Abstract
An automated in-vitro, medium-throughput screening method was designed to compare the rapid actions of 10−9M estradiol to those of modified bisphenol A (BPA) compounds: chlorinated (created due to waste water treatment) and phase II BPA metabolites. GH3/B6/F10 prolactinoma cells were treated with increasing concentrations (10−15‒10−7M) of BPA or modified BPA compounds for 5 min, and phospho-activated kinases assayed via a fixed-cell plate immunoassay. Mono- and di-chlorinated BPAs phospho-activated the extracellular signal-regulated kinase (ERK), while tri-chlorinated BPA dephosphorylated ERK to a level below vehicle-treated controls. When c-Jun-N-terminal kinase (JNK) was examined, mono-chlorinated compounds caused no responses (similar to unmodified BPA), while di- and tri-chlorinated BPAs caused extensive dephosphorylation. When deconjugation of di-sulfated and glucuronidated metabolites were inhibited, these stably conjugated compounds were unable to activate either ERK or JNK, but could inactivate them. This suggests that the modified versions of BPA (extensively chlorinated or conjugated metabolites) may alter the ability of membrane estrogen receptor-α (which mediates these rapid signaling responses) to partner with other signaling proteins. While other studies have examined the estrogenicity of modified (chlorinated and conjugated) BPA compounds in gene expression assays, this is the first to examine their actions via rapid, membrane-initiated signaling pathways.
Introduction
Bisphenol A (BPA) is a well-known and environmentally pervasive endocrine-disrupting chemical (EDC) used commercially for the production of polycarbonate plastic water and food containers, as a monomer in the formation of anti-corrosive epoxy inner linings of food cans, and as an ingredient in coatings for thermal paper cash register receipts and faxes.Citation1-Citation4 BPA had long been considered a weak estrogenic compound compared with estradiol (E2) due to its low affinity (1,000-fold difference) for intracellular estrogen receptors (iERs).Citation5 However, we determined BPA to be equipotent to E2 in initiating rapid signaling via membrane estrogen receptors (mERs)Citation6-Citation8 and, thus, capable of altering associated functional endpoints at low, environmentally relevant concentrations. However, it is unknown if common environmental derivatives of BPA (chlorinated versions) and endogenous phase II metabolites of BPA (glucuronidated and sulfated versions) (see ) can act via these membrane-initiated signaling pathways.
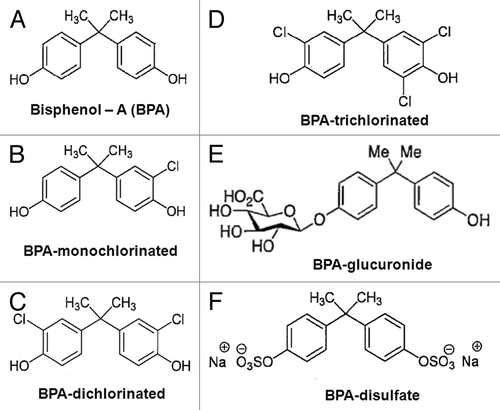
Figure 1. Structure of BPA, its variably chlorinated versions, and BPA phase II metabolites. (A) Bisphenol A; (B) Mono-, (C) Di-, and (D) Tri-chlorobisphenol A; (E) Bisphenol A β-d-glucuronide; (F) Bisphenol A disulfate.
Typical human exposures to BPA occur from consumption of food and drink that have come into contact with packaging containing BPA, especially during the heating of polycarbonate plastic containers.Citation9 Levels of urinary BPA range from 0.4–149 μg/L (1.8–660 nM) in 92.6% of U.S. residents ≥ 6 y of age.Citation10 Several in vivo rodent studies have linked BPA to developmental alterations in reproductive organs,Citation11-Citation13 as well as changes in hormonal signaling.Citation14,Citation15 The presence of BPA has also been associated with the development of chronic diseases such as diabetes,Citation16 asthma,Citation17 ovarian dysfunction, and the onset of obesity.Citation18 In July 2012, the FDA banned BPA from use in the production of plastic baby bottles and drinking cups.Citation19
A major health concern is the presence of BPA in drinking water. Release of BPA into aquatic environments, particularly to waste water treatment plants, occurs primarily from plastic-to-water migrations such as when water runs through poly-vinyl chloride (PVC) pipes in water supply systems, or from discarded plastic in the environment.Citation20 Once in the drinking water, BPA is rapidly biodegraded by microorganisms (e.g., Pseudomonas), with a half-life of 2.5–4 d.Citation21-Citation24 However, because of BPA’s presence in many forms of consumer products and refuse, it is rapidly and repeatedly replaced.
The addition of chlorine to water supply systems as a means of preventing water borne diseases, along with BPA’s constant replenishment from the environment, can result in its reaction with free chlorine, giving rise to a variety of chlorinated forms (, left panels).Citation25-Citation27 Depending on the amount of chlorine present in an aqueous medium, it can rapidly transform BPA within 4 h, producing variably chlorinated forms with half-lives of 10‒20 h (in a controlled environment).Citation28 While an abundance of evidence does exist about other poly-chlorinated biphenols having endocrine-disrupting capabilities, little is known about the estrogenic potential of poly-chlorinated BPA compounds, and nothing about their influence via rapid estrogenic signaling pathways. Our present study therefore uses the well-differentiated GH3/B6/F10 rat prolactinoma cell line as our in-vitro model to examine the ability of progressively chlorinated versions of BPA to initiate rapid kinase signaling.
In addition, we also assessed the ability of phase II glucuronidated and sulfated metabolites of BPA (, right panels) to induce membrane-initiated signaling. While most estrogenic metabolism is mediated via cytochrome P450 enzymes (e.g., E2 to estriol), estrogens also undergo phase II conjugations, which increase their water solubility and facilitate their excretion.Citation29,Citation30 These conjugations prevent endogenous estrogensCitation30 and exogenous estrogens like BPACitation31-Citation33 from binding to intracellular ERs (iERs), thus rendering the compounds biologically inactive in the direct genomic signaling pathway. Previous studies from our group,Citation34-Citation36 and others,Citation37,Citation38 have documented that iERα is the same protein as mERα. Localization of mER’s in lipid membranes (as opposed to iERs’ aqueous environment in the cytosol or nucleus) may yield conformational differences in the receptor that alter the specificity of ligand binding sites. This could allow metabolites of BPA modified by conjugation, while unable to enter cells, still able to bind to mERα and cause membrane-initiated signaling.
As in many of our previous studies, we used a fixed cell-based 96-well plate immunoassay to evaluate protein phosphorylation and dephosphorylation of the mitogen-activated protein kinases (MAPKs).Citation6-Citation8,Citation39-Citation42 While this method has yielded excellent results, it can be a very time consuming process with multiple reagent incubations and the attendant wash steps. To increase efficiency and reproducibility, we designed an automated medium-throughput screening system using a BIOMEK FXP workstation for liquid handling.
