinoin-induced hypertriglyceridema:
Hepatic triglycerides and free fatty acids are elevated by isotretinoin treatment as a result of FoxO1-mediated upregualtion of heme oxigenase-1 with impaired activity of the ETC and diminished fatty acid oxidation.Citation179,Citation180 Increased free fatty acids in the liver are incorporated into triglycerides.
Hepatic very low density lipoprotein (VLDL) production is facilitated by microsomal triglyceride transfer protein (MTP) in a rate-limiting step that is regulated by insulin. In hepatocytes, FoxO1 binds and stimulates MTP promoter activity (). Mice that expressed a constitutively active FoxO1 transgene revealed enhanced MTP expression, augmented VLDL production and elevated plasma triglyceride levels.Citation187 VLDL production is suppressed in response to increased insulin release after meals by insulin-mediated PI3K/Akt activation and reduction of nuclear levels of FoxO1.Citation188 Thus, isotretinoin-induced upregulation of nuclear FoxO1 would nicely explain increased hepatic VLDL synthesis resulting in retinoid-induced hypertriglyceridemia.
A third mechanism provides indirect evidence for isotretinoin's ability to raise nuclear FoxO1 concentrations. Isotretinoin increases the expression of apolipoprotein C-III, a known antagonist of plasma triglyceride catabolism. Apo C-III functions as an inhibitor of lipoprotein lipase and hepatic lipase.Citation189 In fact, isotretinoin treatment resulted in elevated plasma levels of apo C-III.Citation190 Recent studies confirmed that FoxO1 stimulated hepatic apo C-III expression and correlated with the ability of FoxO1 to bind to the apo C-III promoter.Citation191 These observations clearly explain the basic mechanism of isotretinoin-mediated hypertriglyceridemia at the level of upregulated FoxO1-mediated gene transcription and are an excellent proof of the proposed role isotretinoin-induced FoxO transcription in hepatic lipid and lipoprotein metabolism.
Isotretinoin and FoxO1-Mediated Bone Toxicity
Isotretinoin in high doses and given over prolonged periods (>1 mg/kg body weight, >1 year) disturbs the physiological homeostasis of bone metabolism including demineralization, thinning of the bones and premature closure of the epiphyses as well as hyperostosis, periostosis (disseminated idiopathic skeletal hyperostosis, DISH syndrome).Citation192–Citation196 It has been clearly demonstrated that treatment of rats with isotretinoin decreased bone mass.Citation197 Bone mineral density, bone mineral content, bone diameter and cortical thickness of the femur were reduced in rats treated daily with 10 or 15 mg/kg ATRA or 30 mg/kg isotretinoin.Citation197 In acne patients receiving high dose isotretinoin (1 mg/kg of body weight) bone density at the Ward triangle significantly decreased by a mean of 4.4% after 6 months of isotretinoin use and some patients showed decreased density of more than 9% at the Ward triangle.Citation194 However, patients receiving a single course of isotretinoin treatment for 4–6 months until a cumulative dose of 120 mg/kg did not exhibit clinically significant effects on bone metabolism.Citation195 Most hyperostoses are asymptomatic and clinically insignificant.Citation2,Citation196,Citation198 High-dose isotretinoin for a period of over 2 years have been shown to appear to induce skeletal hyperostoses and anterior spinal ligament calcification. Bone abnormalities in children, particularly premature closure of the epiphyses, are associated with high isotretinoin doses (>1 mg/kg/day), vitamin A supplementation and long-term treatment.
Again the question: Is there a link between high levels of isotretinoin, FoxOs and bone metabolism? During the last decade, it has been extensively documented that Wnt/β-catenin signaling is a critical determinant of bone mass.Citation122 The paramount importance of the Wnt/β-catenin/Tcf signaling for bone mass has been explained by the essential role of β-catenin in determining the commitment of multipotential mesenchymal progenitors to the osteoblastic lineage.Citation199,Citation200 In addition to promoting osteoblastogenesis, Wnt/β-catenin signaling inhibits adipogenesis, an alternative fate of the multipotential mesenchymal progenitors, by blocking the expression of PPARγ and C/EBPα as already outline above.Citation201 Similar to the Wnt/β-catenin pathway, oxidative stress influences fundamental cellular processes including stem cell fate and has been linked to aging and the development of age-related diseases like osteoporosis. β-catenin has recently been implicated as a pivotal molecule in defense against oxidative stress by serving as a cofactor of FoxO transcription factors.Citation122 In addition, it has been shown that oxidative stress is a pivotal pathogenetic factor of age-related bone loss and strength in mice, leading to a decrease in osteoblast number and bone formation. These particular cellular changes evidently result from diversion of the limited pool of β-catenin from Tcf- to FoxO-mediated transcription in osteoblastic cells ().Citation135,Citation137,Citation202 Fascinatingly, attenuation of Wnt-mediated transcription has been linked not only to premature osteoporosis, but also to hyperlipidema, insulin resistance and diabetes—observed changes of isotretinoin treatment. It is thus conceivable that bone toxicity of isotretinoin may be mediated by increased nuclear FoxO levels which divert β-catenin from to Tcf-binding to FoxO-binding, thereby attenuating Wnt/β-catenin signaling in the bone ().
Interestingly, ATRA treatment of mouse epiphyseal chondrocytes in culture increased Wnt/β-catenin signaling.Citation203 Cross-regulation of Wnt signaling and retinoid signaling affect chondrocyte function and phenotype and could be quite important in the process of chondrogenesis and proper progression of enchondral ossification during skeletal growth.Citation203 Thus, isotretinoin/FoxO-mediated attenuation of epiphyseal chondrocyte Wnt singaling may be a conceivable mechanism explaining premature closure of the epiphyses by isotretinoin treatment.
Isotretinoin and FoxO-Mediated Adverse Effects on Muscle
Arthralgias and myalgias may occur in up to 2–5% of individuals receiving oral isotretinoin in doses higher than 0.5 mg/kg/day and is more common in adolescents and young adults. In some cases severe muscle pain and temporary disability of movement with early-morning arthralgias were seen. Occasionally, concomitant malaise and fever and increases in creatine phosphokinase (CPK), a specific marker of muscle destruction, may be observed.Citation2,Citation204,Citation205 CPK, has been found to be elevated, occasionally by up to 100 times the normal value with or without muscular symptoms and signs in a variable percentage of patients receiving isotretinoin treatment and particularly in those undergoing vigorous physical exercise.Citation206
Again, the question has to be raised whether isotretinoin affects FoxO transcription in muscle cells? The skeletal muscle is one of the major peripheral tissues that is responsible for insulin-mediated fuel metabolism and energy expenditure. Skeletal muscle accounts for >30% of resting metabolic rate and 80% of whole-body glucose uptake. Expression of FoxO1 is increased in skeletal muscle by energy deprivation such as fasting, suggesting that FoxO1 may mediate the response of skeletal muscle to changes in energy metabolism.Citation207–Citation209 The maintenance of muscle mass is achieved by a dynamic balance of atrophy and hypertrophy.Citation210,Citation211 Activation of FoxO1 or FoxO3a in the skeletal muscle, in fasting or diabetic conditions, can increase protein breakdown through ubiquitin-proteasome and autophagy-lysosome pathways, the two major mechanisms causing muscle atrophy ().Citation212–Citation216 Overexpression of a constitutively active FoxO1 in C2C12 muscle cells promotes expression of atrogin 1 and muscle-specific RING finger protein 1, the two ubiquitin ligases involved in skeletal muscle atrophy.Citation217 Expression of a dominant-negative FoxO1 construct in myotubes or in rodent muscle decreases atrogin-1 expression and muscle atrophy.Citation217 Transgenic FoxO1 in skeletal muscle increases expression of cathepsin L, an atrophy-related lysosomal protease, which is associated with reduced skeletal muscle mass and body weight.Citation218 Moreover, the genes encoding structural proteins of type I muscles (slow twitch, red muscle) are downregulated concomitant with a decreased size of both type I and type II fibers. The coordinate regulation of cathepsin L and type I muscle genes may account at least in part for the loss of muscle mass and glycemic control owing to hyper-activated FoxO.Citation218
Furthermore, FoxO1 suppresses SREBP-1c, the key transcription factor of lipogenesis, in skeletal muscle by disrupting the RXRα/LXR heterodimer on the SREBP-1c promoter ().Citation219 Mice overexpressing FoxO1 lose their glycemic control and display a lower capacity for physical exercise due to severe muscle loss.Citation218 Due to clinical experience, isotretinoin is administerd with caution to atlethes who have to rely on their muscle mass and muscle strength. Skeletal muscle metabolism switches from oxidation of carbohydrates to fatty acids as the major energy source during fasting when the plasma glucose concentration is low. FoxO1 controls this switch by upregulating 3 enzymes: pyruvate dehydrogenase kinase-4 (PDK4) that shuts down glucose oxidation by targeting pyruvate dehydrogenase (PDH), lipoprotein lipase that hydrolyzes plasma triglycerides into fatty acids and fatty acid translocase CD36 that facilitates fatty acid uptake into skeletal muscle.Citation208,Citation220 PDK4 phosphorylates PDH and blocks PDH activity in catalyzing the conversion of pyruvate into acetyl-CoA. This can divert the physiological switch of FoxO1 activity, that is, on in fasting and off in feeding state, which is required for the nutrient/energy homeostasis in the skeletal muscle through carbohydrate/lipid switch. Severe starvation may trigger FoxO1-mediated autophagy and atrophy that break down protein for energy supply, the mechanism that underlies the loss of muscle mass and glycemic control under insulin resistance. It is well known that FoxO1 is a key transcription factor of starvation. Thus, FoxO1 plays a key role in the carbohydrate/lipid metabolic switch in skeletal muscle during fasting/feed cycle. Hyperactivated FoxO1 induces autophagy-related protein degradation through atrogin 1 and muscle-specific RING finger protein 1, which causes atrophy and muscle loss which disturbs metabolic homeostasis ().Citation12
In summary, isotretinoin-induced upregulation of FoxO may impringe an artificial “fasting state” on muscle metabolism switching to catabolic events in muscle cell homeostasis which may very well explain isotretinon-associated myalgias and increases in CPK due to muscle cell degradation.
Isotretinoin, FoxO1 and Mucocutaneous Side Effects
Mucocutaneous adverse effects of oral isotretinoin treatment are dose-dependent and predominantly reflect a decreased production of sebum, reduced stratum corneum thickness, and altered skin barrier function.Citation2,Citation221 Skin xerosis, especially on exposed skin and cheilitis are the earliest and the most frequent side effects that affect almost all treated patients. Staphylococcus aureus colonization correlates with the isotretinoin-induced reduction in sebum production and may lead to overt cutaneous infections. Xerophthalmia due to decreased meibomian gland secretion can lead to blepharoconjunctivitis. Dry genitals and anal mucosa may be a side effect and dry nasal mucosa may lead to epistaxis.Citation2,Citation221
Pioniering studies have focused on the antikeratinizing effect for retinoid activity which comprised dose-dependent alterations in transepidermal water loss and epidermal and stratum corneum loosening associated with loss of epidermal cohesion and abnormal barrier function.Citation222–Citation224 Recently however, using large DNA microarrays it has been shown, that ATRA suppresses genes responsible for biosynthesis of epidermal lipids, long-chain fatty acids, cholesterol and sphingolipids in primary human epidermal keratinocytes.Citation225 Unexpectedly, ATRA regulated many genes associated with the cell cycle and programmed cell death.Citation225
The role of epidermal lipids for skin barrier function and antimicrobial defense has been well established.Citation226,Citation227 The major part of epidermal barrier function is provided by ordered epidermal lipid synthesis and conversion of polar lipids into nonpolar ceramides and acylceramides which constitute the intercorneocyte lipid lamellae important in stratum corneum barrier function and control of transepidermal water loss.Citation226 Free fatty acids, cholesterol and ceramides in appropriate molar ratios are important for barrier function.Citation226
There is recent evidence that RAR, PPARs and LXR are also involved in the regulation of epidermal lipid synthesis and barrier function.Citation226 Whereas activation of PPARα and LXR improve barrier function, RAR activation worsens it.Citation226 Both cholesterol and fatty acid synthesis are regulated by SREBPs.Citation228 Two SREBP genes (SREBP-1 and SREBP-2) encode three proteins: SREBP-1a, SREBP-1c and SREBP-2.Citation229,Citation230 SREBPs bind to the promoters of multiple SREBP-responsive genes stimulating various enzymes of cholesterol and fatty acid synthesis.Citation229,Citation230 SREBP-2 is a more important regulator of cholesterol synthesis. SREBP-1a is as effective as SREBP-2 as a regulator of HMG-CoA synthase and HMG-CoA reductase in cholesterol synthesis, but it has a greater effect on fatty acid synthesis than does SREBP-2. SREBP-1c primarily regulates fatty acid synthesis. Nothing is yet known about the role of FoxOs in the regulation of epidermal lipid homeostasis. In muscle cells however, FoxO1 regulates triglyceride content via the RXRα/LXRα/SREBP-1c pathway and has been shown to suppress RXRα/LXRα-mediated SREBP-1c promoter activity ().Citation219 In FoxO1 transgenic mice, gene expression of SREBP-1c is downregulated in skeletal muscle. During nutritional changes caused by fasting and feeding, gene expression of RXRα and SREBP-1c in mouse skeletal muscle switches off and on, respectively, whereas expression of FoxO1 shows reverse correlation with SREBP-1c expression.Citation219 Supposed that isotretinoin-mediated upregulation of FoxO1 would downregulate SREBP1c-mediated epidermal fatty acid- and cholesterol synthesis in epidermal keratinocytes, a functional disturbance of epidermal barrier function may result. Moreover, alterations in fatty acid synthesis, potentially regulated by FoxOs and SREBPs, could indirectly affect ceramide production as the first biosynthetic step in ceramide synthesis catalyzed by serine palmitoyl transferase requires the presence of sufficient amounts of fatty acids.Citation231–Citation233
In analogy to sebaceous and epidermal lipid biosynthesis during isotretinoin treatment, a FoxO1-mediated downregualtion of lipid synthesis of meibomian glands would explain the isotretinoin-induced blepharoconjunctivitis clinically appearing as “dry eyes.” Indeed, histopathological studies of the eyelids of female New Zealand rabbits after long-term isotretinoin (2 mg/kg) treatment showed “degenerative changes” in the meibomian gland acini, leading to cell necrosis and a decrease in the basaloid cells lining the acini walls without inflammatory changes.Citation234 Systemic administration of isotretinoin caused a reduction of acinar tissue in the hamster meibomian gland. Histologic examination revealed a decrease in the numbers of mature lipid-laden acinar cells and a reduction of up to 75% in mean volume of meibomian acinar tissue from animals fed a high dose of isotretinoin.Citation235 Systemic treatment of adult male New Zealand albino rabbits with isotretinoin resulted in a reduction in the size of the meibomian gland and a decrease in acinar tissue.Citation236
The isotretinoin-induced reduction of acinar tissue and lipid content of meibomian glands in the presented animal models points again to an isotretinoin-induced apoptosis mechanism. This is comparable to isotretinoin's effect on sebocytes and is most likely driven by FoxO-upregulation. Thus, isotretinoin-mediated stimulation of FoxO-mediated gene expression could explain the defects in the quality and composition of the conjunctival lipid film and the resultant blepharoconjunctivitis observed in 20–45% of the patients treated systemically with isotretinoin.Citation234
FoxOs and Isotretinoin-Induced Hair Loss
Long-term use of isotretinoin in higher doses is associated with increased hair loss in susceptible individuals.Citation221 Isotretinoin has been demonstrated to affect hair growth.Citation237 In equine hair follicles in vitro isotretinoin modified sheath-shaft interaction.Citation238 It has recently been shown that ATRA induces premature hair follicle regression and induced a catgen-like stage in human hair follicles.Citation239 Hair shaft elongation declined significantly already after 2 days in the ATRA-treated group, and approximately 80% of the ATRA-treated hair follicles had prematurely entered catagen-like stage at day 6, compared with 30% in the control group. This corresponded to an upregulation of apoptotic and a down-regulation of Ki67-positive cells in ATRA-treated hair follicles,Citation239 thus pointing again to the induction of apoptosis, the hallmark of FoxO signaling.
