Abstract
Polybrominated diphenyl ethers (PBDEs) are a class of brominated flame retardant chemicals that have been used in large quantities and are now detected worldwide in humans and wildlife. To complement reviews of effects on human health, this review discusses and synthesizes current evidence of PBDE toxicokinetics and toxicity mechanisms leading to perturbations of thyroid hormone homeostasis in fish. PBDE disruptions to thyroid signaling in fish appear to proceed through multiple pathways involving declines in circulating thyroid hormones, disrupted deiodination activity, hindered hormone transport, and altered transcriptional regulation of genes involved in thyroid hormone production, transport, and genomic signaling. PBDE exposures have also been linked to impacts on reproductive health with reductions in fecundity, spawning, hatching success, and offspring survival observed in some species, as well as impaired fertility. These studies on PBDE mediated hormone disruption in fish can help inform future studies seeking to understand potential developmental effects in humans.
Introduction
Polybrominated diphenyl ethers (PBDEs) are a class of brominated flame retardant (BFR) chemicals that have been added to many consumer and commercial products including textiles, carpeting, construction materials, and electronics in an effort to reduce their combustibility. Although these compounds have been both banned or phased-out from production in a number of countries, human and environmental exposures continue as products that contain these chemicals are still used and recycled, and present legacy contamination problems upon disposal. As a consequence, PBDEs are widespread and persistent contaminants in both living and non-living parts of the global environment.Citation1-Citation9
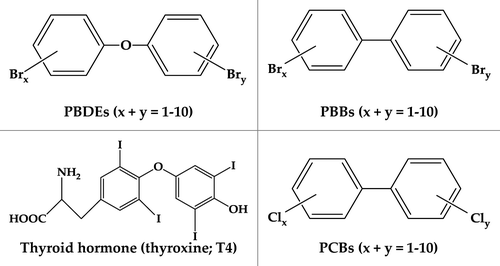
PBDEs can have from 1–10 bromine atoms substituted on diphenyl ether (). There are 209 PBDE congeners (BDE-1 to BDE-209) theoretically possible depending on the number and substitution patterns of bromine. In practice, however, the number of congeners formed is limited based on the chemical properties and composition of the PBDE commercial mixtures. Three PBDE commercial mixtures have been produced: PentaBDE, OctaBDE, and DecaBDE. The PentaBDE commercial mixture is a heterogeneous mix of tetra-, penta-, and hexaBDEs and was added mostly to polyurethane foams and textiles, and to a lesser extent in epoxy and phenolic resins and polyesters. The majority (~95%) of PentaBDE was used in the US where it was added to polyurethane foams in furniture cushioning and could constitute up to 30% by weight of these products.Citation10 The OctaBDE commercial mixture is made up of higher molecular weight (MW) constituents, hepta, octa, and nona-BDEs and was added predominantly to acrylonitrile butadiene styrene (ABS) used in the plastic housing of office equipment and electronics. The production and use of PentaBDE and OctaBDE were phased out in the US and banned in the European Union in 2004 due to concerns about their persistence, bioaccumulation, and toxicity. In 2009, these products were also listed as persistent organic pollutants (POPs) under the United Nations Stockholm Convention.Citation11 The DecaBDE mixture contains the fully brominated congener decabromodiphenyl ether (BDE-209; ~97%) with trace amounts of nonaBDEs. It is used as an additive in high impact polystyrene, polyolefin, and polypropylene plastics used in electronics, automobiles, airplanes, and construction and building materials. To achieve US fire safety standards for plastics, high impact polystyrene plastics typically contain ~10–15% of DecaBDE.Citation12 DecaBDE is one of two dominant BFRs used worldwide with 2007 global consumption estimated at ~73 000 t (~161 million pounds).Citation13,Citation14 The production of DecaBDE was discontinued in the US at the end of 2013 under a voluntary phase-out and has been restricted from use in electronics in the European Union since 2008. It is generally unrestricted from use in Asia.Citation15
PBDEs structurally resemble polybrominated biphenyls (PBBs), polychlorinated biphenyls (PCBs) and some biomolecules, most notably thyroid hormones (THs; ). Because PBDEs are not chemically bound but are rather added to plastics, they can enter the environment during production and may be released into the surrounding environment and biota with the breakdown and volatilization of the parent polymer. Like other persistent, hydrophobic chemicals, the most important route of uptake in aquatic animals appears to be by trophic transfer and consumption of foods contaminated with PBDEs.Citation16,Citation17 This dietary exposure pathway in aquatic animals is distinguished from human uptake that appears to depend on both dietary exposures and the incidental ingestion of PBDE-containing dust.Citation18,Citation19
Despite the discontinued use of PentaBDE, its constituents, including BDE-47 (2,2’4,4’-tetraBDE), BDE-99 (2,2’,4,4’,5-pentaBDE), BDE-100 (2,2’,4,4’,6-pentaBDE), BDE-153 (2,2’,4,4’,5,5′-hexaBDE), and BDE-154 (2,2’,4,4’,5,6’-hexaBDE), continue to be dominant PBDEs frequently detected in humans and wildlife worldwide despite the generally more limited use of PentaBDE outside the US.Citation20,Citation21 Potential sources of these congeners are likely related to the ongoing use and recycling of products that contain PentaBDE as well as their high environmental persistence and long-range global transport potential.Citation22 Another source of these lower MW PBDEs may be attributable to the breakdown of higher PBDEs, such as BDE-209, which can undergo photolytic degradation,Citation23 microbial breakdown,Citation24 and metabolic biotransformationCitation25 to lower MW congeners.