Results
Concentration-dependent changes in phospho-activation of MAPKs by BPA and its progressively chlorinated forms
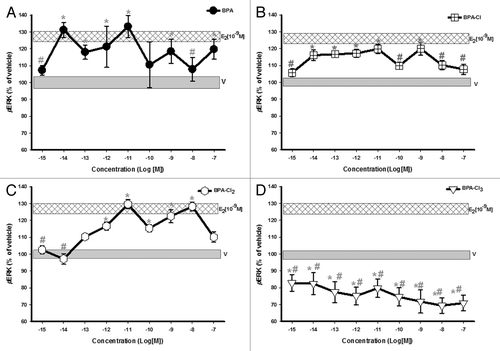
BPA caused ERK activation () in GH3/B6/F10 cells within 5 min, with a non-monotonic response pattern, as expected.Citation6-Citation8 Exposure to mono- and di-chlorinated compounds caused ERK activation at most concentrations, and at some concentrations the responses were equivalent to that of 10−9M E2 (). The dose-responses did not gradually rise and plateau at the highest concentrations, as is seen with classical responses, but instead showed non-monotonic patterns. The tri-chlorinated BPA form did not cause phospho-activation of ERK, but instead led to a dramatic dephosphorylation, to below vehicle control levels ().
Figure 2. Dose-response analysis of ERK phosphorylation (pERK) in GH3/B6/F10 cells after exposure to BPA and its progressively chlorinated versions. The cells were exposed to a range of concentrations (in log increments) of (A) BPA, (B) mono-, (C) di-, and (D) tri-chlorinated BPA. pERK was measured by plate immunoassay at a 5 min exposure time. The widths of the vehicle and E2 (10−9M) bars represent the means ± SE (n = 24 over three experiments) * = p < 0.05 when compared with vehicle (V). # = p < 0.05 when compared with 10−9M E2. E2 (10−9M) is significantly different from vehicle.
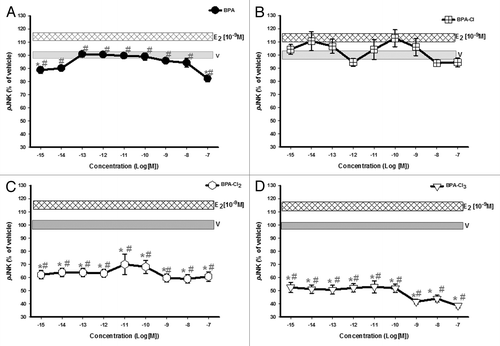
Though unmodified (parent) and mono-chlorinated BPAs had no significant effects on JNK activation (, respectively), di- and tri-chlorinated BPAs caused significant dephosphorylation of JNK (). Thus, chlorination to different degrees had different effects, with the more extensively modified compounds actually disrupting basal levels of MAPK phosphorylation.
Figure 3. Dose-response analysis of JNK phospho-activation (pJNK) in response to varying concentrations of BPA and its progressively chlorinated versions. GH3/B6/F10 cells were exposed to increasing concentrations (in log increments) of (A) BPA, (B) mono-, (C) di-, and (D) tri-chlorinated BPA. pJNK was measured by plate immunoassay at a 5 min exposure time. The widths of the vehicle and E2 (10−9M) bars represent the means ± SE (n = 24 over three experiments) * = p < 0.05 when compared with vehicle (V). # = p < 0.05 when compared with 10−9M E2. E2 (10−9M) is significantly different from vehicle.
Concentration-dependent changes in phospho-activation of MAPKs by BPA phase II metabolites
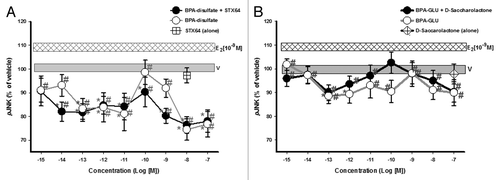
Cells were exposed to BPA phase II metabolites ± β-glucuronidase or sulfatase inhibitors. The inhibitors alone had no significant effect on kinase activation levels. In the presence of the inhibitor STX-64, sulfated-BPA was only able to activate ERK at 10−7M (). However, in the absence of STX-64, the sulfated compound phospho-activated ERK with a non-monotonic dose-response, suggesting some conversion of the compound to the unconjugated form in these pituitary tumor cells. In the presence of the inhibitor D-glucaric acid-1, 4-lactone, stably glucuronidated BPA did not activate ERK, but instead inactivated ERK to significantly below the baseline (vehicle-treated) pERK level, indicating a different and unexpected activity. In the absence of inhibitor, and likely partial deconjugation, we observed significant ERK activation at the lowest concentration (), for which we have previously found BPA to be the most active.Citation6,Citation8,Citation43,Citation44 Variability in the uninhibited activation profiles of these two forms of conjugated compounds may suggest partial deconjugations, as well as differences in the relative rates of bond cleavage by sulfatases vs. glucuronidases.
Figure 4. Dose-response analysis of ERK phospho-activation (pERK) by BPA phase II metabolites. GH3/B6/F10 cells were exposed to increasing concentrations (in log increments) of (A) Bisphenol A- disulfate and (B) Bisphenol A β-d-glucuronide. The cells were pre-incubated ± D-glucaric acid-1,4-lactone (20 mM) or STX-64 (10 nM) to inhibit β-glucuronidase and sulfatase, respectively. pERK was measured by plate immunoassay at a 5 min exposure time. Controls for the inhibitor administered alone are shown by a symbol at the single concentration at which it was used. The widths of the vehicle and E2 (10−9M) bars represent the means ± SE (n = 24 over three experiments) * = p < 0.05 when compared with vehicle (V). # = p < 0.05 when compared with 10−9M E2. E2 (10−9M) is significantly different from vehicle.
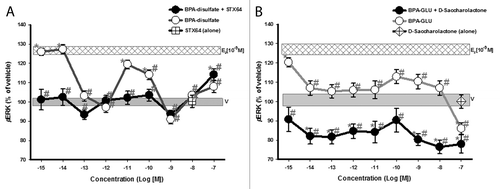
Both conjugated compounds in either the presence or absence of their respective deconjugation inhibitors were unable to activate JNK signaling above vehicle-treated levels, but instead deactivated JNK at some concentrations in a non-monotonic pattern (). The disulfated compound was consistently far more effective in this deactivation. Thus, phase II enzymatic conjugation of BPA with both sulfate and glucuronic acid allows it to suppress MAPK activation levels, disrupting normal actions via mERs and introducing new ones.
Figure 5. Dose-response analysis of JNK phospho-activation (pJNK) by BPA phase II metabolites. GH3/B6/F10 cells were exposed to increasing concentrations (in log increments) of (A) Bisphenol A-disulfate and (B) Bisphenol A β-d-glucuronide. The cells were pre-incubated ± D-glucaric acid-1,4-lactone or STX-64 to inhibit β-glucuronidase and sulfatase, respectively, prior to analysis. Controls for the inhibitor administered alone are shown by a symbol at the single concentration at which it was used . pJNK was measured by plate immunoassay at a 5 min exposure time. The widths of the vehicle and E2 (10−9M) bars represent the means ± SE (n = 24 over three experiments) * = p < 0.05 when compared with vehicle (V). # = p < 0.05 when compared with 10−9M E2. E2 (10−9M) is significantly different from vehicle.