Previous studies have shown that the Wnt signaling pathway plays an important role in the growth and development of hair follicles.Citation99,Citation113,Citation117,Citation118 Wnts are deeply involved in the proliferation and differentiation of skin epithelial cells. The differentiation of cultured primary skin epithelial cells toward hair shaft and inner root sheath of the hair follicle via β-catenin stabilization caused by Wnt10b has been reported. In organ cultures of whisker hair follicles in serum-free conditions no hair shaft growth was observed in the absence of Wnt10b, whereas its addition to the culture promoted elongation of the hair shaft, intensive incorporation of BrdU in matrix cells flanking the dermal papilla, and β-catenin stabilization in dermal papilla and inner root sheath cells. These results suggest a promoting effect of Wnt10b on hair shaft growth that is involved with stimulation of the dermal papilla via Wnt10b/β-catenin signaling, proliferation of matrix cells next to the dermal papilla and differentiation of inner root sheath cells by Wnt10b.Citation240 In contrast, expression of ΔNlef1 transgene in mouse epidermis, which lacks the β-catenin binding site, resulted in differentiation of hair follicles into squamous epidermal cysts and formation of skin tumours.Citation118
Can we explain isotretinoin's adverse effects on hair growth by FoxO-driven apoptosis and induction of catagen? A reasonable explanation would be that isotretinoin-induced FoxO impairs β-catenin signaling which is most important for hair growth. In fact, is has been shown that upregulated nuclear FoxOs binds nuclear β-catenin and divert β-catenin signalling from Tcf/Lef1 interaction ().Citation135 Thus, upregualted FoxOs by isotretinoin would explain impaired β-catenin signaling leading to apoptosis and reduced growth and differentiation of hair follicles, explaining isotretinoin-induced FoxO-mediated hair loss.
Isotretinoin, FoxOs and CNS Side Effects
Clinically observed CNS side effects of isotretinoin are rare. However, isotretinoin, has been associated with various psychiatric side effects such as depression, suicidality and psychotic symptoms. A great number of reports on its CNS effects have been published since its introduction into the market. According to the FDA all patients treated with isotretinoin should be observed closely for symptoms of depression or suicidal thoughts, such as sad mood, irritability, acting on dangerous impulses, anger, loss of pleasure or interest in social or sports activities, sleeping too much or too little, changes in weight or appetite, school or work performance going down, trouble in concentrating, mood disturbances, psychosis or aggression. A causal relationship has yet not been established and the link between isotretinoin use and psychiatric events remains controversial.Citation241 However, six weeks of isotretinoin administration (1 mg/kg) increased depression-related behavior in mice.Citation242
Does isotretinoin modify FoxO regulation in the brain, a metabolically active organ strongly dependent on glucose metabolism? RARs are widely distributed in the brain. The regions of the brain that predominantly exhibit RAR signaling include the limbic system, in particular the hippocampus and the medial prefrontal cortex, the cingulate cortex and subregions of the thalamus and hypothalamus.Citation243,Citation244 Recent studies have demonstrated that the hippocampus is one of the brain regions where new neurons are constantly born, a phenomenon called neurogenesis. One of the theories for the pathogenesis of depression suggests a decreased hippocampal and prefrontal cortex neurogenesis.Citation245,Citation246 Moreover, antidepressant treatment seems to operate by an increase in neurogenesis, which is chronologically seen during the same period as the clinical improvement. Another irregularity in the hippocampus associated with depression is the reduction of the hippocampal volume. Intriguingly, isotretinoin treatment of mice results in both decreased hippocampal neurogenesis and a reduction in the hippocampal volume.Citation247,Citation248 Treatment of GT1–7 hypothalamic cells with 10 µM isotretinoin for 48 h decreased cell growth to 45.6 ± 13% of control. Griffin et al. hypothesized that the ability of isotretinoin to decrease hypothalamic cell number may contribute to the increased depression-related behaviors observed in mice.Citation249 Therefore, the isotretinoin-mediated effect on neurogenesis could provide a plausible biological mechanism mediating depressogenic effects.
Intriguingly, FoxO1 is strongly expressed in the striatum and neuronal subsets of the hippocampus, i.e., the dentate gyrus and the ventral/posterior part of the cornu ammonis regions.Citation250 In wildtype mice, hypothalamic FoxO1 expression is reduced by the anorexigenic hormones insulin and leptin.Citation251 Upregulation of hypocampal FoxO1 levels may inhibit hypocampal neurogenesis and may thus be responsible for the adverse psychiatric drug effects in some disposed individuals. Remarkably, FoxO1 suppresses the transcription of proopiomelanocortin (POMC) by antagonizing the activity of signal transducer and activator of transcription-3 (STAT3).Citation251,Citation252 FoxO1 modulates the melanocortin system by regulating the expression of Agrp and Pomc genes.Citation252 One of the POMC peptide cleavage products is α-melanocyte stimulating hormone (α-MSH). FoxO1 suppresses the expression of both Pomc and Cpe, which is one of the peptidases (carboxypeptidase E) that processes POMC to α-MSH (). Remarkably, the proopiomelanocortin system plays an important role as a neuromediator system in controling the sebaceous gland. It is well known that α-MSH can stimulate sebocyte differentiation and sebaceous lipogenesis.Citation253,Citation254 Thus, isotretinoin-mediated upregulation of hippocampal and hypothalamic FoxO1 could inhibit POMC/α-MSH-signaling to the sebaceous gland.
Antidepressant treatment seems to lead to an increase in neurogenesis, which is chronologically seen during the same period as the clinical improvement.Citation241 Severe acne and acneiform eruptions have been observed with high doses of tricyclic antidepressants and lithium therapy. In brain of mice, lithium significantly decreased FoxO3a levels.Citation255 As already mentioned, FoxO3a activates the promoter of FoxO1 and is an important inducer of FoxO1 gene expression.Citation11 The acneigenic effect of lithium therapy may be related to a lithium-induced nuclear deficiency of FoxO1 by suppression of the FoxO1 promoter. In mice, elevated serotonergic activity increased Akt-mediated phosphorylation of FoxO1 and FoxO3a in various brain regions resulting in nuclear deficieny of FoxO1 and FoxO3a.Citation256 FoxOs in brain of rodents are intensely involved in the regulation of behavioral manifestation.Citation256 Upregulated serotonin levels by antidepressants reduce nuclear concentrations of FoxO1 and FoxO3 in neuronal cells. FoxO1-deficient mice displayed reduced anxiety, whereas FoxO3a-deficient mice presented with a significant anti-depressant-like behavior.Citation256 Thus, elevated nuclear content of FoxO1 and FoxO3a by isotretinoin treatment in the human hippocampus and hypothalamic areas of the brain may explain depression and mood changes observed with isotretinoin therapy in some susceptible individuals.Citation241
As FoxO1 has been shown to be intimately involved in the regulation of brain metabolisms and brain ROS homeostasis, as well as to suppress the transcription of POMC gene, it is conceivable that isotretinoin exerts effects on the hypothalamic-pituitary-adrenal axis (HPA).Citation251,Citation252 Moreover, isotretinoin-induced apoptotic effects on hypothalamic cells may have downstream regulatory effects on the pituitary. Thus, a decrease in α-MSH and adrenocorticotropic hormone (ACTH) and other pituitary hormones may be expected during isotretinoin treatment. Intriguingly, Karadag et al. recently demonstrated that 3 months of isotretinoin treatment in 47 acne patients reduced free triiodothyronine (T3), thyroid-stimulating hormone (TSH), thyroid-stimulating hormone receptor antibody levels, luteinising hormone, prolactin and total testosterone, morning cortisol and ACTH.Citation257 In accordance with isotretinoin, central hypothyroidism is a well known adverse effect of the synthetic RXR-selective retinoid bexarotene, approved for the treatment of cutaneous T-cell lymphoma (CTCL).Citation258–Citation260 Bexarotene was found to cause severe central hypothyroidism with high frequency, associated with marked reductions in serum concentrations of thyroid-stimulating hormone (TSH) and thyroxine.Citation259 Bexarotene-induced apoptosis of CTCL cells has been hypothesiszed to be the major mode of action in CTCL.Citation261 Thus, isotretinoin- and bexarotenemediated effects on secretory cells of the HPA-axis may share a common proapototic mechanism of action. It is thus tempting to speculate that isotretinoin suppresses the HPA-axis. In this regard, recent insights into FoxOs role as controlling system of food intake and the regulation of the circadian clock are further arguments for FoxOs important contribution in CNS homeostasis, regulation of the circadian rhythm and pituitary hormone secretion ().Citation252,Citation262
Isotretinoin, FoxO-Upregulation and Teratogenicity
Isotretinoin is well known to exert teratogenic effects in laboratory animals and humans.Citation263 A characteristic pattern of malformations involving craniofacial, cardiac, thymic and central nervous system structures is seen in humans.Citation264–Citation266 Some of the most characteristic abnormalities include microtia, anotia, micrognathia, conotruncal heart defects and aortic arch abnormalities, thymic ectopia or aplasia, cerebellar vermis agenesis and various neuronal migration anomalies.Citation263
Can we construct a relationship between isotretinoin-mediated upregulation of FoxOs and retinoid-induced teratogenicity during early developmental steps of embryogenesis? FoxOs are expressed especially in adipose, brain, heart, liver, lung, ovary, pancreas, prostate, skeletal muscle, spleen, thymus and testis,Citation58,Citation91,Citation267 thus in those organs affected by isotretinoin teratogenicity. FoxO3a has been associated with the regulation of neuronal survival, vascular integrity, immune function and cellular metabolism and plays an important role in the brain.Citation268 During cardiac development, FoxO proteins appear to be necessary to modulate cardiomyocyte proliferation. Both FoxO1 and FoxO3a are expressed during embryonic development in the developing myocardium. The expression of these FoxO proteins is believed to negatively regulate cardiomyocyte growth, since overexpression of FoxO1 blocks cardiomyocyte proliferation.Citation269 There is accumulating evidence that FoxOs are pivotal regulatory transcription factors of progenitor cell development, thymic T-cell differentiation, T-cell homeostasis, angiogenesis, cardiovascular function and neuronal development and function.Citation269 Malformations other than in the CNS are thought to be due to isotretinoin's interference with migration and/or proliferation of the cranial neural crest cells, leading to deficient mesenchyme in the branchial arches. In animal studies, isotretinoin administration was associated with decreased proliferation rates and increased programmed cell death of neural crest cells ().Citation270,Citation271 Both neural crest and CNS cells expressed high levels of CRABPs during isotretinoin treatment, thus promoting CRABP-mediated proapoptotic signaling.Citation272,Citation273 Isotretinoin-induced FoxO-mediated apoptosis of neuronal crest cells is thus a conceivable mechanism for isotretinoin's teratogenic effect ().
Recently, it has been demonstrated that excess ATRA repressed blastula Wnt signaling and impaired dorsal development in Xenopus embryo.Citation274 ATRA promoted nuclear accumulation of β-catenin, although, surprisingly, Wnt signaling was repressed. The unexpected reduction in Wnt signaling was explained by the ability of liganded RAR to bind to β-catenin thereby inhibiting β-catenin binding with Tcf4. However, there is another reasonable explanation considering ATRA-mediated upregulation of FoxOs which may attract available nuclear β-catenin, thus impairing β-catenin/Tcf signaling ().Citation135 Wnt signaling is of crucial importance for osteoblastogenesis and will affect craniofacial morphogenesis.Citation122,Citation199,Citation200 Isotretinoin-induced diversion of β-catenin from Tcf- to FoxO-mediated transcription may explain the craniofacial and ear abnormalities of retinoid embryopathy. Taken together, an accumulating body of evidence allows the conclusion that overstimulated FoxO-mediated transcriptional regulation with consecutively impaired Wnt/β-catenin signaling may be a major signaling disturbance leading to isotretinoininduced teratogenicity.