Recent attention has focused on the potential for BDE-209 and other highly brominated PBDEs to bioaccumulate. BDE-209 is now the dominant PBDE measured in abiotic compartments, typically at ppb to ppm levels (ng/g dry weight [dw] to low mg/g dw) in dust,Citation18,Citation26 soils and sediments,Citation27-Citation29 and biosolids.Citation30,Citation31 Studies show that BDE-209 is bioaccumulating in a large number and variety of biota residing all over the world, including, for example, in birds and bird eggs,Citation32,Citation33 terrestrial and aquatic mammals,Citation34-Citation36 and plankton, fish and shellfish.Citation20,Citation37 Human body burdens of BDE-209 also appear to be rising including among E-waste workers and people residing near PBDE production facilitiesCitation38,Citation39 as well as among the general population, particularly young children in the US.Citation18,Citation40 People residing in some regions of China with heavy E-waste recycling operations report some of the highest PBDE body burdens in the world, with BDE-209 concentrations in serum at greater than 3000 ng/g lipid. DecaBDE is now more commonly detected among the general population, particularly young children in the US population. For example, recent work in our laboratory,Citation18 in collaboration with the Centers for Disease Control (CDC) and Boston University, measured BDE-209 levels in the serum of a North Carolina cohort of toddlers (3 y old) ranging from <6–68 ng/g lipid. In addition, other highly brominated PBDEs are being detected more frequently in biota, including the hexa- to nonaBDEs, which may reflect the increased use of BDE-209 and its environmental breakdown and biological metabolism.Citation17,Citation41
This review summarizes the biological disposition and toxicity of PBDEs in teleost fishes with particular focus on thyroid disruption mechanisms and interactions as this endocrine system is an important target of the PBDEs. Indeed, most laboratory studies published to date in fish (and other vertebrates) have focused on the potential for PBDEs to perturb thyroid signaling, as well as impair neurological development and reproduction. While these are often the primary endpoints of focus, other toxicity outcomes have been observed in fish as well, including immunotoxicityCitation42,Citation43 and oxidative stress.Citation44-Citation46 The PentaBDE commercial mixture and its component congeners have been the subject of most study to date, with less known about the toxicity of BDE-209 and the other higher MW PBDE congeners. However, BDE-209 is the only PBDE that has been evaluated for carcinogenicity with “suggestive evidence of carcinogenic potential” based on increased thyroid cell hyperplasia and thyroid adenomas/carcinomas in male mice and liver tumors in male rats.Citation47
PBDE data generated in fish not only informs our understanding of potential effects in wild fish species and populations but also provides important information on mechanisms of toxicity and disease in humans due to the high degree of gene and functional conservation shared across vertebrates. Indeed, PBDE toxicity measured in fish shares mutual features and effects to those observed among in vivo and in vitro mammalian models, supporting potentially common biological mechanisms of toxicity that also lend biological plausibility to the human epidemiology data on PBDEs. Therefore, this review includes brief discussion of effects observed in rodent and human epidemiology studies in so far as to frame a fuller picture of the toxicity of these chemicals. PBDE human health effects have been examined in several informative reviews and readers are referred to these papers for more detailed analyses.Citation48-Citation50
Toxicokinetics of PBDEs
Patterns of PBDE toxicokinetics (i.e., absorption, distribution, metabolism, and excretion; ADME) in fishes have been shown to vary depending on the PBDE congener, species, life-stage, and route of exposure. summarizes PBDE toxicokinetic studies in fishes to date.
Table 1. PBDE toxicokinetics measured in teleost fish species
PBDE Uptake and Tissue Distribution
Our understanding of PBDE absorption in fishes is somewhat limited by the species and PBDE congeners studied. Two early studies in Northern Pike (E. lucius) found that BDE-47 was readily absorbed with measured uptake efficiencies of 14C-BDE-47 and unlabeled BDE-47 at 90–100%.Citation51,Citation52 This research group also measured uptake efficiencies of BDE-99 and BDE-153 in pike at ~60% and ~40%, respectively. Studies in rodents have likewise measured absorption of BDE-47, -99, -100, -153, and -154 in the range of ~70–90%.Citation53-Citation55 However, other studies that have exposed fish to unlabeled PBDEs through the diet have measured lower assimilation efficiencies of some congeners, including BDE-99, BDE-153, BDE-183, and BDE-209 suggesting species-specific differences in assimilation efficiencies of PBDEs possibly due to differences in metabolic enzyme systems.Citation56-Citation58 For instance, BDE-209 absorption in some teleost fishes has been shown to occur at a slow rate, which may allow for greater metabolism and elimination than seen in terrestrial species. A dietary study in juvenile rainbow trout receiving 7.5–10 mg/kg bw-day of BDE-209 measured bioavailability at <1%Citation59 with higher bioavailability of 3.2% measured in juveniles of the same species receiving a chronic dietary exposure.Citation60 In fathead minnow juveniles and adults exposed orally to ~10 µg/g ww food, BDE-209 uptake efficiencies were also low at 5.8%Citation61 and 1.3%,Citation62 respectively. Nonetheless, despite its low bioavailability, BDE-209 appears to be bioaccumulating in fish as shown in field measures and laboratory-based studies with BDE-209 spiked sediments.Citation6 While the apparent dichotomy between low bioavailability of some PBDEs, notably BDE-209, and observed bioaccumulation are not fully described, rainbow trout exposed to BDE-209 thru the diet have been shown to bioaccumulate BDE-209 at 1.3 times the concentration of levels in their food, suggesting bioaccumulation that is strongly influenced by tissue composition.Citation60 In addition, recent findings in Chinese sturgeon (A. sinensis) also support that tissue distribution patterns of the higher PBDEs, rather than lipid binding, are important factors influencing their bioaccumulation.Citation63 This study showed low partitioning of the higher PBDEs (heptaBDEs to BDE-209) from blood to tissues that could in turn lead to their slower delivery to metabolically active tissues and thus higher bioaccumulation.
The dominant PBDEs measured in biota (i.e., BDE-47, -99, -100, -153, and -154) are deposited to lipophilic tissue compartments, and these congeners continue to be detected in human serum, breast tissue, and milkCitation18,Citation64,Citation65 and in adipose tissues of a variety of wildlife, including free ranging fish species.Citation17,Citation66,Citation67 PBDEs have been shown to cross the blood–placenta and blood-brain barriers to accumulate in the brains of perinatally exposed rats exposed to the PentaBDE commercial mixtureCitation68 and some birds of prey.Citation69 In addition to accumulation in lipid-rich tissues, the liver is an important target of PBDE disposition and toxicity. The US EPA National Toxicology Program has published findings showing hepatotoxicity, including elevated liver enzyme activity accompanied by hepatic hypertrophy and vacuolizations in mice exposed orally to the PentaBDE mixture for 13 wk.Citation70 The liver also appears to be an important site of PBDE accumulation in fish. In pike fish, 14C-BDE-47 accumulated in the liver and in lipid rich tissues. In rainbow trout (O. mykiss) exposed orally to BDE-209, the highest concentration of BDE-209 was measured in the liver on both a lipid normalized and body weight basis, followed by accumulation in the serum, with less accumulation in the carcass.Citation60 BDE-209 deposition to adipose tissues might be predicted given its low water solubility (<0.1 µg/l) and high octanol-water partitioning coefficient (log Kow; 6–12).Citation71 However, studies have shown that this pattern of preferential deposition to lipid depots does not occur substantially for BDE-209. Rather, BDE-209 preferentially distributes to highly perfused, blood-rich tissues, particularly the liver, kidney, heart, and intestinal wall.Citation25,Citation72,Citation73 The underlying reasons for this distribution pattern appear to be attributable to the large size of BDE-209 and its ability to bind with plasma proteins.Citation72
PBDE Reductive Metabolism in Fish
To understand PBDE metabolic pathways in fish, it is informative to frame the discussion in terms of present knowledge of PBDE metabolism in mammals as this is now fairly well described. The rodent literature supports a PBDE metabolic pathway in mammals that has two major reactions: (1) a cytochrome P450 (CYP450)-mediated epoxidation of PBDE phenyl rings catalyzed predominantly by CYP2B (by constitutive androstane receptor [CAR] inductions) and by CYP3A (by pregnane X receptor [PXR] inductions); and (2) debromination or Phase II conjugation of an OH-intermediate with glucuronides catalyzed by uridine diphosphate glucuronosyl transferases (UDPGTs) and with sulfates by sulfotransferases (SULTs).Citation87,Citation88 Reductive debromination reactions appear to be minor pathways of PBDE metabolism in mammals (e.g., BDE-209 debromination to octa- and nonaBDEs).