Comparison of automated plate assay results with prior manual assays
In designing an automated program for these assays using the BIOMEK software, many adjustments were necessary to match the automated results as closely as possible to our previous manual results.Citation6-Citation8,Citation39, Citation41,Citation44,Citation45 Liquid-handling parameters were optimized, such as the speed of aspiration and dispensing, and determining the adequate depth of tip immersion into the liquid reservoirs. Such adjustments decreased the amount of unwanted liquid carryover that could ultimately build up and increase the final liquid volume in each well, thereby causing added variability. Time adjustments were necessary, taking into consideration the elapsed time from the moment the probe grippers moved or replaced tip boxes, to the point at which the multi-channel probe picked up pipette tips and then finally dispensed the liquids into the wells of the plates. These adjustments made it possible to meet our target times for exposure to estrogens and to precisely terminate the response by fixation. In general, the results of these optimized automated assays agreed very well with previously published MAPK activation/deactivation measurements for both E2 and BPA.Citation6-Citation8,Citation39,Citation41,Citation44,Citation45
Discussion
Drinking water systems that rely on the addition of chlorine as an inexpensive disinfectant are also abundant sources of EDCs, including BPA, which is rapidly chlorinated to other forms.Citation28 Our study demonstrates that environmentally relevant concentrations (femtomolar-picomolar) of chlorinated derivatives of BPA can initiate or alter membrane-initiated actions via mERs in prolactinoma cells. Glucuronidated and sulfated phase II conjugates of BPA often depressed MAPK activities, instead of activating them. Our results thus provide further insights into these metabolites’ biological activities, which differed from those of their parent compounds.Citation30-Citation33 Such actions on MAPKs have the potential to cause inappropriate cellular signaling in humans and other animals, and also to interfere with the actions of natural estrogens (such as E2 and other physiologic estrogenic compounds). Altering these pathways may lead to health changes at downstream functional endpoints such as cell proliferation, and the release of other hormones like prolactin.Citation6,Citation8,Citation43,Citation44 Our automated protocol increased assay efficiency and reproducibility, very important advantages when examining the large number of environmental compounds, and their chemically modified forms that are of concern, for their disruptive actions on kinase activities.
Dose-response studies of BPA and its chlorinated conjugates generated curves with atypical shapes ( and ), now more often recognized as a usual consequence of examining EDCs or endogenous hormones.Citation6,Citation8,Citation43,Citation44 Such non-monotonic dose relationshipsCitation46 are commonly seen when the range of concentrations examined is extended to femtomolar to picomolar levels in sensitive assays. However, the molecular bases for non-monotonicity are largely unknown, and are still a source of much controversy. They could involve protective downregulation of MAPK activities to eliminate unnecessary pathway stimulation; the presence of multiple receptor subtypes that bind the same ligand, each generating a different response pattern (stimulatory or inhibitory); concentration-dependent receptor downregulation or desensitization; and/or stimulation of multiple pathways from the same receptor where signaling can subsequently be redundant, divergent, and convergent.Citation46-Citation50
Phospho-activation of ERK and JNK is often associated with opposing functional responses. For instance, ERK signaling promotes cell growth and differentiation by activating pro-survival enzymesCitation51 and inhibiting apoptotic enzymes.Citation52-Citation54 Conversely, JNK signaling is often associated with inflammation or the initiation of cell death by activating pro-apoptotic proteins, including Bax, Fas, or caspases.Citation55-Citation59 Opposing responses of ERK and JNK can be appreciated in our data by comparing MAPK responses to di-chlorinated BPA where ERK is activated above the vehicle-treated level, and JNK is inactivated. Simultaneous activation of ERK and deactivation of JNK by EDCs have previously been correlated with severe stimulatory effects on downstream functional endpoints such as cell proliferation.Citation60 In contrast, tri-chlorinated BPA suppressed the activities of both MAPKs. Suppression of multiple kinases has been seen with another polychlorinated compound, 2, 3, 7, 8-tetrachlorodibenzo-p-dioxin (TCDD),Citation61,Citation62 where 0.03 and 0.3 nM treatments rapidly (15 min) inhibited phospho-activation of ERK and JNK in CH12.LX B lymphocytes, correlating with decreased cell viability and “impaired plasmacytic differentiation.” Dual suppression of ERK and JNK by tri-chlorinated BPA suggests that it might impair the actions of a common target upstream of ERK and JNK, and probably led to the cancellation of many downstream actions for which these kinases are responsible, dramatically disrupting endocrine function. Therefore, final functional outcomes may depend on the overall balance between ERK- and JNK-related activities.Citation63,Citation64
Early structure-activity studies noted that the 17β-hydroxyl group of E2 was necessary for binding within the ligand-binding pocket of iER.Citation65,Citation66 Therefore, any chemical mimicking this feature of E2, such as in BPA, has the possibility of binding to an ER and inducing an estrogenic response.Citation67 The addition of multiple chlorine atoms at or near these sites might then disrupt this interaction and block or alter downstream responses. Such a trend was shown using a green fluorescent protein expression reporter system to assess nuclear ER actions;Citation68,Citation69 when the number of chlorine substituents added to BPA or nonylphenol increased, their estrogenic activity decreased. Increasing brominated substitutions on BPA also cause attenuation in estrogenic cell proliferation.Citation70
However, our observations are not consistent with merely blocking of ligand binding to the receptor, because these modified BPAs do have additional effects in some cases (e.g., inactivation of MAPKs). Phase II conjugation of both endogenous and exogenous chemicals changes them from hydrophobic to hydrophilic molecules, which while facilitating their elimination from the body, also likely changes their ability to bind certain ER conformations or to alter the receptor shape once bound. The addition of chlorine, sulfate, or glucuronide moieties surrounding the phenolic-hydroxyl groups of BPA could direct different conformations of the ER with resulting changes in the ability to partner with other proteins. In such cases, interacting phosphatases affecting ERK and JNK could be activated instead.Citation71-Citation74
We and others have explored the protein identities of mERα and iERα.Citation34,Citation37 A close protein similarity was established when nine iERα-specific antibodies (Abs) recognized (epitopes on) mERα,Citation34,Citation75 the iERα-specific Ab H151 elicited or blocked membrane-initiated responses to estrogenic ligands,Citation35,Citation75 and disrupting the ERα mRNA (via antisense or siRNA methods), resulted in decreased expression of both the membrane and intracellular versions of ERα.Citation76,Citation77 Phase II conjugates failed to activate rapid kinase responses in our study, indicating another similarity between mER and iER.Citation29,Citation30 However, their ability to inactivate kinases suggests action via an alternative signaling partner. Even though iERs and mERs are modified forms of the same protein, they may differ in conformation, due to their post-translational modificationsCitation78 and their different chemical environments (soluble vs. membrane), and so may accommodate a wider variety of estrogen-like moleculesCitation79 and different signaling partner proteins.