FoxOs and the Chemopreventive Effect of Isotretinoin
Isotretinoin is used as an adjunct in the treatment of pediatric patients with neuroblastoma, acute promyelocytic leukemia and chemoprevention of high-risk patients with non-melanoma skin cancer.Citation275–Citation279 Isomerization of the “prodrug” isotretinoin to ATRA is proposed to be of importance for isotretinoin's superior antitumor activity in neurobalstoma in comparison to treatments with ATRA.Citation280 There is recent evidence that isotretinoin induces gene expression and apoptosis in cancer cell which clearly resemble the transcriptional activity of FoxO proteins. For example, isotretinoin mediates apoptosis in Dalton's lymphoma ascites cells by regulating gene expression with upregulation of caspase-3 and downregulation of bcl-2 expression.Citation281 Similarly, in B16F-10 melanoma cells isotretinoin induces apoptosis and upregulates caspase-3, the tumor suppressor p53 and downregulates bcl-2.Citation282 Gene and protein expression profiling during differentiation of neuroblastoma cells triggered by isotretinoin exhibited a down-regulation of N-myc, cyclin D3 and Wnt10B.Citation283
Is the chemopreventive effect of retinoids in certain tumors related to their ability to induce increased expression of FoxO proteins which are known to lead to apoptosis and block cell cycle progression?Citation11,Citation14,Citation15,Citation80 For example, FoxO3a and FoxO4 can promote cell cycle arrest in mouse myoblastic cell lines through modulation of growth arrest and DNA-damage-response protein 45 (GADD45).Citation284,Citation285 Other work suggests that FoxO proteins utilize the p53 upstream regulator p19(Arf) through myc to block cell cycle induction and lymphoma progression.Citation286 In cell cultures, overexpression of FoxO1 and FoxO3a in prostrate tumor cell lines also leads to apoptosis, suggesting that FoxO1 and FoxO3a are necessary for limiting prostate cell tumor growth.Citation287 In addition, it has been shown that inhibition of FoxO3a activity can result in enhanced prostate tumor cell growth while agents that increase FoxO3a activity in both androgen sensitive and androgen insensitive prostate cancer cell lines prevent prostate cancer cell progression.Citation288 Moreover, it has been shown that astrocyte-elevated gene-1 (AEG-1) can be upregulated in clinical prostate cancer.Citation145 This possibly leads to activation of Akt that suppresses FoxO3a and inhibits apoptosis in prostate tumor cells.Citation289 FoxO proteins can function as redundant repressors of tumor growth. For example, somatic deletion in mice of Foxo1, Foxo3a and Foxo4 results in the growth of thymic lymphomas and hemangiomas.Citation290 In addition, the loss of FoxO3a activity may participate in oncogenic transformation in B-chronic lymphocytic leukemia and in the progression of chronic myelogenous leukemia cell line.Citation291,Citation292 Furthermore, studies suggest that some proteins, such as the Kaposi's sarcoma-associated herpes virus latent protein LANA2, may specifically block the transcriptional activity of FoxO3a to lead to tumor growth.Citation293 In cell models of endometrial cancer, pre-sensitization of cells to block Akt activation and foster transcription activity of FoxO1 enhances the effect of chemotherapy to limit tumor growth.Citation294 It has recently been recognized that ATRA increased the expression of transcription factor FoxO3a in neuroblastoma cells.Citation9 FoxO3a has also been identified as a key regulator for ATRA-induced granulocytic differentiation and apoptosis in acute promyelocytic leukemia.Citation10
Isotretinoin-mediated FoxO signaling just reverses the proposed growth factor/PI3K/Akt pathway of acne which leads to a nuclear deficiency of FoxO proteins.Citation13 As most acneigenic stimuli of acne-associated syndromes with increased growth factor-, insulin-, IGF-1- and FGF-signaling converge in the activation of PI3K/Akt, they are more likely to exert nuclear FoxO deficiencies associated with a higher incidence of cancer.Citation295 Indeed, an epidemiological association between long-lasting acne and prostate carcinoma has been established.Citation296 Chronically upregulated PI3K/Akt signaling might further explain the increased incidence of cancer in patients with other acne-associated diseases like acromegaly,Citation297 polycystic ovary syndrome,Citation298 syndromes with insulin resistance with consecutive hyperinsulinemia,Citation12 and Apert syndrome with increased FGF-signaling.Citation299–Citation303 In this regard, persistent acne in adulthood should be recognized as a serious clinical indicator of dysbalanced growth factor signaling with reduced levels of nuclear FoxOs, an unfavorable condition which increases mitogenic stimulation and cell survival but reduces apoptosis, well-recognized processes in cancer promotion.Citation267
Together, substantial evidence exists for the anti-cancer activity of FoxO transcription factors. Isotretinoin appears to confer its chemopreventive activity by upregulation of FoxO-controlled target genes inducing apoptosis and cell death.Citation267
Conclusion and Future Perspectives
In the beginning of the retinoid research era, isotretinoin's mechanism of action was explained only by modulations of CREBP expression and ATRA/RAR interactions. Today, we begin to understand that retinoids exert most important effects on gene regulatory level by inducing secondary responses due to upregulation of further transcription factors including the FoxO family of transcription factors inducing consecutive molecular crosstalk with other signaling systems like Wnt/β-catenin signaling.Citation8 We have learnt that retinoids provide an essential, early signal that initiates a cascade of events leading to changes in proliferation, differentiation and predominantly apoptosis affecting most CRABP-2 expressing somatic cells as well as the stem cell compartments.Citation8 We have to appreciate that isotretinoin does not “exclusively” targets apoptosis of the sebaceous glands as proapoptotic drug effects have been observed in several unrelated cell systems and explain all adverse effects of isotretinoin and other retinoids (). The asthonishing functional overlap of changes in FoxO-mediated gene transcription and isotretinoinmediated gene transcription () strongly suggests that isotretinoin and its isomerization product ATRA induces upregulation of FoxO-signaling and exerts apoptotic effects in multiple cell types like the muscle, the bone and the brain.
In fact, all isotretinoin-mediated effects on sebocyte apoptosis, sebaceous lipogenesis, anti-inflammatory activity, downregulation of ROS can be explained by upregulation of nuclear levels of FoxO transcription factors. All isotretinoin-induced adverse effect on hepatic glucose and lipid metabolism, retinoid-induced dyslipoproteinemia, loss of bone density, myotoxic effects, mucocutaneous side effects, adverse psychiatric effects, chemopreventive effects and isotretinoin's teratogenicity appear to result from a common mechanism, i.e., FoxO-mediated changes of gene expression. In contrast, increased insulin/IGF-1 signaling of puberty and western diet due to high glycemic load and consumption of insulinotropic milk and milk products downregulates nuclear FoxO levels and thus promotes the development of acne.Citation13,Citation20,Citation21,Citation304 Similar effects are mediated by insulin resistance with consecutive hyperinsulinemia observed in most acne-associated syndromes like polycystic ovary syndrome, HAIRAN-syndrome, congenital adrenal hyperplasia and others recently reviewed elsewhere in reference Citation12. Increased FGF-signaling due to a gain-of-function mutation of FGFR2-downstream signaling in Apert syndrome and acneiform nevus may decrease nuclear levels of FoxO as well.Citation302,Citation305,Citation306 It is thus not surprising that acne in Apert syndrome and acneiform nevus respond very well to isotretinoin treatment,Citation306–Citation309 which counteracts increased FGF/FGFR2-signaling with concomitant depletion of nuclear FoxO levels.Citation305 Thus, isotretinoin corrects through upregulation of FoxOs the relative FoxO deficiency of acne and other acne-associated conditions with increased growth factor singnaling, which all converge in PI3K/Akt-mediated nuclear extrusion of FoxO proteins.Citation295
After more than 30 years of clinicial use of isotretinoin, we are at the beginning to understand isotretinoin's fundamental mode of action at the level of transcriptional regulation of FoxO transcription factors. Using the powerful tool of translational research we are able to understand the complex pathophysiology of acne and retinoid biology.
Abbreviations
| AMP | = | antimicrobial peptide |
| Akt | = | Akt kinase (protein kinase B) |
| Apo CIII | = | apolipoprotein C-III |
| AR | = | androgen receptor |
| Atg1 | = | atrogin 1 (F-box protein 32) |
| ATRA | = | all-trans-retinoic acid |
| CDK | = | cyclin-dependent kinase |
| C/EBPα | = | CCAAT/enhancer binding protein-α |
| CBP | = | CREB (cAMP response element-binding) |
| CNS | = | central nerve system |
| CPK | = | creatine phosphokinase |
| CRABP2 | = | cellular retinoic acid binding protein-2 |
| Cpe | = | carboxypeptidase E |
| DBD | = | DNA binding domain |
| DHT | = | dihydrotestosterone |
| ETC | = | electron transport chain |
| FA | = | fatty acid |
| FABP5 | = | fatty acid binding protein-5 |
| FGF | = | fibroblast growth factor |
| FGFR2 | = | fibroblast growth factor receptor-2 |
| FoxO | = | forkhead box O |
| GSK3β | = | glycogen synthase kinase 3β |
| Hmox1 | = | heme oxigenase 1 |
| IGF-1 | = | insulin-like growth factor-1 |
| IGFBP | = | IGF binding protein |
| IGF1R | = | IGF-1 receptor |
| IκB | = | inhibitor of NFκB |
| IκK | = | IκB kinase |
| IL | = | interleukin |
| IRAK | = | IL-1 receptor associated kinases |
| Isotretinoin | = | 13-cis-retinoic acid |
| Lef1 | = | lymphoid enhancer binding factor-1 |
| LPL | = | lipoprotein lipase |
| LRP5/6 | = | low density receptor-related proteins 5/6 |
| LXR | = | liver X receptor |
| α-MSH | = | α-melanocyte stimulating hormone |
| MnSOD | = | manganese superoxide dismutase |
| MMP | = | matrix metalloproteinase |
| MTP | = | microsomal triglyceride transfer protein |
| MuRF1 | = | muscle-specific RING finger protein 1 |
| NGAL | = | neutrophil gelatinase-associated lipocalin |
| NFκB | = | nuclear factor κB |
| PDK1 | = | 3-phosphoinositide-dependent kinase 1 |
| PDK | = | pyruvate dehydrogenase kinase-4 |
| PEPCK | = | phosphoenolpyruvate carboxykinase |
| PI3K | = | phosphoinositol 3-kinase |
| PML | = | promyelocytic leukemia |
| PMN | = | polymorphonuclear neutrophils |
| POMC | = | proopiomelanocortin |
| PPARγ | = | peroxisome proliferator-activated receptor-γ |
| PPRE | = | PPAR response elements |
| RAR | = | retinoic acid receptor |
| ROS | = | reactive oxygen species |
| RXR | = | retinoid X receptor |
| SREBP-1c | = | sterol-regulatory-element-binding protein 1c |
| TAD | = | transcription activation domain |
| Tcf | = | T cell factor |
| TIMP | = | tissue inhibitor of metalloproteinase |
| TG | = | triglyceride |
| TLR | = | toll-like receptor |
| TNFα | = | tumor necrosis factor-α |
| VDR | = | vitamin D receptor |
| VLDL | = | very-low-density lipoprotein |
| Wnt | = | wingless protein |
Figures and Tables
Figure 1 (A) Nuclear exclusion of FoxO proteins into the cytoplasm by growth factor signaling due to Akt kinase-mediated phosphorylation of nuclear FoxO proteins. (B) Isotretinoin-mediated upregulation of FoxO expression as a secondary response of proapoptotic RAR-signaling. FoxO-regulated genes are switched on. IGF-1, insulin-like growth factor-1; FGFs, fibroblast growth factors; PI3K, phosphoinositol-3 kinase; Akt, Akt kinase (protein kinase B); FoxO, forkhead box class O transcription factor; ATRA, all-trans-retinoic acid; CRABP2, cellular retinoic acid binding protein-2; RAR, retinoic acid receptor.
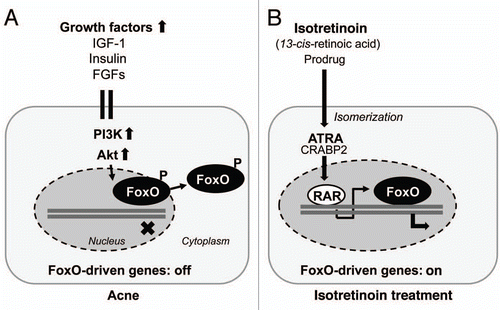
Figure 2 (A) FoxO1-mediated suppression of androgen-receptor (AR) by FoxO1-binding to the AR transcription activation domain (TAD) thereby inhibiting N-/C-terminal interaction of AR resulting in reduced AR transactivation. (B) Direct FoxO1-mediated suppression of PPARγ-regulated target genes. (C) FoxO1-mediated suppression of the PPARγ promoter reducing PPARγ expression. (D) FoxO1-mediated suppression of the SREBP-1c promoter reducing SREBP-1c expression, the key transcription factor of most lipogenic enzymes. DBD, DNA binding domain; DHT, dihydrotestosterone; RXR, retinoid X receptor; PPARγ, peroxisome proliferator-activated receptor-γ.
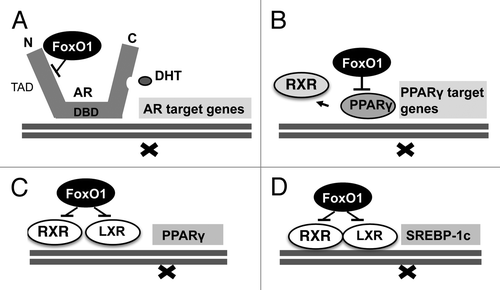
Figure 3 FoxO-induced G1/S arrest of the cell cycle. Isotretinoin-mediated upregulation of cell cycle inhibitors p21 and p27 by FoxO binding to their promoters. Growth factor-mediated nuclear export of FoxO proteins with consecutive downregualtion of p21, p27 and p130. ATRA, all-trans-retinoic acid; Akt, Akt kinase; PI3K, phosphoinositol-3 kinase; IGF-1, insulin-like growth factor-1.
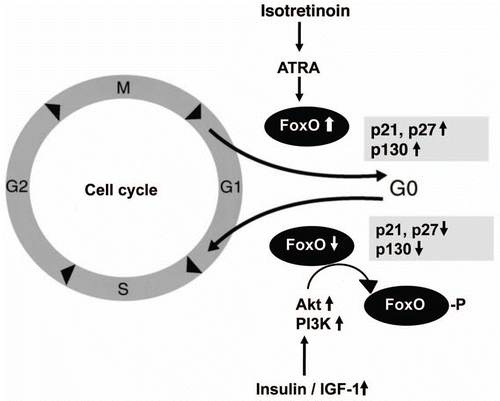
Figure 4 Isotretinoin/FoxO1-mediated upregulation of hepatic gene expression at the promoter level: upregulation of phosphoenolpyruvate carboxykinase (PEPCK) results in gluconeogenesis; upregulation of apolipoprotein C-III (Apo CIII) inhibits the activity of lipoprotein lipase (LPL); upregulation of microsomal triglyceride transfer protein (MTP) leads to increased production and secretion of very low density lipoproteins (VLDL). Increased expression of heme oxigenase 1 (Hmox1) results in cytochrome degradation and mitochondrial damage with impaired β-oxidation of fatty acids (FAs) leading to increased formation of hepatic triglycerides (TG).