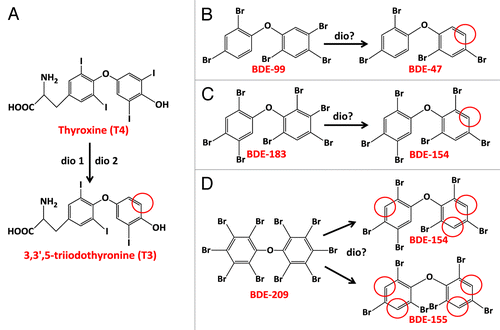
PBDE metabolism in teleost fish appears to be different from that in mammals. As outlined in , a large number of studies have shown reductive debromination of PBDEs to be a major route of metabolism, including in common carp (C. carpio),Citation25 fathead minnow (P. promelas)Citation61,Citation62; rainbow trout,Citation59,Citation60 lake trout (S. namaycush),Citation89 Chinook salmon (O. tshawytscha),Citation76,Citation83 and zebrafish (D. rerio).Citation78 However, while PBDE reductive debromination appears to be a major metabolic pathway in fish, the role of specific enzyme systems in catalyzing this biotransformation remains unclear. One pathway that has been hypothesized to mediate PBDE reductive metabolism in fish is by the activity of iodothyronine deiodinase (Dio) enzymes.Citation25,Citation89 Dios are membrane-bound enzymes that are expressed on plasma membranes and in the endoplasmic reticulum, and regulate TH levels in vertebrates.Citation90 There are three known Dio isoforms in fish, Types 1, 2, and 3 (Dio 1, Dio 2, and Dio 3, respectively) that share functional homology with mammalian Dio isoforms. As illustrated in , the conversion of the TH thyroxine (T4) to the genomically active 3,3′,5-triiodothyronine (T3) hormone is catalyzed by the cleavage of iodine from the meta-position of the outer phenyl ring of T4. The reductive debromination of PBDEs in fishes is also dominated by meta-cleavages of bromine, suggesting a possible role for these enzymes in catalyzing PBDE debromination.Citation25,Citation89 More recent studies have shown that the reductive debromination of BDE-99 to BDE-47 can be substantially inhibited by co-incubating liver microsomes from common carp with THs, suggesting that BDE-99 may be a substrate that competes with THs for Dio enzyme activity.Citation74,Citation81
Figure 2. Outer-ring deiodination of thyroxine (A) catalyzed by Dio 1 and 2 enzymes resulting in meta-cleavage of iodine from T4. Reductive metabolites and meta-position debromination measured in common carp (C. carpio) exposed orally to (B) BDE-99, (C) BDE-183, and (D) BDE-209.Citation25,Citation58,Citation60
Although BDE-glutathione metabolites have been measured in rodentsCitation53,Citation91 and birds,Citation92 glutathione-S-transferases (GSTs) have not been found to be involved in the reductive debromination of BDE-99 to BDE-49 in Chinook salmonCitation76 or of BDE-99 to BDE-47 in common carp,Citation74,Citation81 suggesting that they may not play an important role in PBDE debromination in fish. This finding has been subsequently confirmed in negative results from in vitro testing with liver microsomes from Chinook salmon, rainbow trout, and common carp incubated with several PBDE congenersCitation83 and in juvenile fathead minnows exposed in vivo to BDE-209.Citation61 Taken together, despite these lines of indirect evidence, additional work is needed to better understand the underlying enzymes catalyzing the reductive debromination of PBDEs in fishes, and the potential role of Dio enzymes and/or other possible reductases that have yet to be described.
Mixed Evidence of PBDE Oxidative Metabolism in Fish
Laboratory studies in fish have presented mixed results concerning the formation of hydroxylated PBDE (OH-BDE) metabolites. For instance, no OH-BDEs were detected in the serum of common carp receiving dietary exposures to a mixture of BDE-28, -47, -99, and -153.Citation84 Likewise, OH-BDEs were not detected in liver microsomes from common carp, rainbow trout, or Chinook salmon incubated with various PBDEs, including BDE-209.Citation76,Citation83 In contrast, 11 OH-BDEs were reported in the serum of juvenile common carp exposed to the PentaBDE mixture with no OH-BDEs in DecaBDE exposed fish.Citation86 Another BDE-209 study, however, reported substantial OH-BDE and methoxy PBDE (MeO-BDE) formation in trout.Citation93 OH-BDE metabolites were also reported in juvenile common sole (S. solea L.) exposed to a mixture of congeners (BDE-28, -47, -99, -100, -153, -209).Citation80 In studies showing positive OH-BDE results, the gas chromatography-mass spectrometry (GC/MS) injection techniques used in the PBDE analyses were either not specifiedCitation86 or employed split/splitless injection.Citation80,Citation93 GC/MS splitless injection techniques for PBDE analysis can lead to thermal degradation of parent PBDEs and the formation of byproducts in the GC/MS inlet that may confound identification of MeO-BDEs (GC/MS derivatives of OH-BDE metabolites).Citation94 Although potential analytical confounders were not addressed, if oxidative metabolism occurs in fish, it appears to be a minor metabolic pathway compared with reductive debromination.
In vivo and in vitro studies in fish have shown both weak inductionCitation78,Citation95 and inhibitionCitation82,Citation96 of ethoxyresorufin-O-deethylase (EROD) activity (biomarker of CYP1A and aryl hydrocarbon (AhR) induction) upon exposure to individual PBDE congeners. However, a larger number of PBDE studies in teleost fish have shown no AhR/CYP1A activation.Citation75,Citation89,Citation97,Citation98 Thus, it appears that PBDEs operate predominantly through non-dioxin, AhR independent toxicity mechanisms and are not metabolized by CYP1A. Conversely, the PentaBDE commercial mixture contains small amounts of polybrominated dibenzo-p-dioxins/dibenzofurans (PBDDs/PBDFs) that are trace byproducts formed by thermal stress during production and that activate the AhR. The reasons for the disparate results in the literature are not clear but may be attributable to variations in the purity of formulations tested.
While mammalian studies support PBDE oxidative metabolism by CYP2B and CYP3A catalyzed by CAR/PXR inductions, similar pathways have not been observed in fishes. The expression of CYP2B in fish and the regulatory mechanisms involved in its induction are still unclear. In mammals, phenobarbital (PB) and ortho-substituted halogenated aromatics (e.g., ortho-chlorine-substituted PCBs) are strong inducers of CYP2B through activation of CAR.Citation99 In teleost fish, however, the induction of CYP2B in the presence of PB-type inducers has not been observed.Citation100 Thus, there appear to be important functional differences between piscovorous and mammalian CAR/CYP2B that may play a role in its lack of induction in PBDE-exposed fish, although this has not been examined. With regard to other AhR-independent mechanisms, the function and tissue distribution of enzymes in the CYP3 gene family are not well characterized in fish, but the CYP3A isoforms appear highly versatile with broad substrate affinities.Citation101
PBDE Conjugation in Fish
There has been little research to date to examine the role of UDPGTs and SULTS in the metabolism of PBDEs in fishes, although these enzymes are important catalytic drivers of Phase II metabolism in fishes.Citation100 The UDPGTs catalyze the glucuronidation of an array of endogenous and exogenous substrates to more polar, water-soluble compounds for elimination. The SULTs catalyze the transfer of the sulfonate from 3′-phosphoadenosine-5′-phosphosulfate (PAPS) to hydroxylated and amine substituents on numerous exogenous and endogenous substrates to facilitate elimination. In zebrafish, several UDPGTCitation102 and SULT genesCitation103,Citation104 have been characterized with prototypical substrates such as bilirubin, TH, estradiol (E2), testosterone (T), and phenolic contaminants. As many as 10 different UDPGT isoforms have been identified in zebrafish, with nucleotide similarities to some mammalian UGT1 and UGT2 gene families.Citation105 Two studies have shown a decrease in the relative mRNA abundances of genes encoding UDPGT1ab in zebrafish larvae exposed to BDE-209Citation106 and UGT1 in juvenile Atlantic cod (G. morhua) exposed to BDE-47.Citation82 This decline may be a response to reduced TH levels as UDPGTs are involved in the metabolism of THs.