These changes seen in the phospho-activation of the kinases are smaller compared with some responses, for instance, to epidermal growth factor. However biological responses modified in such small but significant increments, and are no less biologically important than larger changes for initiating functionally consequential activations or de-activations. Such small but significant changes in signaling are known to build by amplification as they progress down a cascade.Citation80 Furthermore, multiple small changes can add up to large ones, with MAPKs acting as response summation nodes.
The studies presented here demonstrate that some chlorinated conjugates of BPA can induce rapid membrane-initiated signaling in estrogen-responsive prolactinoma cells at low (femtomolar-picomolar) concentrations, but that more extensively altered forms (tri-chlorinated, glucuronidated, sulfated) inactivate the same kinases. Further structure-activity studies could provide additional insights and identify the endocrine-disruptive potential of any new chemicals or their modifications.Citation81 With our implementation of the BIOMEK FXP Laboratory Automation Workstation protocol, we have developed a much more efficient and reliable automated screening tool for testing and identifying potential EDCs and their variants, and facilitating large-scale structure-activity studies.
Materials and Methods
Cell culture conditions and experimental compounds
The well-differentiated GH3/B6/F10 rat prolactinoma cell line was used to represent responses of lactotroph anterior pituitary cells, and was selected on the basis of its naturally high expression of mERα.Citation39,Citation82,Citation83 Cells were routinely sub-cultured with phenol red-free Dulbecco’s modification of Eagle’s medium (DMEM, high glucose; 90013PB) containing 12.5% horse serum (SH30074.02), defined supplemented calf (SH30072.03), and fetal serum (SH30088.03) at 2.5% and 1.5%, respectively, all from Fisher Scientific. Cells of passages 10–20 were used for these experiments.
BPA chlorinated compounds and glucuronidated or sulfated metabolites were kindly provided by the National Institute for Environmental Health Sciences (NIEHS). Chemical purity of these compounds was determined by mass spectrometry and nuclear magnetic resonance and ranged from 98–99.7% as indicated by the data sheets provided. Before use on cells, they were dissolved in ethanol, and then diluted in DMEM containing 1% charcoal-stripped serum.
Automated ERK and JNK phosphorylation assays
A fixed cell-based immunoassay was used to quantify phospho-activation of extracellular-regulated kinase (ERK/pERK) and c-jun N-terminal kinases (JNK/pJNK), which we previously developed and optimized, as detailed in references Citation39 and Citation40. These previous studies also showed that ERα is the primary receptor involved in initiating these responses and the very rapid time course of a 5 min activation peak.Citation7,Citation34,Citation39,Citation84-Citation86 This assay automation is simply a mechanical adaption of our previous method, and no changes in the assay parameters were introduced intentionally. 10−9M E2 was used as our positive control in all experiments. Automation of this assay employed the BIOMEK FXP Laboratory Automation Workstation operated by Biomek Software (Biomek Coulter). Our automated assay was programmed to consist of six phases: (1) ligand exposure, (2) Ab incubation, (3) washing, (4) color signal production during the signal amplification incubation, (5) plate reading of signal, and (6) washing to prepare for the subsequent cell staining and extraction steps to estimate cell number. These phases are detailed in the Results section. An example of the “exposure” phase can be viewed in the following link http://www.youtube.com/watch?v=6Jb7xBjWTtA.
The software code used to automate these protocol phases are protected by copyright (2012).
Progressively chlorinated BPA
Cells (104/well) were plated in 96-well plates (Corning Inc.) and allowed to attach for 24 h. The cells were then incubated with DMEM containing 1% charcoal-stripped (4X) serum for 48 h to deprive them of serum hormones. The medium was then removed and cells were exposed to increasing concentrations (10−15‒10−7M) of individual mono-, di-, and tri- chlorinated BPAs for 5 min. The short time point of 5 min was chosen to observe membrane-initiated phospho-activations without interference from later genomic responses. The concentration range was chosen to bracket environmentally relevant concentrations and test very low concentrations that we have shown to activate these responses. Test compounds were dissolved in ethanol and then diluted in DMEM containing 1% charcoal-stripped serum. The vehicle control (V) was 0.001% ethanol in DMEM. To stop mER-initiated signaling, cells were fixed with a 2% paraformaldehyde/0.2% picric acid solution (Ricca Chemical Company; 5860-32) and incubated at 4 °C for 48 h. The cells were then incubated in phosphate-buffered saline (PBS) containing 0.2% fish gelatin (G7765) and 0.1% Triton X-100 (9002931) from Sigma-Aldrich, for 1 h at room temperature (RT), followed by overnight incubation at 4 °C with primary Abs against pERK (4370S) or pJNK (4668S) from Cell Signaling Technology (1:500 in PBS/0.2% fish gelatin/0.1% TritonX-100). The cells were then washed with PBS (3X) before incubation with biotin-conjugated secondary Ab (Vector Labs; BA 2001) (1:500 in PBS/0.2% fish gelatin) for 1 h at RT, then washed again in PBS (3X). The preparation was then incubated with Vectastain ABC-AP solution (50 μL/well) from a kit (Vector Labs; AK5000) for 1 h at RT, followed by addition of 50 μL/well alkaline phosphatase substrate (pNpp solution, Thermo Scientific; 34045). The plates were incubated in the dark for 30 min at 37 °C and the A405 signal due to the para-nitrophenol (pNp) product was measured. The pNp signal was then normalized to the cell number, estimated by the crystal violet (CV) assayCitation40 and measured at A590. Absorbance signals for both assays were read in a model 1420 Wallac microplate reader (Perkin Elmer).
Phase II BPA metabolites
The anterior pituitary is known to contain β-glucuronidaseCitation87 and sulfatases,Citation29,Citation88 which can cause enzymatic hydrolysis and deconjugate glucuronic acid and sulfate from the parent compound, respectively. To maintain the integrity of our conjugated compounds during the analysis, we pre-incubated our cells with 20 mM D-glucaric acid-1,4-lactone (Carbosynth; MG67426), a potent inhibitor of β-glucuronidaseCitation89 or 10 nM 667 Coumate/STX-64 (Sigma-Aldrich; S1950), a sulfatase inhibitor,Citation29 each for 1 h at 37 °C (50 µl/well). Conjugates were then added to wells (50µl/well) at final concentrations of 10−15‒10−7M, incubated for 5 min, and the signaling stopped with 2% paraformaldehyde/0.2% picric acid solution as above. Automated immunoassay methods proceeded as above for the chlorinated compounds.