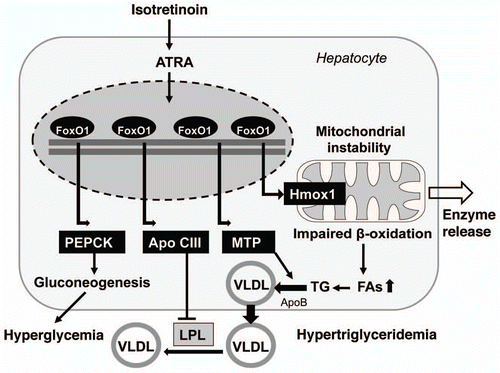
Figure 5 Isotretinoin-mediated overexpression of FoxO proteins and divergence of β-cateinin signaling from Lef1/Tcf-induced transcription by increased binding of β-catenin to nuclear FoxO proteins. ATRA, all-trans-retinoic acid; CRABP2, cellular retinoic acid binding protein-2; Wnts, Wingless proteins; LRP5/6, low density receptor-related proteins 5/6; Frizzled, Wnt receptor Frizzled; β, β-catenin; Lef1, lymphoid enhancer-binding factor-1; Tcf, T cell factor.
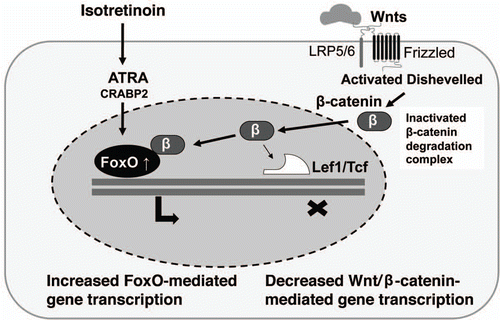
Figure 6 Isotretinoin's effect on muscle homeostasis is mediated by FoxO1-driven upregulation of atrogin-1 (Atg1) and muscle-specific RING finger protein-1 (MuRF1) which both induce autophagy-related protein degradation with muscle loss and release of creatine phosphokinase (CPK).
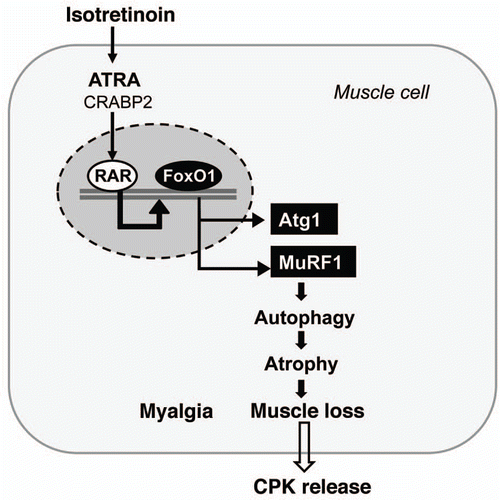
Figure 7 Isotretinoin's effect on the CNS is mediated by FoxO1 upregulation. in the hypothalamus FoxO1 inhibits neurogenesis associated with the risk of mood changes. FoxO1 suppresses the expression of proopiomelanocortin (POMC) and carboxypeptidase E (Cpe). This results in reduced formation of α-melanocyte stimulating hormone (α-MSH) and general suppression of the hypothalamic-pituitary-axis (HPA) with decreased pituitary hormone secretion.
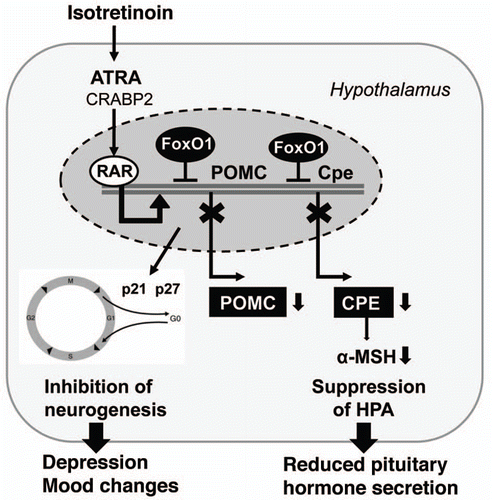
Figure 8 Isotretinoin-induced and FoxO-mediated apoptosis. FoxO1-induced overexpression of heme oxigenase-1 disrupt the electron transport chain (ETC) with increased release of cytochrome c inducing the intrinsic mitochondrial pathway of apoptosis. Formation of the apoptosome activates caspase 9 which finally activates the excutive caspase 3. The pathway explains isotretinoin's teratogenic effects when neuronal crest and CNS cells are affected during embryonic development.
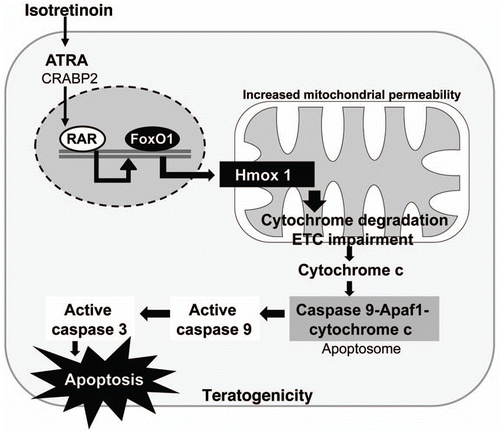
Table 1 Overlapping gene regulatory functions of FoxO proteins and isotretinoin
Table 2 Comparison of retinoid-induced apoptosis in various cell types
References
- Peck GL, Olsen TG, Yoder FM, Strauss JS, Downing DT, Pandya M, et al. Prolonged remissions of cystic acne with 13-cis-retinoic acid. N Engl J Med 1979; 300:329 - 333
- Ganceviciene R, Zouboulis CC. Isotretinoin: state of the art treatment for acne vulgaris. Expert Rev Dermatol 2007; 2:693 - 701
- Layton A. The use of isotretinoin in acne. Dermatoendocrinol 2009; 1:162 - 169
- David M, Hodak E, Lowe NJ. Adverse effects of retinoids. Med Toxicol Adverse Drug Exp 1988; 3:273 - 288
- Tsukada M, Schröder M, Roos TC, Chandraratna RA, Reichert U, Merk HF, et al. 13-cis retinoic acid exerts its specific activity on human sebocytes through selective intracellular isomerization to all-trans retinoic acid and binding to retinoid acid receptors. J Invest Dermatol 2000; 115:321 - 327
- Sitzmann JH, Bauer FW, Cunliffe WJ, Holland DB, Lemotte PK. In situ hybridization analysis of CRABP II expression in sebaceous follicles from 13-cis retinoic acid-treated acne patients. Br J Dermatol 1995; 133:241 - 248
- Zouboulis CC. Isotretinoin revisited: Pluripotent effects on human sebaceous gland cells. J Invest Dermatol 2006; 126:2154 - 2156
- Gudas LJ, Wagner JA. Retinoids regulate stem cell differentiation. J Cell Physiol 2010; 226:322 - 330
- Kim MJ, Ahn K, Park SH, Kang HJ, Jang BG, Oh SJ, et al. SIRT1 regulates tyrosine hydroxylase expression and differentiation of neuroblastoma cells via FOXO3a. FEBS Lett 2009; 583:1183 - 1188
- Sakoe Y, Sakoe K, Kirito K, Ozawa K, Komatsu N. FOXO3A as a key molecule for all-trans retinoic acid-induced granulocytic differentiation and apoptosis in acute promyelocytic leukemia. Blood 2010; 115:3787 - 3795
- Essaghir A, Dif N, Marbehant CY, Coffer PJ, Demoulin JB. The transcription of FOXO genes is stimulated by FOXO3 and repressed by growth factors. J Biol Chem 2009; 284:10334 - 10342
- Chen W, Obermayer-Pietsch B, Hong JB, Melnik B, Yamasaki O, Dessinioti C, et al. Acne-associated syndromes: models for better understanding of acne pathogenesis. J Eur Acad Dermatol Venereol 2011; 25:637 - 646
- Melnik BC. FoxO1—the key for the pathogenesis and therapy of acne?. J Dtsch Dermatol Ges 2010; 8:105 - 114
- Huang H, Tindall DJ. Dynamic FoxO transcription factors. J Cell Sci 2007; 120:2479 - 2487
- Van der Heide LP, Hoekman MF, Smid MP. The ins and outs of FoxO shuttling: mechanisms of FoxO translocation and transcriptional regulation. Biochem J 2004; 380:297 - 309
- Peng SL. Forkhead transcription factors in chronic inflammation. Int J Biochem Cell Biol 2009; 42:482 - 485
- Ouyang W, Beckett O, Flavell RA, Li Mo. An essential role of the Forkhead-box transcription factor Foxo1 in control of T cell homeostasis and tolerance. Immunity 2009; 30:358 - 371
- van der Vos KE, Coffer PJ. The extending network of FOXO transcriptional genes. Antioxid Redox Signal 2011; 14:579 - 592
- Cheng Z, White MF. Targeting forkhead boxO1 from the concept to metabolic diseases: lessons from mouse models. Antioxid Redox Signal 2011; 14:649 - 661
- Melnik B. Milk consumption: aggravating factor of acne and promoter of chronic diseases of western societies. J Dtsch Dermatol Ges 2009; 7:364 - 370
- Melnik BC, Schmitz G. Role of insulin, insulin-like growth factor-1, hyperglycaemic food and milk consumption in the pathogenesis of acne vulgaris. Exp Dermatol 2009; 18:833 - 841
- Smith R, Mann N, Braue A, Mäkeläinen H, Varigos GA. The effect of a high protein, low glycemic load diet versus a conventional, high glycemic load diet on biochemical parameters associated with acne vulgaris: a randomized, investigator-masked, controlled trial. J Am Acad Dermatol 2007; 57:247 - 256
- Smith R, Mann N, Mäkeläinen H, Roper J, Braue A, Varigos G. A pilot study to determine the short-term effects of a low glycemic load diet on hormonal markers of acne: a nonrandomized, parallel, controlled feeding trial. Mol Nutr Food Res 2008; 52:718 - 726
- Choudhry R, Hodgins MB, Van der Kwast TH, Brinkmann AO, Boersma WJ. Localization of androgen receptors in human skin by immunohistochemistry: implications for the hormonal regulation of hair growth, sebaceous glands and sweat glands. J Endocrinol 1992; 133:467 - 475
- Pelletier G, Ren L. Localization of sex steroid receptors in human skin. Histol Histopathol 2004; 19:629 - 636
- Rosignoli C, Nicolas JC, Jomard A, Michel S. Involvement of the SREBP pathway in the mode of action of androgens in sebaceous glands in vivo. Exp Dermatol 2003; 12:480 - 489
- Imperato-McGinley J, Gautier T, Cai LQ, Yee B, Epstein J, Pochi P. The androgen control of sebum production. Studies of subjects with dihydrotestosterone deficiency and complete androgen insensitivity. J Clin Endocrinol Metab 1993; 76:524 - 528
- Schmidt JB, Spona J, Huber J. Androgen receptor in hisutism and acne. Gynecol Obest Invest 1986; 22:206 - 211
- Li J, Al-Azzawi F. Mechanism of androgen receptor action. Maturitas 2009; 63:142 - 148
- He B, Minges JT, Lee LW, Wilson EM. The FXXLF motif mediates androgen receptor-specific interactions with coregulators. J Biol Chem 2002; 277:10226 - 10235
- Heemers HV, Tindall DJ. Androgen receptor (AR) coregulators: a diversity of functions converging on and regulating the AR transcriptional complex. Endocr Rev 2007; 28:778 - 808
- Ma Q, Fu W, Li P, Nicosia SV, Jenster G, Zhang X, et al. FoxO1 mediates PTEN suppression of androgen receptor N- and C-terminal interactions and coactivator recruitment. Mol Endocrinol 2009; 23:213 - 225
- Yanase T, Fan WQ. Modification of androgen receptor function by IGF-1 signaling: implications in the mechanism of refractory prostate carcinoma. Vit Horm 2009; 80:649 - 666
- Fan W, Yanase T, Morinaga H, Okabe T, Nomura M, Daitoku H, et al. Insulin-like growth factor 1/insulin signaling activates androgen signaling through direct interactions of Foxo1 with androgen receptor. J Biol Chem 2007; 282:7329 - 7338
- Nantermet P, Xu J, Yu Y, Hodor P, Holder D, Adamski S, et al. Identification of genetic pathways activated by the androgen receptor during the induction of proliferation in the ventral prostate gland. J Biol Chem 2004; 279:1310 - 1322
- Karadag AS, Ertugrul DT, Tutal E, Akin KO. Short-term isotretinoin treatment decreases insulin-like growth factor-1 and insulin-like growth factor binding protein-3 levels: does isotretinoin affect growth hormone physiology?. Br J Dermatol 2010; 162:798 - 802
- Boudou P, Soliman H, Chivot M, Villette JM, Vexiau P, Belanger A, et al. Effect of oral isotretinoin treatment on skin androgen receptor levels in male acneic patients. J Clin Endocinol Metab 1995; 80:1158 - 1161
- Horton R, Pasupuletti V, Antonipillai I. Androgen induction of 5α-reductase may be mediated via insulin-like growth factor-I. Endocrinology 1993; 133:447 - 451
- Boudou P, Chivot M, Vexiau P, Soliman H, Villette JM, Julien R, et al. Evidence for decreased androgen 5α-reduction in skin and liver of men with severe acne after 13-cis retinoic acid treatment. J Clin Endocr Metab 1994; 78:1064 - 1069
- Hembree JR, Harmon CS, Nevins TD, Eckert RL. Regulation of human dermal papilla cell production of insulin-like growth factor binding protein-3 by retinoic acid, glucocorticoids and insulin-like growth factor-1. J Cell Physiol 1996; 167:556 - 561
- Collier CN, Harper JC, Cafardi JA, Cantrell WC, Wang W, Foster KW, et al. The prevalence of acne in adults 20 years and older. J Am Acad Dermatol 2008; 58:56 - 59
- Dréno B. Recent data on epidemiology of acne. Ann Dermatol Venereol 2010; 137:49 - 51
- Walton S, Wyatt EH, Cunliffe WJ. Genetic control of sebum excretion and acne—a twin study. Br J Dermatol 1988; 118:393 - 396
- Stewart ME, Grahek MO, Cambier LS, Wertz PW, Downing DT. Dilutional effect of increased sebaceous gland activity on the proportion of linoleic acid in sebaceous wax esters and in epidermal acylceramides. J Invest Dermatol 1986; 87:733 - 736
- Bataille V, Snieder H, MacGregor AJ, Sasieni P, Spector TD. The influence of genetics and environmental factors in the pathogenesis of acne: A twin study of acne in women. J Invest Dermatol 2002; 119:1317 - 1322
- Ballanger F, Baudry P, N'Guyen JM, Khammari A, Dréno B, et al. Heredity: A prognostic factor for acne. Dermatology 2006; 212:145 - 149
- Sawaya ME, Shalita AR. Androgen receptor polymorphisms (CAG repeat lengths) in androgenetic alopecia, hirsutism and acne. J Cutan Med Surg 1998; 3:9 - 15
- Pang Y, He CD, Liu Y, Wang KB, Xiao T, Wang YK. Combination of short CAG and GGN repeats in the androgen receptor gene is associated with acne risk in North East China. JEADV 2008; 22:1445 - 1451
- Yang Z, Cheng B, Tang W, Tang W, Dong Y, Xiao C. Relationship between the CAG repeat polymorphism in the androgen receptor gene and acne in the Han ethnic group. Dermatology 2009; 218:302 - 306
- Pinsky L, Beitel LK, Trifiro MA. Scriver CR, Beaudet AL, Sly WS, et al. Spinobulbar muscular atrophy. The Metabolic & Molecular Bases of Inherited Disease 2001; 3:New York McGraw-Hill 4147 - 4157 Eights, Ed.