PBDE Elimination
provides whole body elimination half-lives (t1/2) reported in fish for PBDEs frequently detected in the environment and biota, with inclusion of data in humans and rodents for comparison. In rodents, the major route of PBDE elimination is by the fecal route with low levels of excretion in the urine and bile depending on the PBDE congener.Citation88,Citation107 In fish, routes of PBDE elimination have not been targeted specifically but the early studies in pike suggest that biliary and fecal excretion also occurs.Citation52 Some reports estimate apparent half-lives in humans for the tetra - hexaBDEs that are substantially longer than those reported in rodents and some fish. Conversely, some data in rodents suggest relatively short half-lives (e.g., BDE-99) that are incongruous with the human and fish data. Thus, there continues to be uncertainty about PBDE elimination half-lives in fish and other biota with substantial species variability apparent that appears related to differential metabolism.
Table 2. Whole-body elimination half-lives (t1/2) of environmentally relevant PBDEs measured and estimated in humans, laboratory rodents, and teleost fish species
Fish Thyroid System
To discuss PBDE-related TH disruption, it is informative to broadly highlight current knowledge of the structure and function of the fish thyroid with some comparison to the mammalian thyroid. The vertebrate thyroid is well-conserved across taxa, and chemical effects in lower level vertebrates like fish can reveal mechanisms of thyroid dysregulation in higher level species. THs are key regulators of vertebrate development, endothermic basal metabolism, and organ system physiology. The importance of TH in brain and somatic development is well established, and small changes in maternal or fetal TH can cause severe motor skill deficiencies and irreversible cognitive impairments.Citation114 Recent attention has focused on the permissive role of THs in regulating physiological processes in adults, including neurological plasticity, mood, cognition, and reproduction.Citation90,Citation115 In fish, THs are important mediators of many physiological, developmental, and behavioral processes, including growth and metamorphic transitioning,Citation116,Citation117 osmoregulation,Citation118 olfactory imprinting,Citation119 interrenal regulation,Citation120 otolith formation,Citation121 and reproduction,Citation90,Citation122 often acting in concert with other hormones.Citation123,Citation124
Structure and Function of Fish Thyroid
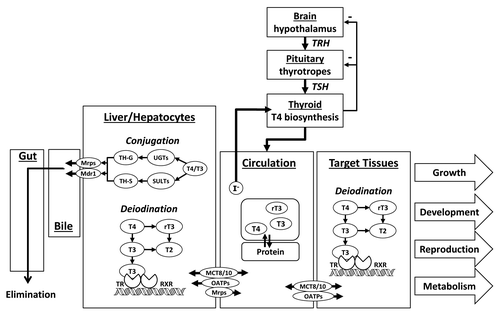
The general architecture of the thyroid appears to be similar across vertebrates whereby circulating levels of TH are tightly controlled by both a centrally operating hypothalamic-pituitary-thyroid (HPT) axis and in peripheral tissues through the activity of Dio enzymes, among other dynamically operating regulatory processes (). The functional unit of the central HPT is the thyroid follicle where the THs T4 and 3,5,3′-triiodothyronine (T3) are synthesized and secreted into circulation. However, there continue to be gaps in our understanding of the fish thyroid in comparison to the more thoroughly studied mammalian and amphibian thyroid systems. Typically in teleost fish, thyroid follicles are found dispersed predominantly in the ventral pharyngeal region, rather than being organized in a compact lobular gland as seen in higher vertebrates. One important differentiating feature of the fish thyroid may relate to how THs are produced and regulated. In fish, T4 may be the primary, possibly only TH produced in the thyroid where it is under negative feedback control by the HPT axis. The production of T3 in fish, in contrast, is thought to be under exclusive control of peripheral tissues, although this has not been studied recently or beyond Salmonid fishes.Citation125 In some contrast, the mammalian thyroid gland produces both T4 and to a lesser extent T3 under negative feedback control by the central HPT. Thus, while extrathyroidal regulation of T3 is important in all vertebrates, including for instance in the brains of developing mammals, in fish the formation and regulation of T3 may be dominated by local control in response to the needs of individual tissues rather than by T4 availability and through the central HPT axis. THs circulate in plasma bound to TH binding proteins, including thyroid binding globulin (TBG), transthyretin (TTR), and albumin. In humans, the primary transporter of TH is TBG while in rodents it is TTR.Citation126 Less is known about the dominant transporters in fish although it has recently been shown that TTR may bind TH in some species.Citation127,Citation128 Most TH in fish circulation is bound to protein with only a small amount (< 1%) thought to be free and available for uptake into cells. As demonstrated in rodents, the cellular transport of THs in fish is mediated largely by membrane bound transporters, including the high affinity monocarboxylate transporter 8 (MCT8) and organic anion transporter polypeptides (e.g., OATP1c1), among others.Citation129-Citation132
Figure 3. Overview of TH regulation and signaling in teleost fishes. TRH = thyrotropin releasing hormone; TSH = thyroid stimulating hormone; T4 = thyroxine; T3 = 3,3′,5-triiodothyronine; rT3 = 3,3′,5′-reverse T3; T2 = 3,3′-diiodothyronine; UGT = uridine diphosphate glucuronosyl transferase; SULT = sulfotransferase; TH-G = glucuronidated thyroid hormone; TH-S = sulfated thyroid hormone; Mrp = multidrug resistance associated protein; Mdr1 = multidrug resistance protein 1 or P-glycoproteins; MCT = monocarboxylate transporter; OATP = organic anion transport polypeptide; TR = thyroid hormone receptor; RXR = retinoic x receptor.