Statistical analysis
Statistical analysis was performed using Sigmaplot version 12.3 (Systat Software Inc.). One-way analysis of variance (ANOVA) was applied to the dose-dependent and timed studies to assess the statistical significance of mean values produced by varying exposures. A Holm-Sidak comparison against vehicle control or against 10−9M E2 treatment was used to evaluate significance. The overall α level selected for the statistical analysis was 0.05.
| Abbreviations: | ||
| Ab | = | antibody |
| BPA | = | bisphenol A |
| JNK | = | c-Jun-N-terminal kinase |
| CV | = | crystal violet |
| DMEM | = | Dulbecco’s modified eagle medium |
| EDC | = | endocrine-disrupting compounds |
| ERKs | = | extracellular signal regulated kinases |
| E2 | = | estradiol |
| ER | = | estrogen receptor |
| iERα | = | intracellular estrogen receptor-α |
| mERα | = | membrane estrogen receptor-α, MAPKs, mitogen-activated protein kinases |
| PBS | = | phosphate-buffered saline |
| pERK | = | phosphorylated ERK |
| pJNK | = | phosphorylated JNK |
| RT | = | room temperature |
Acknowledgments
These studies were supported by National Institutes of Health grant ES015292, NIH Ruth L. Kirschstein National Research Service Award F31ES021164-01, and the Colgate-Palmolive Award for Student Research Training in Alternative Methods. The parent BPA and all of the conjugates were provided by the NIEHS. This work utilized the University of Texas Medical Branch/Gulf Coast Consortium Core in High Throughput Screening for Chemical Biology, which is supported in part by the John S. Dunn Foundation through the Gulf Coast Consortium for Chemical Genomics. We also thank Dr David Konkel for critically editing the manuscript.
Disclosure of Potential Conflicts of Interest
The authors declare that they have no conflict of interest.
References
- Zalko D, Jacques C, Duplan H, Bruel S, Perdu E. Viable skin efficiently absorbs and metabolizes bisphenol A. Chemosphere 2011; 82:424 - 30; http://dx.doi.org/10.1016/j.chemosphere.2010.09.058; PMID: 21030062
- Willhite CC, Ball GL, McLellan CJ. Derivation of a bisphenol A oral reference dose (RfD) and drinking-water equivalent concentration. J Toxicol Environ Health B Crit Rev 2008; 11:69 - 146; http://dx.doi.org/10.1080/10937400701724303; PMID: 18188738
- Liao C, Liu F, Alomirah H, Loi VD, Mohd MA, Moon HB, et al. Bisphenol S in urine from the United States and seven Asian countries: occurrence and human exposures. Environ Sci Technol 2012; 46:6860 - 6; http://dx.doi.org/10.1021/es301334j; PMID: 22620267
- Liao C, Liu F, Kannan K. Bisphenol s, a new bisphenol analogue, in paper products and currency bills and its association with bisphenol a residues. Environ Sci Technol 2012; 46:6515 - 22; http://dx.doi.org/10.1021/es300876n; PMID: 22591511
- Kuiper GG, Lemmen JG, Carlsson B, Corton JC, Safe SH, van der Saag PT. van der BB, and Gustafsson JA. Interaction of estrogenic chemicals and phytoestrogens with estrogen receptor beta. Endocr 1998; 139:4252 - 63; http://dx.doi.org/10.1210/en.139.10.4252
- Wozniak AL, Bulayeva NN, Watson CS. Xenoestrogens at picomolar to nanomolar concentrations trigger membrane estrogen receptor-alpha-mediated Ca2+ fluxes and prolactin release in GH3/B6 pituitary tumor cells. Environ Health Perspect 2005; 113:431 - 9; http://dx.doi.org/10.1289/ehp.7505; PMID: 15811834
- Jeng YJ, Watson CS. Combinations of physiologic estrogens with xenoestrogens alter ERK phosphorylation profiles in rat pituitary cells. Environ Health Perspect 2011; 119:104 - 12; http://dx.doi.org/10.1289/ehp.1002512; PMID: 20870566
- Kochukov MY, Jeng Y-J, Watson CS. Alkylphenol xenoestrogens with varying carbon chain lengths differentially and potently activate signaling and functional responses in GH3/B6/F10 somatomammotropes. Environ Health Perspect 2009; 117:723 - 30; PMID: 19479013
- Kubwabo C, Kosarac I, Stewart B, Gauthier BR, Lalonde K, Lalonde PJ. Migration of bisphenol A from plastic baby bottles, baby bottle liners and reusable polycarbonate drinking bottles. Food Addit Contam Part A Chem Anal Control Expo Risk Assess 2009; 26:928 - 37; http://dx.doi.org/10.1080/02652030802706725; PMID: 19680968
- Calafat AM, Ye X, Wong LY, Reidy JA, Needham LL. Exposure of the U.S. population to bisphenol A and 4-tertiary-octylphenol: 2003-2004. Environ Health Perspect 2008; 116:39 - 44; http://dx.doi.org/10.1289/ehp.10753; PMID: 18197297
- Fernández M, Bourguignon N, Lux-Lantos V, Libertun C. Neonatal exposure to bisphenol a and reproductive and endocrine alterations resembling the polycystic ovarian syndrome in adult rats. Environ Health Perspect 2010; 118:1217 - 22; http://dx.doi.org/10.1289/ehp.0901257; PMID: 20413367
- Newbold RR, Jefferson WN, Padilla-Banks E. Prenatal exposure to bisphenol a at environmentally relevant doses adversely affects the murine female reproductive tract later in life. Environ Health Perspect 2009; 117:879 - 85; PMID: 19590677
- Berger RG, Foster WG, deCatanzaro D. Bisphenol-A exposure during the period of blastocyst implantation alters uterine morphology and perturbs measures of estrogen and progesterone receptor expression in mice. Reprod Toxicol 2010; 30:393 - 400; http://dx.doi.org/10.1016/j.reprotox.2010.06.006; PMID: 20599497
- Fernández M, Bianchi M, Lux-Lantos V, Libertun C. Neonatal exposure to bisphenol a alters reproductive parameters and gonadotropin releasing hormone signaling in female rats. Environ Health Perspect 2009; 117:757 - 62; PMID: 19479018
- Abrahám IM, Han SK, Todman MG, Korach KS, Herbison AE. Estrogen receptor beta mediates rapid estrogen actions on gonadotropin-releasing hormone neurons in vivo. J Neurosci 2003; 23:5771 - 7; PMID: 12843281