- Landthaler M, Kummermehr J, Wagner A, Plewig G. Inhibitory effects of 13-cis-retinoic acid on human sebaceous glands. Arch Dermatol Res 1980; 269:297 - 309
- Orfanos CE, Zouboulis CC, Almond Roesler B, Geilen CC. Current use and future potential role of retinoids in dermatology. Drugs 1997; 53:358 - 388
- Melnik B, Kinner T, Plewig G. Influence of oral isotretinoin treatment on the composition of comedonal lipids. Implications for comedogenesis in acne vulgaris. Arch Dermatol Res 1988; 280:97 - 102
- Zouboulis CC, Krieter A, Gollnick H, Orfanos CE. Progressive differentiation of human sebocytes in vitro is characterized by increased cell size and altered antigenic expression and is regulated by culture duration and retinoids. Exp Dermatol 1994; 3:151 - 160
- Zouboulis CC, Korge B, Akamatsu H, Xia L, Schiller S, Gollnick H, et al. Effects of 13-cis-retinoic acid, all-trans-retinoic acid and acitretin on the proliferation, lipid synthesis and keratin expression of cultured human sebocytes in vitro. J Invest Dermatol 1991; 96:792 - 797
- Nelson AM, Zhao W, Gilliland KL, Zaenglein AL, Liu W, Thiboutot DM. Temporal changes in gene expression in the skin of patients treated with isotretinoin provide insight into its mechanism of action. Dermatoendocrinol 2009; 1:177 - 187
- Nelson AM, Zhao W, Gilliland KL, Zaenglein AL, Liu W, Thiboutot DM. Isotretinoin temporally regulates distinct sets of genes in patient skin. J Invest Dermatol 2009; 129:1038 - 1042
- Nakae J, Oki M, Cao Y. The FoxO transcription factors and metabolic regulation. FEBS Lett 2008; 582:54 - 67
- Akamatsu H, Zouboulis CC, Orfanos CE. Control of human sebocyte proliferation in vitro by testosterone and 5-alpha-dihydrotestosterone is dependent on the localization of the sebaceous glands. J Invest Dermatol 1992; 99:509 - 511
- Chen W, Yang CC, Sheu HM, Seltmann H, Zouboulis CC. Expression of peroxisome proliferator-activated receptor and CCAAT/enhancer binding protein transcription factors in cultured human sebocytes. J Invest Dermatol 2003; 121:441 - 447
- Makrantonaki E, Zouboulis CC. Testosterone metabolism to 5α-dihydrotestosterone and synthesis of sebaceous lipids is regulated by the peroxisome proliferator-activated receptor ligand linoleic acid in human sebocytes. Br J Dermatol 2007; 156:428 - 432
- Zhang Q, Seltmann H, Zouboulis CC, Konger RL. Involvement of PPARgamma in oxidative stress-mediated prostaglandin E(2) production in SZ95 human sebaceous gland cells. J Invest Dermatol 2006; 126:42 - 48
- Trivedi NR, Cong Z, Nelson AM, Albert AJ, Rosamilia LL, Sivarajah S, et al. Peroxisome proliferator-activated receptors increase human sebum production. J Invest Dermatol 2006; 126:2002 - 2009
- Armoni M, Harel C, Karni S, Chen H, Bar-Yoseph F, Ver MR, et al. FOXO1 represses peroxisome proliferator-activated receptor-gamma1 and -gamma2 gene promoters in primary adipocytes. A novel paradigm to increase insulin sensitivity. J Biol Chem 2006; 281:19881 - 19891
- Dowell P, Otto TC, Adi S, lane MD. Convergence of peroxisome proliferator-activated receptor gamma and Foxo1 signaling pathways. J Biol Chem 2003; 278:45485 - 45491
- Fan WQ, Imamura T, Sonoda N, Sears DD, Patsouris D, Kim JJ, et al. FOXO1 transrepresses peroxisome proliferator-activated receptor γ transactivation, coordinating an insulin-induced feed-forward response in adipocytes. J Biol Chem 2009; 284:12188 - 12197
- Vora S, Ovhal A, Jerajani H, Nair N, Chakrabortty A. Correlation of facial sebum to serum insulin-like growth factor-1 in patients with acne. Br J Dermatol 2008; 159:990 - 991
- Hong I, Lee MH, Na TY, Zouboulis CC, Lee MO. LXRalpha enhances lipid synthesis in SZ95 sebocytes. J Invest Dermatol 2008; 128:1266 - 1272
- Schultz JR, Tu H, Luk A, Repa JJ, Medina JC, Li L, et al. Role of LXRs in control of lipogenesis. Genes Dev 2000; 14:2831 - 2838
- Seo JB, Moon HM, Kim WS, Lee YS, Jeong HW, Yoo EJ, et al. Activated liver X receptors stimulate adipocyte differentiation through induction of peroxisome proliferator-activated receptor gamma expression. Mol Cell Biol 2004; 24:3430 - 3444
- Harrison WJ, Bull JJ, Seltmann H, Zouboulis CC, Philpott MP. Expression of lipogenic factors galectin-12, resistin, SREBP-1 and SCD in human sebaceous glands and cultured sebocytes. J Invest Dermatol 2007; 127:1309 - 1317
- Smith TM, Gilliland K, Clawson GA, Thiboutot D. IGF-1 induces SREBP-1 expression and lipogenesis in SEB-1 sebocytes via activation of the phosphoinosit-ide-3-kinase/Akt pathway. J Invest Dermatol 2008; 128:1286 - 1293
- Kamei Y, Miura S, Suganami T, Akaike F, Kanai S, Sugita S, et al. Regulation of SREBP1c gene expression in skeletal muscle: role of retinoid X receptor/liver X receptor and forkhead-O1 transcription factor. Endocrinology 2008; 149:2293 - 2305
- Zouboulis CC, Orfanos CE. Millikan LE. Retinoids. Drug Therapy in Dermatology 2000; New York/Basel Marcel Dekker 171 - 233
- Kim MJ, Ciletti N, Michel S, Reichert U, Rosenfield RL. The role of specific retinoid receptors in sebocyte growth and differentiation in culture. J Invest Dermatol 2000; 114:349 - 353
- Nelson AM, Gilliland KL, Cong Z, Thiboutot DM. 13-cis Retinoic acid induces apoptosis and cell cycle arrest in human SEB-1 sebocytes. J Invest Dermatol 2006; 126:2178 - 2189
- Zouboulis CC, Seltmann H, Neitzel H, Orfanos CE. Establishment and characterization of an immortalized human sebaceous gland cell line (SZ95). J Invest Dermatol 1999; 113:1011 - 1020
- Nelson AM, Zhao W, Gilliland KL, Zaenglein AL, Liu W, Thiboutot DM. Early gene changes induced by isotretinoin in the skin provide clues to its mechanism of action. Dermatoendocrinol 2009; 1:100 - 101
- Grana X, Garriga J, Mayol X. Role of the retinoblastoma protein family, pRB, p107 and p130 in the negative control of cell growth. Oncogene 1998; 17:3365 - 3383
- Kops GJ, Medema RH, Glassford J, Essers MA, Dijkers PF, Coffer PJ, et al. Control of cell cycle exit and entry by protein kinase B-regulated forkhead transcription factors. Mol Cell Biol 2002; 22:2025 - 2203
- Sitzmann JH, Bauer FW, Cunliffe WJ, Holland DB, Lemotte P. In situ hybridization analysis of CRABP II expression in sebaceous follicles from 13-cis retinoic acid-treated acne patients. Br J Dermatol 1995; 133:241 - 248
- Noy N. Between death and survival: retinoic acid in regulation of apoptosis. Ann Rev Nutr 2010; 30:201 - 217
- Battle TE, Roberson MS, Zhang T, Varvayanis S, Yen A. Retinoic acid-induced blr1 expression requires RARalpha RXR and MAPK activation and uses ERK2 but not JNK/SAPK to accelerate cell differentiation. Eur J Cell Biol 2001; 80:59 - 67
- Wróbel A, Seltmann H, Fimmel S, Müller-Decker K, Tsukada M, Bogdanoff B, et al. Differentiation and apoptosis in human immortalized sebocytes. J Invest Dermatol 2003; 120:175 - 181
- Nelson AM, Zhao W, Gilliland KL, Zaenglein AL, Liu W, Thiboutot M. Neutrophil gelatinase-associated lipocalin mediates 13-cis retinoic acid-induced apoptosis of human sebaceous gland cells. J Clin Invest 2008; 118:1468 - 1478
- Liu B, Lee HY, Weinzimer SA, Powelli DR, Clifford JL, Kurie JM, et al. Direct functional interactions between insulin-like growth factor-binding protein-3 and retinoid X receptor-α regulate transcriptional signaling and apoptosis. J Biol Chem 2000; 275:33607 - 33613
- Lee KW, Cohen P. Nuclear effects: unexpected intracellular actions of insulin-like growth factor binding protein-3. J Endocrinol 2002; 175:33 - 40
- Becker T, Loch G, Beyer M, Zinke I, Aschenbrenner AC, Carrera1 P, et al. FOXO-dependent regulation of innate immune homeostasis. Nature 2010; 463:369 - 373
- Edmondson SR, Thumiger SP, Kaur P, Loh B, Koelmeyer R, Li A, et al. Insulin-like growth factor binding protein-3 (IGFBP-3) localizes to and modulates proliferative epidermal keratinocytes in vivo. Br J Dermatol 2005; 152:225 - 230
- Plewig G, Fulton JE, Kligman AM. Cellular dynamics of comedo formation in acne vulgaris. Arch Dermatol Forsch 1971; 242:12 - 29
- Ikezoe T, Tanosaki S, Krug U, Liu B, Cohen P, Taguchi H, et al. Insulin-like growth factor binding protein-3 antagonizes the effects of retinoids in myeloid leukemia cells. Blood 2004; 104:237 - 242
- Oh Y, Gucev Z, Ng L, Müller HL, Rosenfeld RG. Antiproliferative actions of insulin-like growth factor binding protein (IGFBP)-3 in human breast cancer cells. Prog Growth Factor Res 1995; 6:503 - 512
- Niemann C. Differentiation of the sebaceous gland. Dermatoendocrinol 2009; 1:64 - 67
- Arnold I, Watt FM. c-Myc activation in transgenic mouse epidermis results in mobilization of stem cells and differentiation of their progeny. Curr Biol 2001; 11:558 - 568
- Waikel RL, Kawachi Y, Waikel PA, Wang XJ, Roop DR. Deregulated expression of c-myc depletes epidermal stem cells. Nat Genet 2001; 28:165 - 168
- Braun KM, Niemann C, Jensen UB, Sundberg JP, Watt FM. Manipulation of stem cell proliferation and lineage commitment in mouse epidermis: Visualisation of label-retaining cells in whole mounts of mouse epidermis. Development 2003; 130:5241 - 5255
- He TC, Sparks AB, Rago C, Hermeking H, Zawel L, da Costa LT, et al. Identification of c-MYC as a target of the APC pathway. Science 1998; 281:1509 - 1512
- Clevers H. Wnt/beta-catenin signalling in development and disease. Cell 2006; 127:469 - 480
- Lo Celso C, Berta MA, Braun KM, Frye M, Lyle S, Zouboulis CC, Watt FM. Characterization of bipotent epidermal progenitors derived from human sebaceous gland: Contrasting roles of c-myc and β-catenin. Stem Cells 2008; 26:1241 - 1252
- Braun KM, Niemann C, Jensen UB, Sundberg JP, Silva-Vargas V, Watt FM. Manipulation of stem cell proliferation and lineage commitment: visualisation of label retaining cells in whole mounts of mouse epidermis. Development 2003; 130:5241 - 5255
- Horsley V, O'Carroll D, Tooze R, Ohinata Y, Saitou M, Obukhanych T, et al. Blimp1 defines a progenitor population that governs cellular input to the sebaceous gland. Cell 2006; 126:597 - 609
- Sellheyer K, Krahl D. Blimp-1: a marker of terminal differentiation but not of sebocytic progenitor cells. J Cutan Pathol 2010; 37:362 - 370
- Tothova Z, Gilliland DG. FoxO transcription factors and stem cell homeostasis: insights from the hematopoietic system. Stem Cell 2007; 1:140 - 152
- Delpuech O, Griffiths B, East P, Essafi A, Lam EW, Burgering B, et al. Induction of Mxi1-SRalpha by FOXO3a contributes to repression of Myc-dependent gene expression. Mol Cell Biol 2007; 27:4917 - 4930
- Chandramohan V, Jeay S, Pianetti S, Sonenshein GE. Reciprocal control of forkhead box O 3a and c-myc via the phosphatidylinositol-3-kinase pathway coordinately regulates p27Kip1 levels. J Immunol 2004; 172:5522 - 5527
- Dijkers PF, Medema RH, Pals C, Banerji L, Thomas NS, Lam EW, et al. Forkhead transcription factor FKHR-L1 modulates cytokine-dependent transcriptional regulation of p27(KIP1). Mol Cell Biol 2000; 20:9138 - 9148
- Sherr CJ, Roberts JM. CDK inhibitors: positive and negative regulators of G1-phase progression. Genes Dev 1999; 13:1501 - 1512
- Medema RH, Kops GJ, Bos JL, Burgering BM. AFX-like Forkhead transcription factors mediate cell cycle regulation by Ras and PKB through p27kip1. Nature 2000; 404:782 - 787
- Nakamura N, Ramaswamy S, Vazquez F, Signoretti S, Loda M, Sellers WR. Forkhead transcription factors are critical effectors of cell death and cell cycle arrest downstream of PTEN. Mol Cell Biol 2000; 20:8969 - 8982
- Burgering BM, Kops GJ. Cell cycle and death control: long live Forkheads. Trends Biochem Sci 2002; 27:352 - 360
- Furukawa-Hibi Y, Kobayashi Y, Chen C, Motoyama N. FOXO transcription factors in cell cycle regulation and the response to oxidative stress. Antioxid Redox Signal 2005; 7:752 - 760
- Martinez-Gac L, Marques M, Garcia Z, Campanero MR, Carrera AC. Control of cyclin G2 mRNA expression by forkhead transcription factors: novel mechanism for cell cycle control by phosphoinositide-3-kinase and forkhead. Mol Cell Biol 2004; 24:2181 - 2189