Peripheral TH Regulation and Signaling in Fish
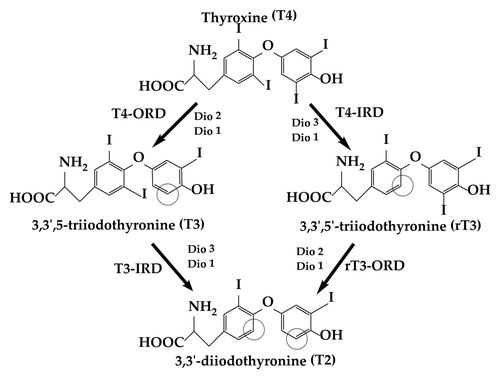
Once in the cell, T4 can be deiodinated to the active T3 hormone or inactivated to 3,3′,5′-triiodothyronine (rT3) or 3,3′-diiodothyronine (T2) (). Dio 1 and Dio 2 enzymes in vertebrates may catalyze T4-outer ring deiodination (ORD) to produce the active T3 hormone, while Dio 1 and Dio 3 catalyze T4-inner ring deiodination (IRD) to inactive rT3. Thus, Dio 1 can be involved in both ORD and IRD. In addition, T3-IRD and rT3-ORD can metabolize hormone to T2. THs in vertebrates are further conjugated in the liver to glucuronides or sulfates catalyzed by UDPGTs and SULTs, respectively, and excreted through the bile and urine. The transcriptional activity of T3 is mediated by complexing with nuclear thyroid receptors (TRs) that bind to TH response elements (TREs) to induce the expression of TH responsive genes.Citation133 Increased attention is also focusing on nongenomic pathways of THs signaling by integrin mediated signaling and kinase activation.Citation134,Citation135
Figure 4. Pathways of outer and inner ring deiodination of thyroid hormones in extrathyroidal peripheral tissues of vertebrates.
While Dio enzymes in fish and mammals are believed to share many functional features, relative tissue distributions and activity vary, which may have implications for their role in TH regulation.Citation136,Citation137 For instance, Dio 1 has been localized to the kidneys and liver of fish and mammals (as well as in the mammalian thyroid gland).Citation138 However, hepatic T4-ORD activity is thought to be catalyzed mostly by Dio 1 in mammals and Dio 2 in fish.Citation136,Citation139 One notable exception to this peripheral TH production may occur in the teleost fish brain with its relatively low T4-ORD activity. Early studies that measured deiodination in rainbow trout attributed reduced brain T4-ORD in fish to absent or negligible Dio 2 expression.Citation140 However, more recent studies using quantitative PCR have localized mRNA expression of dio2 genes in the fish brain, although at comparatively lower levels than in other tissues.Citation62,Citation141,Citation142 Limited evidence also suggests that the transcriptional response of Dio 2 in the fish brain may be more sensitive to systemic TH changes than Dio 2 in the liver.Citation141 Likewise, in mammals, Dio 2 has demonstrated substantial physiological plasticity in the brain with a short half-life of ∼40 min suggesting that it might also be an important regulator of intracellular T3 in these tissues.Citation143
PBDE Thyroid Disruption in Fish
Because PBDEs, particularly OH-BDE metabolites, are similar in structure to THs, concerns have been raised about their effects on thyroid system functioning in both mammalian and non-mammalian vertebrates.Citation50 In fish, PBDEs have been shown to perturb the thyroid system at several points along the central HPT and in extrathyroidal tissues with most study to date focused on the PentaBDE mixture, BDE-47, and BDE-209. Many of these laboratory studies in fish have shown declines in circulating levels of THs and altered TH signaling in some species exposed to these PBDE congeners and mixtures. provides a summary of studies in fish conducted to date. Similar to results in fish, in vivo rodent studies and cell-based assays have reported PBDE-induced disruption of thyroid homeostasis, including declines in circulating THsCitation144-Citation146; altered expression and activity of TH metabolizing enzymes,Citation147,Citation148 and competitive binding with plasma transporters.Citation149 Human epidemiology studies have shown associations between altered plasma concentrations of THs in adults and PBDE levels in serum and dust.Citation150-Citation152
Table 3. PBDE effects on thyroid endocrine systems of teleost fish
BDE-47, PentaBDE, and OH-BDE Thyroid Dysfunction
In one of the earlier studies in fish, depressed levels of circulating free T4 and T3 were measured in the plasma of lake trout (S. namaycush) dosed through the diet with a mixture of 13 PBDE congeners (e.g., BDE-28, -47, -99, -100, -153, -154, -209) at 2.5 ng/g and 25 ng/g per congener for 56 d.Citation89 In fathead minnows, dietary exposures to BDE-47 at 2.4 and 12.3 µg/breeding pair-day for 21 d elicited depressed total T4 (TT4) but not total T3 (TT3) that was accompanied by elevated mRNA transcripts encoding TSHβ in the pituitary of low dose fish.Citation154 In addition, elevated and reduced levels of mRNA transcripts encoding TRα and TRβ, respectively, were detected in the brain but not the liver, suggesting that the adult fish brain may be a sensitive target of BDE-47.Citation154 These results in minnows and trout are consistent with observations in European flounder (P. flesus) whereby declines in circulating TT4 with no change in TT3 were detected after a 101 d dietary exposure to the PentaBDE commercial mixture purified to remove polybrominated dibenzo-p-dioxins/dibenzofurans (PBDDs/Fs).Citation96
Reductions in whole fish T4 have also been measured in zebrafish larvae subjected to waterborne exposures of the PentaBDE commercial mixture.Citation157 In some divergence, however, this same research group measured elevated whole fish T4 and T3 in zebrafish offspring exposed to PentaBDE at the same dose as their previous study (Yu et al., 2010) but by a different exposure route (maternal) and longer duration (5 mo). The opposing effects of PentaBDE on TH levels that were measured in the Yu et al. (2010–11) zebrafish work demonstrate potentially important differences in PBDE impacts on vertebrate thyroid signaling that may be influenced by the pathway and duration of exposure (e.g., aqueous, short-term vs. maternal, chronic). BDE-209 and the PentaBDE mixture also have been found to enhance the relative mRNA expression of genes encoding dio1 and dio2 in zebrafish larvaeas well as thyroidal genes encoding the sodium iodide symporter (NIS), thyroglobulin (TG) and other transcription factors regulating NIS and TG expression (Nkx2.1a, Pax8).Citation106,Citation157
There has been only limited thyroid-related study in fish to date focused on the OH-BDEs. Recent work in our laboratory using whole mount in situ hybridization (WISH) measured significantly upregulated mRNA expression of dio1, but not dio2 or dio3, in the periventricular brains of 22 hpf embryos exposed to the hydroxylated metabolite 6-OH-BDE-47. An increase in dio3 mRNA expression was also detected in the pronephric duct, which is the earliest form of the kidney in vertebrates and constitutes the central component of the excretory system. Thus, this study demonstrated that effects of 6-OH-BDE-47 on the developing zebrafish thyroid may elicit localized and age-specific transcriptional responses that then potentially contribute to downstream effects on neurological, renal, and reproductive development. To better understand the implications of these findings, additional data are needed to clarify the cellular and tissue distribution of Dios during ontogeny of the fish brain. In the mammalian brain, Dio 2 is expressed in glial cells (astrocytes, tanycytes), which could play a role in transporting and maintaining T3 supplies to neurons.Citation159
BDE-209 Thyroid Dysfunction
BDE-209 reduced circulating levels of free T4 and T3 in early life-stages of rainbow troutCitation93 with declines in whole fish T4 also reported in zebrafish larvae exposed aqueously to BDE-209.Citation106 In addition, an increase in dio2 mRNA transcripts have been measured in the larvae of Chinese rare minnows exposed to BDE-209 with a decrease in dio2 transcripts measured in the brains of adults minnows.Citation155 Recently published data of ours has shown TH regulation and signaling in juvenile and adult fathead minnows to be disrupted by low dose exposures to BDE-209. Specifically, in juvenile fathead minnows, compared with vehicle controls, the activity of Dio enzymes (T4-ORD and T4-IRD) declined by ~74% in fish dosed with 9.8 µg/g ww food at 3% bw/day for 28 d.Citation61 This extrathyroidal perturbation was accompanied by evidence of thyroid follicle hypertrophy indicative of over-stimulation and injury. In adult male fathead minnows, BDE-209 caused a > 53% and > 46% decline in circulating total T4 and T3, respectively, upon a 28-d exposure to low doses of BDE-209 at ~3 and 300 ng/g bw-day.Citation62 Depressed levels of circulating THs were accompanied by a 65% decline in Dio activity (T4-ORD) in the brains of treated fish at both BDE-209 doses tested. This hypothyroid response in BDE-209 exposed minnows was accompanied by possible localized compensatory signaling, including increased T4-ORD activity in the liver and transient, tissue-specific upregulation of genes encoding several important thyroidal proteins (). However, similar to results in minnows exposed to BDE-47,Citation154 this study suggested that the fish brain may be particularly sensitive to BDE-209 based on severe reductions in brain Dio activity (T4-ORD) and potentially muted adaptive responses of the brain to reduced TH levels. Consistent with observations in the brains of adult fish, data collected in developing rodents suggest weak adaptive responses of the brains of younger mammals to TH insufficiency caused by low level chemical exposures.Citation160-Citation162 Additional work is needed to better understand how tissues, especially the brains, of developing and adult animals adapt or not to contaminant-induced TH insufficiency and whether this ameliorates downstream apical endpoints.