- Nadal A, Alonso-Magdalena P, Soriano S, Quesada I, Ropero AB. The pancreatic beta-cell as a target of estrogens and xenoestrogens: Implications for blood glucose homeostasis and diabetes. Mol Cell Endocrinol 2009; 304:63 - 8; http://dx.doi.org/10.1016/j.mce.2009.02.016; PMID: 19433249
- Midoro-Horiuti T, Tiwari R, Watson CS, Goldblum RM. Maternal bisphenol a exposure promotes the development of experimental asthma in mouse pups. Environ Health Perspect 2010; 118:273 - 7; http://dx.doi.org/10.1289/ehp.0901259; PMID: 20123615
- Takeuchi T, Tsutsumi O, Ikezuki Y, Takai Y, Taketani Y. Positive relationship between androgen and the endocrine disruptor, bisphenol A, in normal women and women with ovarian dysfunction. Endocr J 2004; 51:165 - 9; http://dx.doi.org/10.1507/endocrj.51.165; PMID: 15118266
- Indirect Food FDA. Additives: Polymers.Docket No. FDA-2012-F-0031. Available: http://www.gpo.gov/fdsys/pkg/FR-2012-07-17/pdf/2012-17366.pdf. Last accessed: 10-8-2012. 2012
- Yamamoto T, Yasuhara A. Quantities of bisphenol a leached from plastic waste samples. Chemosphere 1999; 38:2569 - 76; http://dx.doi.org/10.1016/S0045-6535(98)00464-0; PMID: 10204238
- Dorn PB. Chi-Su Chou, and Joseph J.Gentempo. Degradation of Bisphenol A in Natural Waters. Chemosphere 1987; 16:1501 - 7; http://dx.doi.org/10.1016/0045-6535(87)90090-7
- Staples CA, Dorn PB, Klecka GM, O’Block ST, Harris LR. A review of the environmental fate, effects, and exposures of bisphenol A. Chemosphere 1998; 36:2149 - 73; http://dx.doi.org/10.1016/S0045-6535(97)10133-3; PMID: 9566294
- Gibson D. Microbial Biodegradation of Organic Compounds. 1984; 13:535
- Kang JH, Kondo F. Bisphenol a degradation by bacteria isolated from river water. Arch Environ Contam Toxicol 2002; 43:265 - 9; http://dx.doi.org/10.1007/s00244-002-1209-0; PMID: 12202920
- Kuruto-Niwa R, Nozawa R, Miyakoshi T, Shiozawa T, Terao Y. Estrogenic activity of alkylphenols, bisphenol S, and their chlorinated derivatives using a GFP expression system. Environ Toxicol Pharmacol 2005; 19:121 - 30; http://dx.doi.org/10.1016/j.etap.2004.05.009; PMID: 21783468
- Yamamoto T, Yasuhara A. Chlorination of bisphenol A in aqueous media: formation of chlorinated bisphenol A congeners and degradation to chlorinated phenolic compounds. Chemosphere 2002; 46:1215 - 23; http://dx.doi.org/10.1016/S0045-6535(01)00198-9; PMID: 11951989
- Gallard H, Leclercq A, Croué JP. Chlorination of bisphenol A: kinetics and by-products formation. Chemosphere 2004; 56:465 - 73; http://dx.doi.org/10.1016/j.chemosphere.2004.03.001; PMID: 15212912
- Gallard H, Leclercq A, Croué JP. Chlorination of bisphenol A: kinetics and by-products formation. Chemosphere 2004; 56:465 - 73; http://dx.doi.org/10.1016/j.chemosphere.2004.03.001; PMID: 15212912
- Reed MJ, Purohit A, Woo LW, Newman SP, Potter BV. Steroid sulfatase: molecular biology, regulation, and inhibition. Endocr Rev 2005; 26:171 - 202; http://dx.doi.org/10.1210/er.2004-0003; PMID: 15561802
- Zhu BT, Conney AH. Functional role of estrogen metabolism in target cells: review and perspectives. Carcinogenesis 1998; 19:1 - 27; http://dx.doi.org/10.1093/carcin/19.1.1; PMID: 9472688
- Matthews JB, Twomey K, Zacharewski TR. In vitro and in vivo interactions of bisphenol A and its metabolite, bisphenol A glucuronide, with estrogen receptors alpha and beta. Chem Res Toxicol 2001; 14:149 - 57; http://dx.doi.org/10.1021/tx0001833; PMID: 11258963
- Shimizu M, Ohta K, Matsumoto Y, Fukuoka M, Ohno Y, Ozawa S. Sulfation of bisphenol A abolished its estrogenicity based on proliferation and gene expression in human breast cancer MCF-7 cells. Toxicol In Vitro 2002; 16:549 - 56; http://dx.doi.org/10.1016/S0887-2333(02)00055-3; PMID: 12206822
- Snyder RW, Maness SC, Gaido KW, Welsch F, Sumner SC, Fennell TR. Metabolism and disposition of bisphenol A in female rats. Toxicol Appl Pharmacol 2000; 168:225 - 34; http://dx.doi.org/10.1006/taap.2000.9051; PMID: 11042095
- Campbell CH, Watson CS. A comparison of membrane vs. intracellular estrogen receptor-alpha in GH(3)/B6 pituitary tumor cells using a quantitative plate immunoassay. Steroids 2001; 66:727 - 36; http://dx.doi.org/10.1016/S0039-128X(01)00106-4; PMID: 11522334
- Norfleet AM, Clarke CH, Gametchu B, Watson CS. Antibodies to the estrogen receptor-α modulate rapid prolactin release from rat pituitary tumor cells through plasma membrane estrogen receptors. FASEB J 2000; 14:157 - 65; PMID: 10627290
- Norfleet AM, Thomas ML, Gametchu B, Watson CS. Estrogen receptor-α detected on the plasma membrane of aldehyde-fixed GH3/B6/F10 rat pituitary tumor cells by enzyme-linked immunocytochemistry. Endocrinology 1999; 140:3805 - 14; http://dx.doi.org/10.1210/en.140.8.3805; PMID: 10433242
- Powell CE, Soto AM, Sonnenschein C. Identification and characterization of membrane estrogen receptor from MCF7 estrogen-target cells. J Steroid Biochem Mol Biol 2001; 77:97 - 108; http://dx.doi.org/10.1016/S0960-0760(01)00040-1; PMID: 11377974
- Razandi M, Alton G, Pedram A, Ghonshani S, Webb P, Levin ER. Identification of a structural determinant necessary for the localization and function of estrogen receptor alpha at the plasma membrane. Mol Cell Biol 2003; 23:1633 - 46; http://dx.doi.org/10.1128/MCB.23.5.1633-1646.2003; PMID: 12588983
- Bulayeva NN, Gametchu B, Watson CS. Quantitative measurement of estrogen-induced ERK 1 and 2 activation via multiple membrane-initiated signaling pathways. Steroids 2004; 69:181 - 92; http://dx.doi.org/10.1016/j.steroids.2003.12.003; PMID: 15072920
- Campbell CH, Bulayeva N, Brown DB, Gametchu B, Watson CS. Regulation of the membrane estrogen receptor-alpha: role of cell density, serum, cell passage number, and estradiol. FASEB J 2002; 16:1917 - 27; http://dx.doi.org/10.1096/fj.02-0182com; PMID: 12468456