- Nowak JA, Polak L, Pasolli HA, Fuchs E. Hair follicle stem cells are specified and function in early skin morphogenesis. Stem Cell 2008; 3:33 - 43
- Kormish JD, Sinner D, Zorn AM. Interactions between SOX factors and Wnt/β-catenin signaling in development and disease. Dev Dynamics 2010; 239:56 - 68
- Bastide P, Darido C, Pannequin J, Kist R, Robine S, Marty-Double C, et al. Sox9 regulates cell proliferation and is required for Paneth cell differentiation in the intestinal epithelium. J Cell Biol 2007; 178:635 - 648
- Blache P, van de Wetering M, Duluc I, Domon C, Berta P, Freund JN, et al. SOX9 is an intestine crypt transcription factor, is regulated by the Wnt pathway and represses the CDX2 and MUC2 genes. J Cell Biol 2004; 166:37 - 47
- Merrill BJ, Gat U, DasGupta R, Fuchs E. Tcf3 and Lef1 regulate lineage differentiation of multipotent stem cells in skin. Genes Dev 2001; 15:1688 - 1705
- Niemann C, Owens DM, Hülsken J, Birchmeier W, Watt FM. Expression of ΔNLef1 in mouse epidermis results in differentiation of hair follicle keratinocytes into squamous epidermal cysts and formation of skin tumours. Development 2002; 129:95 - 109
- Takeda H, Lyle S, Lazar AFJ, Zouboulis CC, Smyth I, Watt FM. Human sebaceous tumors harbour inactivating mutations in Lef1. Nat Med 2006; 12:395 - 397
- Han G, Li AG, Liang YY, Owens P, He W, Lu S, et al. Smad7-induced β-catenin degradation alters epidermal appendage development. Dev Cell 2006; 11:301 - 312
- Quan T, He T, Kang S, Voorhees JJ, Fisher GJ. Ultraviolet irradiation alters transforming growth factor beta/smad pathway in human skin in vivo. J Invest Dermatol 2002; 119:499 - 506
- Manolagas SC, Almeida M. Gone with the Wnts: beta-catenin, T-cell factor, forkhead box O and oxidative stress in age-dependent diseases of bone, lipid and glucose metabolism. Mol Endocrinol 2007; 21:2605 - 2614
- Beildeck ME, Gelmann EP, Byers SW. Cross-regulation of signaling pathways: an example of nuclear hormone receptors and the canonical Wnt pathway. Exp Cell Res 2010; 316:1763 - 1772
- Easwaran V, Pishvaian M, Salimuddin, Byers S. Cross-regulation of beta-catenin-LEF/TCF and retinoid signaling pathways. Curr Biol 1999; 9:1415 - 1418
- Mulholland DJ, Dedhar S, Coetzee GA, Nelson CC. Interaction of nuclear receptors with the Wnt/betacatenin/Tcf signaling axis: Wnt you like to know?. Endocr Rev 2005; 26:898 - 915
- Chesire DR, Isaacs WB. Ligand-dependent inhibition of beta-catenin/TCF signaling by androgen receptor. Oncogene 2002; 21:8453 - 8469
- Song LN, Herrell R, Byers S, Shah S, Wilson EM. Gelmann EP Beta-catenin binds to the activation function 2 region of the androgen receptor and modulates the effects of the N-terminal domain and TIF2 on ligand-dependent transcription. Mol Cell Biol 2003; 23:1674 - 1687
- Mulholland DJ, Read JT, Rennie PS, Cox ME, Nelson CC. Functional localization and competition between the androgen receptor and T-cell factor for nuclear beta-catenin: a means for inhibition of the Tcf signaling axis. Oncogene 2003; 22:5602 - 5613
- Ross SE, Hemati N, Longo KA, Bennett CN, Lucas PC, Erickson RL, et al. Inhibition of adipogenesis by Wnt signaling. Science 2000; 289:950 - 953
- Chen W, Yang CC, Sheu HM, Seltmann H, Zouboulis CC. Expression of peroxisome proliferator-activated receptor and CCA AT/enhancer binding protein transcription factors in cultured human sebocytes. J Invest Dermatol 2003; 121:441 - 447
- Nakae J, Kitamura T, Kitamura Y, Biggs WH 3rd, Arden KC, Accili D. The forkhead transcription factor Foxo1 regulates adipocyte differentiation. Dev Cell 2003; 4:119 - 129
- Gerin I, Bommer GT, Lidell ME, Cederberg A, Enerback S, Macdougald OA. On the role of FOX transcription factors in adipocyte differentiation and insulin-stimulated glucose uptake. J Biol Chem 2009; 284:10755 - 10763
- Armoni M, Harel C, Karni S, Chen H, Bar-Yoseph F, Ver MR, Quon MJ, et al. FOXO1 represses peroxisome proliferator-activated receptor-gamma1 and -gamma2 gene promoters in primary adipocytes. A novel paradigm to increase insulin sensitivity. J Biol Chem 2006; 281:19881 - 19891
- Onai T, Lin HC, Schubert M, Koop D, Osborne PW, Alvarez S, Alvarez R, et al. Retinoic acid and Wnt/betacatenin have complementary roles in anterior/posterior patterning embryos of the basal chordate amphioxus. Dev Biol 2009; 332:223 - 233
- Essers MA, de Vries-Smits LM, Barker N, Polderman PE, Burgering BM, Korswagen HC. Functional interaction between beta-catenin and FOXO in oxidative stress signaling. Science 2005; 308:1181 - 1184
- Jin T, Fantus GI, Sun J. Wnt and beyond Wnt: multiple mechanisms control the transcriptional property of β-catenin. Cell Signal 2008; 20:1697 - 1704
- Hoogeboom D, Essers MAG, Polderman PE, Voets E, Smits LMM, Burgering BMT. Interaction of FOXO with β-catenin inhibits β-catenin/T cell factor activity. J Biol Chem 2008; 283:9224 - 9230
- Kang S, Cho S, Chung JH, Hammerberg C, Fisher GJ, Voorhees JJ. Inflammation and extracellular matrix degradation mediated by activated transcription factors nuclear factor κB and activator protein-1 in inflammatory acne lesions in in vivo. Am J Pathol 2005; 166:1691 - 1699
- Papakonstantinou E, Aletras AJ, Glass E, Tsogas P, Dionyssopoulos A, Adjaye J, et al. Matrix metalloproteinases of epithelial origin in facial sebum of patients with acne and their regulation by isotretinoin. J Invest Dermatol 2005; 125:673 - 684
- Tanaka H, Murakami Y, Ishii I, Nakata S. Involvement of a forkhead transcription factor, FOXO1A, in UV-induced changes of collagen metabolism. J Invest Dermatol Symp Proc 2009; 14:60 - 62
- Leyden JJ. Treatment of photodamaged skin with topical tretinoin: an update. Plast Reconstr Surg 1998; 102:1667 - 1671
- Singh M, Griffiths CE. The use of retinoids in the treatment of photoaging. Dermatol Ther 2006; 19:297 - 305
- Abid MR, Shih SC, Otu HH, Spokes KC, Okada Y, Curiel DT, et al. A novel class of vascular endothelial growth factor-responsive genes that require forkhead activity for expression. J Biol Chem 2006; 281:35544 - 35553
- Ganapathy S, Chen Q, Singh KP, Shankar S, Srivastava RK. Resveratrol enhances antitumor activity of TRAIL in prostate cancer xenografts through activation of FOXO transcription factor. PLoS One 2010; 5:15627
- Kikuno N, Shiina H, Urakami S, Kawamoto K, Hirata H, Tanaka Y, et al. Knockdown of astrocyte-elevated gene-1 inhibits prostate cancer progression through upregulation of FOXO3a activity. Oncogene 2007; 26:7647 - 7655
- Li H, Liang J, Castrillon DH, DePinho RA, Olson EN, Liu ZP. FoxO4 regulates tumor necrosis factor alpha-directed smooth muscle cell migration by activating matrix metalloproteinase 9 gene transcription. Mol Cell Biol 2007; 27:2676 - 2686
- Salminen A, Kaarniranta K. Insulin/IGF-1 paradox of aging: regulation via AKT/IKK/NFκB signaling. Cell Signal 2009; 22:573 - 577
- Perkins ND. Integrating cell-signalling pathways with NFkappaB and IKK function. Nat Rev Moll Cell Biol 2007; 8:49 - 62
- Vallabhapurapu S, Karin M. Regulation and function of NFκB transcription factors in the immune system. Annu Rev Immunol 2009; 27:693 - 733
- Jugeau S, Tenaud I, Knol AC, Jarrousse V, Quereux G, Khammari A, et al. Induction of toll-like receptors by Propionibacterium acnes. Br J Dermatol 2005; 153:1109 - 1113
- Kim J, Ochoa MT, Krutzik SR, Takeuchi O, Uematsu S, Legaspi AJ, et al. Activation of toll-like receptor 2 in acne triggers inflammatory cytokine responses. J Immunol 2002; 169:1535 - 1541
- Nagy I, Pivarcsi A, Koreck A, Széll M, Urbán E, Kemény L. Distinct strains of Propionibacterium acnes indcuce selective human beta-defensin-2 and interleukin-8 expression in human keratinocytes through toll-like receptors. J Invest Dermatol 2005; 124:931 - 939
- Kim J. Review of the innate immune response in acne vulgaris: Activation of toll-like receptor 2 in acne triggers inflammatory cytokine responses. Dermatology 2005; 211:193 - 198
- Arbibe L, Mira JP, Teusch N, Kline L, Guha M, Mackman N, et al. Toll-like receptor 2-mediated NFkappaB activation requires a Rac1-dependent pathway. Nat Immunol 2000; 1:533 - 540
- Li X, Jiang S, Tapping RI. Toll-like receptor signaling in cell proliferation and survival. Cytokine 2010; 49:1 - 9
- Laird MH, Rhee SH, Perkins DJ, Medvedev AE, Piao W, Fenton MJ, et al. TLR4/MyD88/PI3K interactions regulate TLR4 signaling. J Leukoc Biol 2009; 85:966 - 977
- Oeff MK, Seltmann H, Hiroi N, Nastos A, Makrantonaki E, Bornstein SR, et al. Differential regulation of Toll-like receptor and CD14 pathways by retinoids and corticosteroids in human sebocytes. Dermatology 2006; 213:266
- Iinuma K, Sato T, Akimoto N, Noguchi N, Sasatsu M, Nishijima S, et al. Involvement of Propionibacterium acnes in the augmentation of lipogenesis in hamster sebaceous glands in vivo and in vitro. J Invest Dermatol 2009; 129:2113 - 2119
- Dejean AS, Hedrick SM, Kerdiles YM. Highly specialized role of Foxo transcription factors in the immune system. Antioxid Redox Signal 2011; 14:663 - 674
- Gan L, Li L. Regulations and roles of the interleukin-1 receptor associated kinases (IRAKs) in innate and adaptive immunity. Immunol Res 2006; 35:295 - 302
- Liu PT, Krutzik SR, Kim J, Modlin RL. Cutting edge: all-trans retinoic acid downregulates TLR2 expression and function. J Immunol 2005; 174:2467 - 2470
- Jeremy AH, Holland DB, Roberts SG, Thomson KF, Cunliffe WJ. Inflammatory events are involved in acne lesion initiation. J Invest Dermatol 2003; 121:20 - 27
- Kerdiles YM, Beisner DR, Tinoco R, Dejean AS, Castrillon DH, DePinho RA, et al. Foxo1 links homing and survival of naive T cells by regulating L-selectin, CCR7 and interleukin 7 receptor. Nat Immunol 2009; 10:176 - 184
- Akamatsu H, Horio T. The possible role of reactive oxygen species generated by neutrophils in mediating acne inflammation. Dermatology 1998; 196:82 - 85
- Akamatsu H, Horio T, Hattori K. Increased hydrogen peroxide generation by neutrophils from patients with acne inflammation. Int J Dermatol 2003; 42:366 - 369
- Kurutas EB, Arican O, Sasmaz S. Superoxide dismutase and myeloperoxidase activities in polymorphonuclear leukocytes in acne vulgaris. Acta Dermatovenerol Alp Panonica Adriat 2005; 14:39 - 42
- Sarici G, Cinar S, Armutcu F, Altinyazar C, Koca R, Tekin NS. Oxidative stress in acne vulgaris. J Eur Acad Dermatol Venereol 2010; 24:763 - 767
- Arican O, Kurutas EB, Sasmaz S. Oxidative stress in patients with acne vulgaris. Mediators Inflamm 2005; 2005:380 - 384
- Bohne M, Struy H, Gerber A, Gollnick H. Effects of retinoids on the generation of neutrophil-derived reactive oxygen species studied by EPR spin trapping techniques. Inflamm Res 1997; 46:423 - 424
- Balaban RS, Nemoto S, Finkel T. Mitochondria, oxidants and aging. Cell 2005; 120:483 - 495
- Kops GJ, Dansen TB, Polderman PE, Saarloos I, Wirtz KW, Coffer PJ, et al. Forkhead transcription factor FOXO3a protects quiescent cells from oxidative stress. Nature 2002; 419:316 - 321
- Nemoto S, Finkel T. Redox regulation of forkhead proteins through a p66shc-dependent signaling pathway. Science 2002; 295:2450 - 2452
- Cheng Z, Guo S, Copps K, Dong X, Kollipara R, Rodgers JT, et al. Foxo1 integrates insulin signaling with mitochondrial function in the liver. Nat Med 2009; 15:1307 - 1311
- Penniston KL, Tanumihardjo SA. The acute and chronic toxic effects of vitamin A. Am J Clin Nutr 2006; 83:191 - 201
- Zane LT, Leyden WA, Marqueling AL, Manos MM. A population-based analysis of laboratory abnormalities during isotretinoin therapy for acne vulgaris. Arch Dermatol 2006; 142:1016 - 1022
- Heliövaara MK, Remitz A, Reitamo S, Teppo AM, Karonen SL, Ebeling P. 13-cis-Retinoic acid therapy induces insulin resistance, regulates inflammatory parameters and paradoxically increases serum adiponectin concentration. Metabolism 2007; 56:786 - 791