Table 4. Relative expression of genes encoding deiodinase (dio) enzymes, nuclear thyroid receptors (tr), monocarboxylate transporters (mct), and organic anion transporter polypeptides (oatp) in brains and livers of adult male fathead minnows exposed orally to BDE-209 and the positive control 6-propyl-2-thiouracil (PTU) for 28 d with a 14-d depuration.Citation62*
Mechanisms of PBDE Thyroid Disruption
The vertebrate thyroid system maintains normal physiological functioning by responding to endogenous and exogenous perturbations with changes in TH production from the thyroid and through changes in the capacity and sensitivity of peripheral tissues. Such integrated compensatory responses at the central HPT and in peripheral target tissues make it challenging to evaluate mechanisms of action for thyroid disruptors like PBDEs. Nonetheless, several mechanisms appear to play a role in the thyroid perturbations measured in fish (and other vertebrates) exposed to PBDEs including: interference with Dio enzyme activity/expression; enhanced metabolism and elimination of THs; altered expression and activity of plasma transporters and membrane bound transporters; and altered genomic signaling.
Interference with Dio Enzymes
One mechanism that might be contributing to thyroid perturbations in fish and other vertebrates could involve PBDEs interfering with the expression and activity of Dio enzymes. PBDEs may be acting as competitive substrates for Dio enzymes or otherwise altering the expression and activity of these enzymes. As discussed, altered mRNA expression and enzymatic activity of some Dio isoforms has been observed in fish exposed to BDE-209Citation61,Citation62,Citation155 and 6-OH-BDE-47,Citation153 as well as in rodents exposed to PentaBDECitation148 and human microsomes incubated with 5′-OH-BDE-99 and 2,4,6-TBP.Citation147 However, it remains unclear whether PBDEs (or OH-BDEs) can bind directly to Dio enzymes or whether they may elicit other allosteric effects that affect the capacity of Dios to mediate TH regulation.
Induction of TH Metabolizing Enzymes
Another hypothesis for PBDE-induced thyroid disruption is that PBDE detoxification responses may induce the expression and/or activity of TH catabolizing enzymes. In some support of this hypothesis, studies have measured increased mRNA expression of TH-conjugating UDPGT and SULT enzymes in rodents exposed to BDE-47Citation145 and the PentaBDE commercial mixture.Citation148,Citation163 In these studies, declines in circulating levels of T4 from PBDEs were linked to enhanced glucuronidation associated with UDPGT inductions.Citation148,Citation163,Citation164 Other studies, however, have shown little to no change in UDPGT levels in rodents following exposure to PBDEs despite decreased T4 levels in circulation,Citation145,Citation165 although increased mRNA expression of UDPGT transcripts has been observed in some of this work.Citation145 In contrast to the rodent data, limited evidence in fish has shown declines in mRNA transcript abundances of some UDPGT isoforms suggesting that PBDEs may be acting as TH mimics that then downregulate the expression of these TH metabolizing enzymes in PBDE exposed fish.Citation82,Citation106
Altered Expression/Activity of Plasma and Cellular Transporters
Few studies have explored the role of plasma and membrane bound transporters in PBDE metabolic detoxification pathways and in contributing to or ameliorating PBDE effects on TH signaling.Citation166 OH-BDE metabolites produced in rat liver microsomes enriched with CYP2b (i.e., PB-induced) have been found to compete with THs for binding to the plasma transport protein TTR, potentially leading to greater elimination of TH and hypothyroidism.Citation149,Citation167 Likewise in a recombinant sea bream TTR assay, several parent PBDEs (BDE-28, -49, -47, -99) were shown to be potent inhibitors of T3 binding to TTR, suggesting competitive interferences, while 6-OH-BDE-47 had less affinity for sea bream TTR than T3 or T4.Citation156 Other studies have also used biosensor screening methods to show that the OH-BDEs may bind to TTR and TBG with high potency.Citation166 More recently, newly designed fluorescent probes and competitive binding assays have shown that the binding of OH-BDEs with TBG and TTR increases with bromine number and OH position (i.e., 3-meta OH).Citation168
In addition to plasma transport, a few studies have explored PBDE effects on cell membrane bound transporters of TH. Our recently published data in fathead minnows measured upregulated mRNA transcripts encoding mct8 and oatp1c1 in the brains and livers of fish exposed to BDE-209 ().Citation62 As observed with dio transcription, upregulated mRNA expression patterns of these transporters in minnows exposed to BDE-209 may be indicative of additional compensatory responses to hypothyroidism as mct8 and oatp1c1 are specific and active transporter of THs in fishCitation129,Citation169 and mammals.Citation170,Citation171 Only a limited number of the OATP transporters have been characterized in vertebrates with recent work in zebrafishCitation131 and fathead minnowsCitation169 to clarify their tissue distribution and function. Some OATPs have been found to be potentially important PBDE transporters. BDE-47, -99, and -153 have been shown to be effective substrates for human OATP1B1, OATP1B3, and OATP2B1 expressed in Chinese hamster ovary (CHO) cells.Citation172 OATP1B1 and OATP1B3 were found to transport BDE-47 with the highest affinity, while OATP2B1 was found to transport all three tested congeners with similar affinities. Using human embryonic kidney cells transiently expressing mouse hepatic OATPs, this same research group also measured that OATP1a4, OATP1b2, and OATP2b1 were able to bind and transport BDE-47, -99, and -153.Citation173 Consistent with these results, upregulated mRNA expression of genes encoding OATP1a4, which transports THs, bile acids, and xenobiotics, was also detected in young rats exposed to PentaBDE.Citation148 PBDEs have also been shown to affect the Phase III hepatic efflux transporters, P-glycoproteins (i.e., Pgp; Mdr1) and multidrug resistance-associated proteins (Mrps) in rodents. Pgp and Mrp transporters are members of the ATP-binding cassette (ABC) superfamily, are regulated by AhR, CAR, and PXR, and play important roles in the efflux of xenobiotics and THs into the bile for elimination.