- Jeng YJ, Kochukov MY, Watson CS. Membrane estrogen receptor-alpha-mediated nongenomic actions of phytoestrogens in GH3/B6/F10 pituitary tumor cells. J Mol Signal 2009; 4:2; http://dx.doi.org/10.1186/1750-2187-4-2; PMID: 19400946
- Jeng YJ, Kochukov M, Nauduri D, Kaphalia BS, Watson CS. Subchronic exposure to phytoestrogens alone and in combination with diethylstilbestrol - pituitary tumor induction in Fischer 344 rats. Nutr Metab (Lond) 2010; 7:40; http://dx.doi.org/10.1186/1743-7075-7-40; PMID: 20459739
- Jeng YJ, Watson CS. Proliferative and anti-proliferative effects of dietary levels of phytoestrogens in rat pituitary GH3/B6/F10 cells - the involvement of rapidly activated kinases and caspases. BMC Cancer 2009; 9:334; http://dx.doi.org/10.1186/1471-2407-9-334; PMID: 19765307
- Jeng YJ, Kochukov M, Watson CS. Combinations of physiologic estrogens with xenoestrogens alter calcium and kinase responses, prolactin release, and membrane estrogen receptor trafficking in rat pituitary cells. Environ Health 2010; 9:61; http://dx.doi.org/10.1186/1476-069X-9-61; PMID: 20950447
- Bulayeva NN, Watson CS. Xenoestrogen-induced ERK-1 and ERK-2 activation via multiple membrane-initiated signaling pathways. Environ Health Perspect 2004; 112:1481 - 7; http://dx.doi.org/10.1289/ehp.7175; PMID: 15531431
- Vandenberg LN, Colborn T, Hayes TB, Heindel JJ, Jacobs DR Jr., Lee DH, et al. Hormones and endocrine-disrupting chemicals: low-dose effects and nonmonotonic dose responses. Endocr Rev 2012; 33:378 - 455; http://dx.doi.org/10.1210/er.2011-1050; PMID: 22419778
- Conolly RB, Lutz WK. Nonmonotonic dose-response relationships: mechanistic basis, kinetic modeling, and implications for risk assessment. Toxicol Sci 2004; 77:151 - 7; http://dx.doi.org/10.1093/toxsci/kfh007; PMID: 14600281
- Watson CS, Jeng YJ, Kochukov MY. Nongenomic signaling pathways of estrogen toxicity. Toxicol Sci 2010; 115:1 - 11; http://dx.doi.org/10.1093/toxsci/kfp288; PMID: 19955490
- Weltje L, vom Saal FS, Oehlmann J. Reproductive stimulation by low doses of xenoestrogens contrasts with the view of hormesis as an adaptive response. Hum Exp Toxicol 2005; 24:431 - 7; http://dx.doi.org/10.1191/0960327105ht551oa; PMID: 16235731
- Hunter T. Protein kinases and phosphatases: the yin and yang of protein phosphorylation and signaling. Cell 1995; 80:225 - 36; http://dx.doi.org/10.1016/0092-8674(95)90405-0; PMID: 7834742
- McCubrey JA, Steelman LS, Chappell WH, Abrams SL, Wong EW, Chang F, et al. Roles of the Raf/MEK/ERK pathway in cell growth, malignant transformation and drug resistance. Biochim Biophys Acta 2007; 1773:1263 - 84; http://dx.doi.org/10.1016/j.bbamcr.2006.10.001; PMID: 17126425
- Allan LA, Morrice N, Brady S, Magee G, Pathak S, Clarke PR. Inhibition of caspase-9 through phosphorylation at Thr 125 by ERK MAPK. Nat Cell Biol 2003; 5:647 - 54; http://dx.doi.org/10.1038/ncb1005; PMID: 12792650
- Allan LA, Clarke PR. Phosphorylation of caspase-9 by CDK1/cyclin B1 protects mitotic cells against apoptosis. Mol Cell 2007; 26:301 - 10; http://dx.doi.org/10.1016/j.molcel.2007.03.019; PMID: 17466630
- Allan LA, Clarke PR. Apoptosis and autophagy: Regulation of caspase-9 by phosphorylation. FEBS J 2009; 276:6063 - 73; http://dx.doi.org/10.1111/j.1742-4658.2009.07330.x; PMID: 19788417
- Junttila MR, Li SP, Westermarck J. Phosphatase-mediated crosstalk between MAPK signaling pathways in the regulation of cell survival. FASEB J 2008; 22:954 - 65; http://dx.doi.org/10.1096/fj.06-7859rev; PMID: 18039929
- Nordström E, Fisone G, Kristensson K. Opposing effects of ERK and p38-JNK MAP kinase pathways on formation of prions in GT1-1 cells. FASEB J 2009; 23:613 - 22; http://dx.doi.org/10.1096/fj.08-115360; PMID: 18824519
- Xia Z, Dickens M, Raingeaud J, Davis RJ, Greenberg ME. Opposing effects of ERK and JNK-p38 MAP kinases on apoptosis. Science 1995; 270:1326 - 31; http://dx.doi.org/10.1126/science.270.5240.1326; PMID: 7481820
- Meloche S, Pouysségur J. The ERK1/2 mitogen-activated protein kinase pathway as a master regulator of the G1- to S-phase transition. Oncogene 2007; 26:3227 - 39; http://dx.doi.org/10.1038/sj.onc.1210414; PMID: 17496918
- Ip YT, Davis RJ. Signal transduction by the c-Jun N-terminal kinase (JNK)--from inflammation to development. Curr Opin Cell Biol 1998; 10:205 - 19; http://dx.doi.org/10.1016/S0955-0674(98)80143-9; PMID: 9561845
- Viñas R, Watson CS. Chemical Mixtures of Bisphenol-A and Bisphenol-S disrupt E2-induced non-genomic signaling in mERá-enriched (GH3/B6/F10) rat pituitary cell line. Abstract: Endocrine Society Annual Meeting June 2012. Endocrine Society Annual Meeting June (2012) 2012
- Lu H, Crawford RB, Kaplan BL, Kaminski NE. 2,3,7,8-Tetrachlorodibenzo-p-dioxin-mediated disruption of the CD40 ligand-induced activation of primary human B cells. Toxicol Appl Pharmacol 2011; 255:251 - 60; http://dx.doi.org/10.1016/j.taap.2011.06.026; PMID: 21807014
- North CM, Crawford RB, Lu H, Kaminski NE. 2,3,7,8-tetrachlorodibenzo-p-dioxin-mediated suppression of toll-like receptor stimulated B-lymphocyte activation and initiation of plasmacytic differentiation. Toxicol Sci 2010; 116:99 - 112; http://dx.doi.org/10.1093/toxsci/kfq095; PMID: 20348231
- Dhanasekaran DN, Reddy EP. JNK signaling in apoptosis. Oncogene 2008; 27:6245 - 51; http://dx.doi.org/10.1038/onc.2008.301; PMID: 18931691
- Sánchez-Perez I, Murguía JR, Perona R. Cisplatin induces a persistent activation of JNK that is related to cell death. Oncogene 1998; 16:533 - 40; http://dx.doi.org/10.1038/sj.onc.1201578; PMID: 9484843
- Brzozowski AM, Pike AC, Dauter Z, Hubbard RE, Bonn T, Engstrom O, et al. Molecular basis of agonism and antagonism in the oestrogen receptor. Nature 1997; 389 - 8; PMID: 9311781