- Koistinen HA, Remitz A, Gylling H, Miettinen TA, Koivisto VA, Ebeling P. Dyslipidemia and a reversible decrease in insulin sensitivity induced by therapy with 13-cis-retinoic acid. Diabetes Metab Res Rev 2001; 17:391 - 395
- Guo S, Copps KD, Dong X, Park S, Cheng Z, Pocai A, et al. The Irs1 branch of the insulin signaling cascade plays a dominant role in hepatic nutrient homeostasis. Mol Cell Biol 2009; 29:5070 - 5083
- Cheng Z, White MF. Foxo1 in hepatic lipid metabolism. Cell Cycle 2010; 9:219 - 220
- Matsumoto M, Han S, Kitamura T, Accili D. Dual role of transcription factor FoxO1 in controlling hepatic insulin sensitivity and lipid metabolism. J Clin Invest 2006; 116:2464 - 2472
- Converso DP, Taille C, Carreras MC, Jaitovich A, Poderoso JJ, Boczkowski J. HO-1 is located in liver mitochondria and modulates mitochondrial heme content and metabolism. FASEB J 2006; 20:1236 - 1238
- Schalinske KL, Steele RD. 13-cis-retinoic acid and hepatic steatosis in rats. Biochem Pharmacol 1993; 46:319 - 325
- Rigobello MP, Scutari G, Friso A, Barzon E, Artusi S, Bindoli A. Mitochondrial permeability transition and release of cytochrome c induced by retinoic acids. Biochem Pharmacol 1999; 58:665 - 670
- Bershad S, Rubinstein A, Paterniti JR, Le NA, Poliak SC, Heller B, et al. Changes in plasma lipids and lipoproteins during isotretinoin therapy for acne. N Engl J Med 1985; 313:981 - 985
- Melnik BC, Bros U, Plewig G. Evaluation of the atherogenic risk of isotretinoin-induced and etretinate-induced alterations of lipoprotein cholesterol metabolism. J Invest Dermatol 1987; 88:39 - 43
- Melnik B, Bros U, Plewig G. Characterization of apoprotein metabolism and atherogenic lipoproteins during oral isotretinoin treatment. Dermatologica 1987; 175:158 - 168
- Kamagate A, Qu S, Perdomo G, Su D, Kim DH, Slusher S, et al. FoxO1 mediates insulin-dependent regulation of hepatic VLDL production in mice. J Clin Invest 2008; 118:2347 - 2364
- Kamagate A, Dong HH. FoxO1 intergrates insulin signaling to VLDL production. Cell Cycle 2008; 7:3162 - 3170
- Wang CS, McConathy WJ, Kloer HJ, Alaupovic P. Modulation of lipoprotein lipase activity by apolipoproteins: effect of apolipoprotein C-III. J Clin Invest 1985; 75:384 - 390
- Vu-Dac N, Gervois P, Torra IP, Fruchart JC, Kosykh V, Kooistra T, et al. Retinoids increase human apo C-III expression at the transcriptional level via the retinoid X receptor. Contribution to the hypertriglyceridemic action of retinoids. J Clin Invest 1998; 102:625 - 632
- Altomonte J, Cong L, Harbaran S, Richter A, Xu J, Meseck M, et al. Foxo1 mediates insulin action on apo C-III and triglyceride metabolism. J Clin Invest 2004; 114:1493 - 1503
- McGuire J, Lawson JP. Skeletal changes associated with chronic isotretinoin and etretinate administration. Dermatologica 1987; 175:169 - 181
- Melnik B, Plewig G. Unwanted bone changes in systemic treatment with synthetic retinoids. Hautarzt 1987; 38:193 - 197
- Leachman SA, Insogna KL, Katz L, Ellison A, Milstone LM. Bone densities in patients receiving isotretinoin for cystic acne. Arch Dermatol 1999; 135:961 - 965
- Tekin NS, Ozdolap S, Sarikaya S, Keskin SI. Bone mineral density and bone turnover markers in patients receiving a single course of isotretinoin for nodulocystic acne. Int J Dermatol 2008; 47:622 - 625
- Ling TC, Parkin G, Islam J, Seukeran DC, Cunliffe WJ. What is the cumulative effect of long-term, low dose isotretinoin on the development of DISH?. Br J Dermatol 2001; 144:630 - 632
- Hotchkiss CE, Latendresse J, Ferguson SA. Oral treatment with retinoic acid decreases bone mass in rats. Comp Med 2006; 56:502 - 511
- DiGiovanna JJ. Isotretinoin effects on bone. J Am Acad Dermatol 2001; 45:176 - 182
- Kolpakova E, Olsen BR. Wnt/β-catenin-a canonical tale of cell-fate choice in the vertebrate skeleton. Dev Cell 2005; 8:626 - 627
- Hartmann C. A Wnt canon orchestrating osteoblastogenesis. Trends Cell Biol 2006; 16:151 - 158
- Rosen ED, MacDougald OA. Adipocyte differentiation from the inside out. Nat Rev Mol Cell Biol 2006; 7:885 - 896
- Almeida M, Han L, Martin-Millan M, O'Brien CA, Manolagas SC. Oxidative stress antagonizes WNT signaling in osteoblast precursors by diverting β-catenin from T cell factor- to Forkhead box O-mediated transcription. J Biol Chem 2007; 282:27298 - 27305
- Yasuhara R, Yuasa T, Williams JA, Byers SW, Shah S, Pacifici M, et al. Wnt/β-catenin and retinoic acid receptor signaling pathways interact to regulate chondrocyte function and matrix turnover. J Biol Chem 2010; 285:317 - 327
- Kaymak Y. Creatine phosphokinase values during isotretinoin treatment for acne. Int J Dermatol 2008; 47:398 - 401
- Heudes AM, Laroche L. Muscular damage during isotretinoin treatment. Ann Dermatol Venereol 1998; 125:94 - 97
- Chroni E, Monastirli A, Tsambaos D. Neuromuscular adverse effects associated with systemic retinoid dermatotherapy: monitoring and treatment algorithm for clinicians. Drug Saf 2010; 33:25 - 34
- Chiba T, Kamei Y, Shimizu T, Shirasawa T, Katsumata A, Shiraishi L, et al. Overexpression of FOXO1 in skeletal muscle does not alter longevity in mice. Mech Ageing Dev 2009; 130:420 - 428
- Furuyama T, Kitayama K, Yamashita H, Mori N. Forkhead transcription factor FOXO1 (FKHR)-dependent induction of PDK4 gene expression in skeletal muscle during energy deprivation. Biochem J 2003; 375:365 - 371
- Kamei Y, Mizukami J, Miura S, Suzuki M, Takahashi N, Kawada T, et al. A forkhead transcription factor FKHR upregulates lipoprotein lipase expression in skeletal muscle. Growth Regul 2003; 536:232 - 236
- Glass DJ. Signalling pathways that mediate skeletal muscle hypertrophy and atrophy. Nat Cell Biol 2003; 5:87 - 90
- Sandri M. Signaling in muscle atrophy and hypertrophy. Physiology (Bethesda) 2008; 23:160 - 170
- Crossland H, Constantin-Teodosiu D, Gardiner SM, Constantin D, Greenhaff PL. A potential role for Akt/FOXO signalling in both protein loss and the impairment of muscle carbohydrate oxidation during sepsis in rodent skeletal muscle. J Physiol 2008; 586:5589 - 5600
- Mammucari C, Milan G, Romanello V, Masiero E, Rudolf R, Del PP, et al. FoxO3 controls autophagy in skeletal muscle in vivo. Cell Metab 2007; 6:458 - 471
- Masiero E, Agatea L, Mammucari C, Blaauw B, Loro E, Komatsu M, et al. Autophagy is required to maintain muscle mass. Cell Metab 2009; 10:507 - 515
- Sandri M, Sandri C, Gilbert A, Skurk C, Calabria E, Picard A, et al. Foxo transcription factors induce the atrophy related ubiquitin ligase atrogin-1 and cause skeletal muscle atrophy. Cell 2004; 117:399 - 412
- Zhao J, Brault JJ, Schild A, Cao P, Sandri M, Schiaffino S, et al. FoxO3 coordinately activates protein degradation by the autophagic/lysosomal and proteasomal pathways in atrophying muscle cells. Cell Metab 2007; 6:472 - 483
- Stitt TN, Drujan D, Clarke BA, Panaro F, Timofeyva Y, Kline WO, et al. The IGF-1/PI3K/Akt pathway prevents expression of muscle atrophy-induced ubiquitin ligases by inhibiting FOXO transcription factors. Mol Cell 2004; 14:395 - 403
- Kamei Y, Miura S, Suzuki M, Kai Y, Mizukami J, Taniguchi T, et al. Skeletal muscle FOXO1 (FKHR) transgenic mice have less skeletal muscle mass, down-regulated type I (slow twitch/red muscle) fiber genes and impaired glycemic control. J Biol Chem 2004; 279:41114 - 41123
- Kamei Y, Miura S, Suganami T, Akaike F, Kanai S, Sugita S, et al. Regulation of SREBP1c gene expression in skeletal muscle: role of retinoid X receptor/liver X receptor and forkhead-O1 transcription factor. Endocrinology 2008; 149:2293 - 2305
- Bastie CC, Nahle Z, McLoughlin T, Esser K, Zhang W, Unterman T, et al. FoxO1 stimulates fatty acid uptake and oxidation in muscle cells through CD36-dependent and -independent mechanisms. J Biol Chem 2005; 280:14222 - 14229
- Shalita AR. Mucocutaneous and systemic toxicity of retinoids: monitoring and management. Dermatologica 1987; 175:151 - 157
- Elias PM, Fritsch PO, Lampe M, Williams ML, Brown BE, Nemanic M, et al. Retinoid effects on epidermal structure, differentiation and permeability. Lab Invest 1981; 44:531 - 540
- Elias PM. Retinoid effects on the epidermis. Dermatologica 1987; 175:28 - 36
- Eichner R. Epidermal effects of retinoids: in vitro studies. J Am Acad Dermatol 1986; 15:789 - 797
- Lee DD, Stojadinovic O, Krzyzanowska A, Vouthounis C, Blumenberg M, Tomic-Canic M. Retinoid-responsive transcriptional changes in epidermal keratinocytes. J Cell Physiol 2009; 220:427 - 439
- Elias PM. Stratum corneum defensive functions: an integrated view. J Invest Dermatol 2005; 125:183 - 200
- Melnik B. Disturbances of antimicrobial lipids in atopic dermatitis. J Dtsch Dermatol Ges 2006; 4:114 - 123
- Brown MS, Goldstein JL. Sterol regulatory element binding proteins (SREBPs): controllers of lipid synthesis and cellular uptake. Nutr Rev 1998; 56:1 - 3
- Smith JR, Osborne TF, Brown MS, Goldstein JL, Gil G. Multiple sterol regulatory elements in promoter for hamster 3-hydroxy-3-methylglutaryl-coenzyme A synthase. J Biol Chem 1988; 263:18480 - 18487
- Vallett SM, Sanchez HB, Rosenfeld JM, Osborne TF. A direct role for sterol regulatory element binding protein in activation of 3-hydroxy-3-methyl-glutaryl coenzyme A reductase gene. J Biol Chem 1996; 271:12247 - 12253
- Harris IR, Farrell AM, Holleran WM, Jackson S, Grunfeld C, Elias PM, et al. Parallel regulation of sterol regulatory element binding protein-2 and the enzymes of cholesterol and fatty acid synthesis but not ceramide synthesis in cultured human keratinocytes and murine epidermis. J Lipid Res 1998; 39:412 - 422
- Narce M, Poisson JP. Lipid metabolism. Regulation of lipid metabolism gene expression by peroxisome proliferator-activated receptor alpha and sterol regulatory element binding proteins. Curr Opin Lipidol 2002; 13:445 - 447
- Holleran WM, Feingold KR, Man MQ, Gao WN, Lee JM, Elias PM. Regulation of epidermal sphingolipid synthesis by permeability barrier function. J Lipid Res 1991; 32:1151 - 1158
- Kremer I, Gaton DD, David M, Gaton E, Shapiro A. Toxic effects of systemic retinoids on meibomian glands. Ophthalmic Res 1994; 26:124 - 128
- Lambert RW, Smith RE. Effects of 13-cis-retinoic acid on the hamster meibomian gland. J Invest Dermatol 1989; 92:321 - 325
- Lambert RW, Smith RE. Pathogenesis of blepharoconjunctivitis complicating 13-cis-retinoic acid (isotretinoin) therapy in a laboratory model. Invest Ophthalmol Vis Sci 1988; 29:1559 - 1564
- Heilgemeir GP, Braun-Falco O, Plewig G, Sund M. Effect of 13-cis-retinoic acid on hair growth. Hautarzt 1982; 33:533 - 536
- Williams D, Siock P, Stenn K. 13-cis-Retinoic acid affect sheath-shaft interaction of equine hair follicles in vitro. J Invest Dermatol 1996; 106:356 - 361
- Foitzik K, Spexard T, Nakamura M, Halsner U, Paus R. Towards dissecting the pathogenesis of retinoidinduced hair loss: all-trans retinoic acid induces premature hair follicle regression (catagen) by upregulation of transforming growth factor-beta2 in the dermal papilla. J Invest Dermatol 2005; 124:1119 - 1126
- Ouji Y, Yoshikawa M, Moriya K, Ishizaka S. Effects of Wnt-10b on hair shaft growth in hair follicle cultures. Biochem Biophys Res Commun 2007; 359:516 - 522
- Kontaxakis VP, Skourides D, Ferentinos P, Havaki-Kontaxaki BJ, Papadimitriou GN. Isotretinoin and psychopathology: a review. Ann Gen Psych 2009; 8:2
- O'Reilly KC, Shumake J, Gonzalez-Lima F, Lane MA, Bailey SJ. Chronic administration of 13-cis-retinoic acid increases depression-related behavior in mice. Neuropsychopharmacol 2006; 31:1919 - 1927
- Zetterstrom RH, Lindqvist E, Mata de Urquiza A. Role of the retinoids in the CNS: differential expression of retinoid binding proteins and receptors and evidence for presence of retinoic acid. Eur J Neurosci 1999; 11:407 - 416