Binding to Thyroid Receptors
Limited evidence in fish and other vertebrates supports that PBDEs may, in part, be mediating effects on the thyroid by altering TR expression and signaling. The transcriptional activity of nuclear TRs is thought to be mediated by both the presence and absence of T3 due to its ability to bind to TRE regions of regulated genes in both the presence and absence of ligand. As in other vertebrates, two genetically distinct receptors TRα and TRβ have been identified in teleost fishes, including zebrafish,Citation174,Citation175 flounder (P. olivaceus)Citation176; goldfish,Citation177 fathead minnows,Citation178,Citation179 Nile tilapia (O. niloticus),Citation180 and Atlantic salmon.Citation180 Additional receptor subtypes with the capacity to bind TH have also been identified attributable to gene duplication and alternative mRNA splicing. Two trα genes, thraa (original) and thrab (duplication) have been described in zebrafish with the thraa gene shown to encode two protein variants, TRαA1 and TRαA1–2.Citation181,Citation182 Two TRβ isoforms have also been identified in teleosts.Citation175,Citation180 While TR variants arising from TRα and TRβ have also been described in other vertebrates, including humans, the general structure and function of TRs appears well conserved across vertebrates, which has been the topic of several reviews.Citation183-Citation185
Questions remain, however, concerning whether and how parent PBDEs and their OH-BDE metabolites interact with and bind to TRs. For instance, BDE-47 was reported to not interact as either an agonist or antagonist with TRβ1 in an in vitro binding assay and did not interfere with TRβ-responsive gene expression.Citation186 However, altered expression in thyroid-responsive genes was observed in the brain and livers of rodent pups exposed perinatally to BDE-47, suggesting that BDE-47 operated through alternative mechanisms to TRβ signaling. Studies in rat pituitary GH3 cell proliferation assays have shown BDE-127 and BDE-185 to be TR agonists while BDE-206 was a TR antagonist.Citation167,Citation187,Citation188 Cell based assays have shown that some OH-BDEs, including 3-OH-BDE-47, can inhibit T3 binding to TRs by antagonizing the receptor, whereas several other parent PBDEs and OH-BDEs have shown no TR affinity.Citation189,Citation190 Another study showed TR antagonistic activity for BDE-209, -153, -154, -100, and PentaBDE.Citation191 Limited evidence has shown some OH-BDEs to behave as weak TR agonists.Citation192-Citation194 It has been suggested that hydroxy moieties in the 3 or 4 position of the phenyl ring along with two adjacent bromine substituents are necessary for OH-BDE to bind to TRs.Citation189 In addition, recent molecular docking assays have shown that OH-BDEs may have varied interactions with TR binding pockets depending on the degree of bromination with lower OH-BDEs (e.g., 6-OH-BDE-47, 5-OH-BDE-47) showing weak TR agonism while higher OH-BDEs (e.g., 3-OH-BDE-100, 3′-OH-BDE-154) antagonized TRs.Citation194 Taken together, data on TR binding of PBDEs and OH-BDEs continue to be inconsistent and have demonstrated both antagonistic and weak agonistic activities toward TRs, as well as no interactions, that may be attributable to hydroxylation and bromination patterns that influence binding geometries with the receptor.
PBDEs also may alter patterns of TR-responsive gene transcription, although this remains understudied. For instance, reductions in relative mRNA transcript abundances of brain transcription element binding protein (BTEB) were measured in adult fathead minnows exposed to BDE-47.Citation154 The downregulated expression of BTEB, which is a thyroid-responsive transcription factor involved in neurogenesis, was accompanied by declines in circulating T4 and reductions in trβ gene transcripts in the brains of treated minnows, suggesting that hypothyroidism in BDE-47 treated fish may elicit downstream effects on neurogenic capacity in adults. Similarly, in primary rat cerebellar granule cell cultures, BDE-99 was found to disrupt trα1 and trα2 mRNA transcript abundances, alter TR-responsive gene transcription (e.g., brain-derived neurotrophic factor), and increase the production of reactive oxygen species.Citation195 Finally, a study with CV-1 cell cultures measured suppressed TR binding with TREs through the DNA binding domain (vs. between THs and TRs) upon exposure to several PBDEs and OH-BDEs, with BDE-209 showing the greatest suppression at the lowest dose.Citation191 The suppressed TR-TRE binding was then shown to inhibit TH-dependent dendrite arborization of cerebellar Purkinje cells, suggesting TR-TRE mediated impacts on PBDE neurotoxicity.
Recently, a second potential binding site for T3 and T4 was suggested in the ligand binding domain (LBD) of TRα 196. This second binding site was identified on the surface of the TRα LBD in the same region where the F-domain (i.e., additional C-terminal amino acids) is located in some species. While human TRs do not appear to have F-domains, it has been identified within the TRαA1 isoform of zebrafish where it has been shown to constrain transcriptional activity by altering TR coactivator recruitment.Citation182 Thus, one hypothesis put forth is that this second binding site within TRα may serve to suppress TR activation when elevated concentrations of TH are present.Citation196 While more work is needed, this second binding site could also play a role in mediating how environmental contaminants like PBDEs interact with TRs and alter the functioning of these nuclear receptors.
Neurodevelopmental Toxicity
Limited evidence of PBDE effects on neurodevelopment of fishes has been observed in early life stages of fish (zebrafish typically) for a subset of PBDE congeners. Specifically, neurodevelopmental abnormalities, including impaired normal motor behavior and inhibited neuron growth and differentiation, as well as morphological deformities have been measured in zebrafish larvae exposed to: PentaBDECitation157,Citation197,Citation198; a mixture of BDE-47, -99, -100, -153, and -183Citation199; BDE-47,Citation200-Citation203 BDE-49Citation204; and BDE-209Citation205 ().