- Tabira Y, Nakai M, Asai D, Yakabe Y, Tahara Y, Shinmyozu T, et al. Structural requirements of para-alkylphenols to bind to estrogen receptor. Eur J Biochem 1999; 262:240 - 5; http://dx.doi.org/10.1046/j.1432-1327.1999.00422.x; PMID: 10231387
- Nakai M, Tabira Y, Asai D, Yakabe Y, Shimyozu T, Noguchi M, et al. Binding characteristics of dialkyl phthalates for the estrogen receptor. Biochem Biophys Res Commun 1999; 254:311 - 4; http://dx.doi.org/10.1006/bbrc.1998.9928; PMID: 9918834
- Kuruto-Niwa R, Terao Y, Nozawa R. Identification of estrogenic activity of chlorinated bisphenol A using a GFP expression system. Environ Toxicol Pharmacol 2002; 12:27 - 35; http://dx.doi.org/10.1016/S1382-6689(02)00011-X; PMID: 21782620
- Kuruto-Niwa R, Nozawa R, Miyakoshi T, Shiozawa T, Terao Y. Estrogenic activity of alkylphenols, bisphenol S, and their chlorinated derivatives using a GFP expression system. Environ Toxicol Pharmacol 2005; 19:121 - 30; http://dx.doi.org/10.1016/j.etap.2004.05.009; PMID: 21783468
- Samuelsen M, Olsen C, Holme JA, Meussen-Elholm E, Bergmann A, Hongslo JK. Estrogen-like properties of brominated analogs of bisphenol A in the MCF-7 human breast cancer cell line. Cell Biol Toxicol 2001; 17:139 - 51; http://dx.doi.org/10.1023/A:1011974012602; PMID: 11693576
- Canesi L, Lorusso LC, Ciacci C, Betti M, Zampini M, Gallo G. Environmental estrogens can affect the function of mussel hemocytes through rapid modulation of kinase pathways. Gen Comp Endocrinol 2004; 138:58 - 69; http://dx.doi.org/10.1016/j.ygcen.2004.05.004; PMID: 15242752
- Liu R, Xing L, Kong D, Jiang J, Shang L, Hao W. Bisphenol A inhibits proliferation and induces apoptosis in micromass cultures of rat embryonic midbrain cells through the JNK, CREB and p53 signaling pathways. Food Chem Toxicol 2013; 52:76 - 82; http://dx.doi.org/10.1016/j.fct.2012.10.033; PMID: 23146694
- Song KH, Lee K, Choi HS. Endocrine disrupter bisphenol a induces orphan nuclear receptor Nur77 gene expression and steroidogenesis in mouse testicular Leydig cells. Endocrinology 2002; 143:2208 - 15; http://dx.doi.org/10.1210/en.143.6.2208; PMID: 12021184
- Xu X, Li T, Luo Q, Hong X, Xie L, Tian D. Bisphenol-A rapidly enhanced passive avoidance memory and phosphorylation of NMDA receptor subunits in hippocampus of young rats. Toxicol Appl Pharmacol 2011; 255:221 - 8; http://dx.doi.org/10.1016/j.taap.2011.06.022; PMID: 21763338
- Watson CS, Campbell CH, Gametchu B. Membrane oestrogen receptors on rat pituitary tumour cells: immuno-identification and responses to oestradiol and xenoestrogens. Exp Physiol 1999; 84:1013 - 22; http://dx.doi.org/10.1111/j.1469-445X.1999.01903.x; PMID: 10564698
- Norfleet AM, Thomas ML, Watson CS. Modulation of membrane estrogen receptor-α levels by nuclear estrogen receptor-α antisense oligodeoxynucleotides in the rat pituitary tumor cell line, GH3/B6/F10. Endocrine Society Meeting 1999
- Pedram A, Razandi M, Levin ER. Nature of functional estrogen receptors at the plasma membrane. Mol Endocrinol 2006; 20:1996 - 2009; http://dx.doi.org/10.1210/me.2005-0525; PMID: 16645038
- Pedram A, Razandi M, Deschenes RJ, Levin ER. DHHC-7 and -21 are palmitoylacyltransferases for sex steroid receptors. Mol Biol Cell 2012; 23:188 - 99; http://dx.doi.org/10.1091/mbc.E11-07-0638; PMID: 22031296
- Watson CS, Gametchu B. Membrane-initiated steroid actions and the proteins that mediate them. Proc Soc Exp Biol Med 1999; 220:9 - 19; http://dx.doi.org/10.1046/j.1525-1373.1999.d01-2.x; PMID: 9893163
- Watson CS, Lange CA. Steadying the boat: integrating mechanisms of membrane and nuclear-steroid-receptor signalling. EMBO Rep 2005; 6:116 - 9; http://dx.doi.org/10.1038/sj.embor.7400336; PMID: 15678158
- Schug TT, Abagyan R, Blumberg B, Collins T, Crews D, DeFur P, et al. Designing endocrine disruption out of the next generation of chemicals. Green Chem 2012; 1:181 - 98
- Pappas TC, Gametchu B, Yannariello-Brown J, Collins TJ, Watson CS. Membrane estrogen receptors in GH3/B6 cells are associated with rapid estrogen-induced release of prolactin. Endocrine 1994; 2:813 - 22
- Pappas TC, Gametchu B, Watson CS. Membrane estrogen receptor-enriched GH(3)/B6 cells have an enhanced non-genomic response to estrogen. Endocrine 1995; 3:743 - 9; http://dx.doi.org/10.1007/BF03000207; PMID: 21153164
- Bulayeva NN, Wozniak AL, Lash LL, Watson CS. Mechanisms of membrane estrogen receptor-alpha-mediated rapid stimulation of Ca2+ levels and prolactin release in a pituitary cell line. Am J Physiol Endocrinol Metab 2005; 288:E388 - 97; http://dx.doi.org/10.1152/ajpendo.00349.2004; PMID: 15494610
- Viñas R, Watson CS. Bisphenol S disrupts estradiol-induced nongenomic signaling in a rat pituitary cell line: effects on cell functions. Environ Health Perspect 2013; 121:352 - 8; http://dx.doi.org/10.1289/ehp.1205826; PMID: 23458715
- Vinas R, Watson CS. Mixtures of xenoestrogens disrupt estradiol-induced nongenomic signaling and functions in pituitary cells. BMC Environmental Health 2013
- Demarest KT, Riegle GD, Moore KE. Pharmacological manipulation of anterior pituitary dopamine content in the male rat: relationship to serum prolactin concentration and lysosomal enzyme activity. Endocrinology 1984; 115:493 - 500; http://dx.doi.org/10.1210/endo-115-2-493; PMID: 6745164
- Connolly PB, Resko JA. Estrone sulfatase activity in rat brain and pituitary: effects of gonadectomy and the estrous cycle. J Steroid Biochem 1989; 33:1013 - 8; http://dx.doi.org/10.1016/0022-4731(89)90254-9; PMID: 2601328
- Oleson L, Court MH. Effect of the beta-glucuronidase inhibitor saccharolactone on glucuronidation by human tissue microsomes and recombinant UDP-glucuronosyltransferases. J Pharm Pharmacol 2008; 60:1175 - 82; http://dx.doi.org/10.1211/jpp.60.9.0009; PMID: 18718121