- Krezel W, Kastner P, Chambon P. Differential expression of retinoid receptors in the adult mouse central nervous system. Neuroscience 1999; 89:1291 - 1300
- Duman RS. Depression: a case of neuronal life and death?. Biol Psychiatry 2004; 56:140 - 145
- Sapolsky RM. Depression, antidepressants and the shrinking hippocampus. Proc Natl Acad Sci USA 2001; 98:12320 - 12322
- Sakai Y, Crandall JE, Brodsky J, McCaffery P. 13-cis retinoic acid (Accutane) suppresses hippocampal cell survival in mice. Ann NY Acad Sci 2004; 1021:436 - 440
- Crandall JE, Sakai Y, Zhang J, Koul O, Mineur Y, Crusio WE, McCaffery P. 13-cis retinoic acid suppresses hippocampal cell division and hippocampal-dependent learning in mice. Proc Nat Acad Sci USA 2004; 101:5111 - 5116
- Griffin JN, Pinali D, Olds K, Lu N, Appleby L, Doan L, et al. 13-Cis-retinoic acid decreases hypothalamic cell number in vitro. Neurosci Res 2010; 68:185 - 190
- Hoekman MF, Jacobs FM, Smidt MP, Burbach JP. Spatial and temporal expression of FoxO transcription factors in the developing and adult murine brain. Gene Expr Patterns 2006; 6:134 - 140
- Kim MS, Pak YK, Jang PG, Namkoong C, Choi YS, Won JC, et al. Role of hypothalamic Foxo1 in the regulation of food intake and energy homeostasis. Nat Neurosci 2006; 9:901 - 906
- Sasaki T, Kitamura T. Roles of FoxO1 and Sirt1 in the central regulation of food Intake. Endocr J 2010; 57:939 - 946
- Huang Q, Tatro JB. Alpha-melanocyte stimulating hormone suppresses intracerebral tumor necrosis factor-alpha and interleukin-1beta gene expression following transient cerebral ischemia in mice. Neurosci Lett 2002; 334:186 - 190
- Zhang L, Anthonavage M, Huang Q, Li WH, Eisinger M. Proopiomelanocortin peptides and sebogenesis. Ann NY Acad Sci 2003; 994:154 - 161
- Mao Z, Liu L, Zhang R, Li X. Lithium reduces FoxO3a transcriptional activity by decreasing its intracellular content. Biol Psychiatry 2007; 62:1423 - 1430
- Polter A, Yang S, Zmijewska AA, van Groen T, Paik JH, Depinho RA, et al. Forkhead box, class O transcription factors in brain: regulation and behavioral manifestation. Biol Psychiatry 2009; 65:150 - 159
- Karadag AS, Ertugrul DT, Tutal E, Akin KO. Isotretinoin influences pituitary hormone levels in acne patients. Acta Derm Venereol 2011; 91:31 - 34
- Martin C, Bach-Ngohou K, Perrin B, Masson D. Central hypothyroidism associated with bexarotene therapy. Ann Biol Clin (Paris) 2006; 64:331 - 334
- Sherman SI. Etiology, diagnosis and treatment recommendations for central hypothyroidism associated with bexarotene therapy for cutaneous T-cell lymphoma. Clin Lymphoma 2003; 3:249 - 252
- Sherman SI, Gopal J, Haugen BR, Chiu AC, Whaley K, Nowlakha P, Duvic M. Central hypothyroidism associated with retinoid X receptor-selective ligands. N Engl J Med 1999; 340:1075 - 1079
- Zhang C, Hazarika P, Ni X, Weidner DA, Duvic M. Induction of apoptosis by bexarotene in cutaneous T-cell lymphoma cells: relevance to mechanism of therapeutic action. Clin Cancer Res 2002; 8:1234 - 1240
- Zheng X, Yang Z, Yue Z, Alvarez JD, Sehgal A. FOXO and insulin signaling regulate sensitivity of the circadian clock to oxidative stress. Proc Natl Acad Sci USA 2007; 104:15899 - 15904
- Coberly S, Lammer E, Alashari M. Retinoic acid embryopathy: Case report and review of literature. Pediatr Pathol Lab Med 1996; 16:823 - 836
- Lammer EJ, Chen DT, Hoar RM, Agnish ND, Benke PJ, Braun JT, et al. Retinoic acid embryopathy. N Engl J Med 1985; 313:837 - 841
- Lynburg MC, Khoury MJ, Lammer EJ, Waller KO, Codero JF, Erickson JD. Sensitivity, specificity and positive predictive value of malformations in isotretinoin embryopathy. Teratology 1990; 42:513 - 519
- Fernhoff PM, Lammer EJ. Craniofacial features of isotretinoin embryopathy. J Pediatr 1984; 5:595 - 597
- Maiese K, Chong ZZ, Shang YC, Hou J. Clever cancer strategies with FoxO transcription factors. Cell Cycle 2008; 7:3829 - 3839
- Maiese K, Chong ZZ, Shang YC. “SLY AS A FOXO”: New paths with forkhead signaling in the brain. Curr Neurovasc Res 2007; 4:295 - 302
- Evans-Anderson HJ, Alfieri CM, Yutzey KE. Regulation of cardiomyocyte proliferation and myocardial growth during development by FOXO transcription factors. Circ Res 2008; 102:686 - 694
- Johnston MC, Bronsky PT. Animal models for human craniofacial malformations. J Craniofac Genet Dev Biol 1991; 11:227 - 291
- Watanabe T, Goulding EH, Pratt RM. Alteration in craniofacial growth induced by isotretinoin (13-cis retinoic acid) in mouse whole embryo and primary mesenchymal cell culture. J Craniofac Genet Dev Biol 1988; 8:21 - 33
- Lammer EJ, Armstrong DL. Morriss-Kay G. Malformations in hindbrain structures among humans exposed to isotretinoin (13-cis-retinoic acid) during early embryogenesis. Retinoids in Normal Development and Teratogenesis 1991; New York Oxford University Press 281 - 295
- Dencker L, Gustafson AL, Annerwall E, Busch C, Erickson U. Retinoid-binding proteins in craniofacial development. J Craniofac Genet Dev Biol 1991; 11:303 - 314
- Li S, Lou X, Wang J, Liu B, Ma L, Su Z, et al. Retinoid signaling can repress blastula Wnt signaling and impair dorsal development in Xenopus embryo. Differentiation 2008; 76:897 - 907
- Matthay KK, Reynolds CP, Seeger RC, Shimada H, Adkins ES, Haas-Kogan D, et al. Long-term results for children with high-risk neuroblastoma treated on a randomized trial of myeloablative therapy followed by 13-cis-retinoic acid: a children's oncology group study. J Clin Oncol 2009; 27:1007 - 1013
- Veal G, Rowbotham S, Boddy A. Pharmacokinetics and pharmacogenetics of 13-cis-retinoic acid in the treatment of neuroblastoma. Therapy 2007; 62:91 - 93
- Campbell RM, DiGiovanna JJ. Skin cancer chemoprevention with systemic retinoids: an adjunct in the management of selected high-risk patients. Dermatol Ther 2006; 19:306 - 314
- Kraemer KH, DiGiovanna JJ, Moshell AN, Tarone RE, Peck GL. Prevention of skin cancer in xeroderma pigmentosum with the use of oral isotretinoin. N Engl J Med 1988; 318:1633 - 1637
- Jones E, Korzenko A, Kriegel D. Oral isotretinoin in the treatment and prevention of cutaneous squamous cell carcinoma. J Drugs Dermatol 2004; 3:498 - 500
- Armstrong JL, Redfern CP, Veal GA. 13-cis retinoic acid and isomerization in paediatric oncology—is changing shape the key to success?. Biochem Pharmacol 2005; 69:1299 - 1306
- Guruvayoorappan C, Pradeep CR, Kuttan G. 13 cisretinoic acid mediates apoptosis in Dalton's lymphoma ascites cells by regulating gene expression. J Basic Clin Physiol Pharmacol 2007; 18:267 - 276
- Guruvayoorappan C, Pradeep CR, Kuttan G. 13-cisretinoic acid induces apoptosis by modulating caspase-3, bcl-2 and p53 gene expression and regulates the activation of transcription factors in B16F-10 melanoma cells. J Environ Pathol Toxicol Oncol 2008; 27:197 - 207
- Yuza Y, Agawa M, Matsuzaki M, Yamada H, Urashima M. Gene and protein expression profiling during differentiation of neuroblastoma cells triggered by 13-cis retinoic acid. J Pediatr Hematol Oncol 2003; 25:715 - 720
- Maiese K, Chong ZZ, Shang YC. OutFOXOing disease and disability: the therapeutic potential of targeting FoxO proteins. Trends Mol Med 2008; 14:219 - 227
- Maiese K, Chong ZZ, Li F, Shang YC. Erythropoietin: Elucidating new cellular targets that broaden therapeutic strategies. Prog Neurobiol 2008; 85:194 - 191
- Bouchard C, Lee S, Paulus-Hock V, Loddenkemper C, Eilers M, Schmitt CA. FoxO transcription factors suppress Myc-driven lymphomagenesis via direct activation of Arf. Genes Dev 2007; 21:2775 - 2787
- Modur V, Nagarajan R, Evers BM, Milbrandt J. FOXO proteins regulate tumor necrosis factor-related apoptosis inducing ligand expression. Implications for PTEN mutation in prostate cancer. J Biol Chem 2002; 277:47928 - 47937
- Li Y, Wang Z, Kong D, Murthy S, Dou QP, Sheng S, et al. Regulation of FOXO3a/beta-catenin/GSK-3beta signaling by 3,3′-diindolylmethane contributes to inhibition of cell proliferation and induction of apoptosis in prostate cancer cells. J Biol Chem 2007; 282:21542 - 21550
- Trotman LC, Alimonti A, Scaglioni PP, Koutcher JA, Cordon-Cardo C, Pandolfi PP. Identification of a tumour suppressor network opposing nuclear Akt function. Nature 2006; 441:523 - 527
- Paik JH, Kollipara R, Chu G, Ji H, Xiao Y, Ding Z, et al. FoxOs are lineage-restricted redundant tumor suppressors and regulate endothelial cell homeostasis. Cell 2007; 128:309 - 323
- Ticchioni M, Essafi M, Jeandel PY, Davi F, Cassuto JP, Deckert M, et al. Homeostatic chemokines increase survival of B-chronic lymphocytic leukemia cells through inactivation of transcription factor FOXO3a. Oncogene 2007; 26:7081 - 7091
- Kikuchi S, Nagai T, Kunitama M, Kirito K, Ozawa K, Komatsu N. Active FKHRL1 overcomes imatinib resistance in chronic myelogenous leukemia-derived cell lines via the production of tumor necrosis factor-related apoptosis-inducing ligand. Cancer Sci 2007; 98:1949 - 1958
- Munoz-Fontela C, Marcos-Villar L, Gallego P, Arroyo J, Da Costa M, Pomeranz KM, et al. Latent protein LANA2 from Kaposi's sarcoma-associated herpesvirus interacts with 14-3-3 proteins and inhibits FOXO3a transcription factor. J Virol 2007; 81:1511 - 1516
- Hoekstra AV, Ward EC, Hardt JL, Lurain JR, Singh DK, Buttin BM, et al. Chemosensitization of endometrial cancer cells through AKT inhibition involves FOXO1. Gynecol Oncol 2008; 108:609 - 618
- Melnik BC. Acneigenic stimuli converge in phosphoinositol-3-kinase/Akt/FoxO1 signal transduction. J Clin Exp Dermatol 2010; 1:101
- Sutcliffe S, Giovannucci E, Isaacs WB, Willett WC, Platz EA. Acne and risk of prostate cancer. Int J Cancer 2007; 121:2688 - 2692
- Kauppinen-Mäkelin R, Sane T, Välimäki MJ, Markkanen H, Niskanen L, Ebeling T, et al. Increased cancer incidence in acromegaly—a nationwide survey. Clin Endocrinol (Oxf) 2010; 72:278 - 279
- Chittenden BG, Fullerton G, Maheshwari A, Bhattacharya S. Polycystic ovary syndrome and the risk of gynaecological cancer: a systematic review. Reprod Biomed Online 2009; 19:398 - 405
- Pollock PM, Gartside MG, Dejeza LC, Powell MA, Mallon MA, Davies H, et al. Frequent activating FGFR2 mutations in endometrial carcinomas parallel germline mutations associated with craniosynostosis and skeletal dysplasia syndromes. Oncogene 2007; 26:7158 - 7162
- Rouzier C, Soler C, Hofman P, Brennetot C, Bieth E, Pedeutour F. Ovarian dysgerminoma and Apert syndrome. Pediatr Blood Cancer 2008; 50:696 - 698
- Andreou A, Lamy A, Layet V, Cailliez D, Gobet F, Pfister C, et al. Early-onset low-grade papillary carcinoma of the bladder associated with Apert syndrome and a germline FGFR2 mutation (Pro253Arg). Am J Med Genet 2006; 140:2245 - 2257
- Melnik BC. Role of FGFR2-signaling in the pathogenesis of acne. Dermatoendocrinol 2009; 1:141 - 156
- Pollak M. Insulin and insulin-like growth factor signalling in neoplasia. Nature Rev Cancer 2008; 8:915 - 928
- Melnik BC. Milk signalling in the pathogenesis of type 2 diabetes. Med Hypotheses 2011; 76:553 - 559
- Ahmed Z, Schuller AC, Suhling K, Tregidgo C, Ladbury JE. Extracellular point mutations in FGFR2 elicit unexpected changes in intracellular signalling. Biochem J 2008; 413:37 - 49
- Melnik BC, Vakilzadeh F, Aslanidis C, Schmitz G. Unilateral segmental acneiform naevus: a model disorder towards understanding fibroblast growth factor receptor 2 function in acne?. Br J Dermatol 2008; 158:1397 - 1399
- Cuerda E, del Pozo J, Rodrìguez-Lozano J, Peña-Penabad C, Fonseca E. Acne in Apert's syndrome: treatment with isotretinoin. J Dermatolog Treat 2003; 14:43 - 45
- Campanati A, Marconi B, Penna L, Paolinelli M, Offidani A. Pronounced and early acne in Apert's syndrome: a case successfully treated with oral isotretinoin. Eur J Dermatol 2002; 12:496 - 498
- Downs AM, Condon CA, Tan R. Isotretinoin therapy for antibiotic-refractory acne in Apert's syndrome. Clin Exp Dermatol 1999; 24:461 - 463