Table 5. PBDE neurodevelopmental/developmental malformations measured in teleost fish
PentaBDE Neurotoxicity
For instance, in 96-hpf zebrafish parentally exposed to the PentaBDE mixture, several genes involved in central nervous system development were downregulated, including α1-tubulin, synapsin IIa, and myelin basic protein.Citation198 This decline in mRNA expression was accompanied by reduced protein expression of α1-tubulin and synapsin IIa as well as reduced locomotor activity among treated larvae. In another study by this same research group, the PentaBDE commercial mixture also caused a downregulation in mRNA transcript abundances of sonic hedgehog (Shh).Citation197 This suggests a possible contributory role for thyroid dysregulation in some PBDE related neurodevelopmental toxicity as the Shh pathway, as well as its coreceptors patched (Ptc) and smoothened (Smo), have been shown to be regulated by THs in embryonic forebrain signaling and development in mammals.Citation206 Only one study has examined neurotoxicity endpoints in a fish species beyond zebrafish. This study, conducted in mummichogs (F. heteroclitus) detected hindered behavior and learning ability, as well as dorsal curvatures, in fish exposed to PentaBDE.Citation98
BDE-47, BDE-49, and BDE-209 Neurotoxicity
In BDE-47 exposed zebrafish, delayed hatching, neural defects, and cardiac arrhythmias were measured at 168 hpf in larvae exposed to 5 mg/l of BDE-47.Citation202 The cardiac toxicity and dorsal curvature deformities observed in this study were coupled with reduced flow rates of cerebrospinal fluid in neural tubes and brain ventricles of the hindbrain. The reduced flow rates of cerebrospinal fluid were postulated to be related to the dorsal curvatures that then possibly contributed to the measured neurotoxicity. Dorsal curvatures, attenuated heart rates, and impaired touch-escape responses were also measured in zebrafish larvae exposed to another tetra PBDE congener BDE-49.Citation204 Moreover, neurodevelopmental impairments, including delayed hatching and hindered motor neuron development, loose muscle fiber deformities and slow locomotion, and hyperactivity under a light/dark stimulation test, have also been observed in zebrafish larvae exposed maternally to BDE-209.Citation205 These behavioral findings with BDE-209 are consistent with another recent study in which zebrafish larvae exposed to sediment spiked with 12.5 mg/kg of BDE-209 from 4–192 hpf experienced hyperactive responses to light stimulation that may be linked to impaired neurodevelopment.Citation6 Additional in silico binding assays in this study also predicted BDE-209 binding with several human proteins involved in neurological functioning, including: tubulin α1A involved in microtubule formation; acetylcholinesterase (AChE) involved in the breakdown of acetylcholine; 5HTR2A, 5HTR2C, and 5HTR3A, which are serotonin receptor system genes; and cocaine esterases.
Toxicity Mechanisms and Thyroid Interactions
In mammals, like in fish, neurodevelopmental toxicity of PBDEs is also an important toxicological endpoint of concern. For instance, PBDEs (BDE-47, -99, and -100) measured in umbilical cord blood of women have been found to be correlated with reduced performance of gestationally-exposed children (aged 0–6) on mental performance tests.Citation207 Moreover, maternal prenatal and childhood PBDE exposures have been associated with reduced attention, fine motor coordination, and cognition (declines in IQ scores) among a California cohort of Mexican-American children.Citation64 A substantial number of studies in rodents, spanning different laboratories, have demonstrated also that PBDEs can elicit adverse neurobehavioral outcomes in early development.Citation208-Citation210 Recent in vitro work has even shown that the prominent OH-BDE metabolite in humans, 6-OH-BDE-47, can disrupt adult neurogenesis by inhibiting neuronal differentiation and oligodendrocyte differentiation, proliferation, and survival of primary cultured adult neural stem/progenitor cells isolated from the brains of adult mice.Citation213 Other in vitro study has shown that the hydroxylated metabolites of BDE-47 may disturb intracellular calcium release.Citation214
Some neurological deficits and alterations measured in fishCitation154 and rodentsCitation191,Citation211,Citation212 have been accompanied by reductions in circulating T4 and altered TH signaling, suggesting that one of the contributing neurotoxicity mechanisms may proceed through PBDE interference with TH regulation and signaling. However, PBDE neurotoxicity has been observed absent impacts on TH regulation, suggesting other mechanistic pathways. Indeed, a growing body of evidence suggests that PBDE mechanisms of neurotoxicity may operate by several pathways that include disrupted TH signaling, altered cholinergic neurotransmissionsCitation215,Citation216; impaired neuronal proliferation and plasticity,Citation191,Citation214,Citation217 and oxidative stress.Citation218,Citation219 While the underlying mechanisms of PBDE neurotoxicity are unclear, continued testing in fish would be informative to better understand these underlying mechanisms of PBDEs effects on the development and functioning of the central and peripheral nervous systems of vertebrates.
Reproductive Toxicity
PBDE impacts on fish reproduction and reproductive development have been evaluated in a limited number of studies and congeners (). Studies that have examined PBDE effects on fish reproduction have presented mixed evidence of reduced fecundity, spawning, hatching success, and offspring survival as well as impaired fertility, particularly among male fish, with PBDE-induced alterations in spermatogenesis, declines in sperm counts, and feminization possibly playing important roles.
Table 6. PBDE reproductive alterations measured in teleost fish
PentaBDE Reproductive Effects
Reduced fecundity and larval survival have been measured in zebrafish exposed orally to the PentaBDE mixture purified of PBDDs/PBDFs.Citation96 Reductions in larval survival in this and other studies may be partly attributable to maternal transfer of PBDEs to eggs, which has been shown in zebrafish and marine medaka (O. melastigma) and could hinder normal developmental progression.Citation221,Citation222 A study in zebrafish adults with the PentaBDE mixture reported sex-specific alterations in the relative expression of genes encoding an array of reproductive hormones and receptors along the HPG axis, as well as disruption in circulating levels of E2, T, and 11-keto-testosterone in males.Citation223 In a second related study, this same research group also measured PentaBDE-induced reductions in spawning, fertilization, and hatching success along with reduced larval survival and higher percentages of male offspring.Citation224 These reproductive impairments were accompanied by altered counts of spermatogonia, spermatocytes, and spermatids in the testis of treated fish. In some contradiction, however, this study reported an increase in the gonado-somatic index (GSI) in treated males.
BDE-47 Reproductive Effects
With regard to constituents of the PentaBDE commercial mixture, most of the limited research to date that has studied PBDE impacts on reproduction has been conducted with BDE-47. Reduced spawning has been observed in adult fathead minnow male/female pairs exposed orally to ~14 µg/fish of BDE-47 for 25 d, with reproduction completely ceased within 10 d of exposure.Citation108 The impaired reproduction may have been attributable to selective toxicity in male fathead minnows as decreased mature spermatozoa and reduced condition factors were noted in these fish. In another study, declines in mature spermatozoa also were measured, but with no effect on fecundity, in adult male fathead minnows exposed orally to ~6 µg/fish-day of BDE-47 for 21 d.Citation154 BDE-47 also elicited no effects on the morphological development of offspring from adult male rainbow trout exposed to 55 µg/kg-day of BDE-47 for 17 d and then bred with untreated females.Citation225 These data suggest that other components in the PentaBDE mixture in addition to BDE-47 may play an important role in affecting fecundity, embryonic development, and adult male reproduction.
PBDE Effects on Male Fish Reproduction
Some studies have suggested a role for PBDEs in the feminization of male fish. Specifically, dose-dependent increases in relative mRNA transcripts encoding vitellogenin (Vtg; egg yolk precursor protein) and zona radiata protein (eggshell protein) were measured in hepatocytes of juvenile male Atlantic salmon (S. solar) exposed to BDE-47 and a PBDE mixture (BDE-47, 153, -154).Citation226 However, some of these in vitro findings have not been reproduced with in vivo studies of juvenile Atlantic salmon exposed to the PentaBDE mixtureCitation75 or in zebrafish larvae exposed to BDE-47,Citation95 whereby no change in the expression of Vtg mRNA transcript abundances were detected. Thus, while the in vitro evidence is limited because it may not account for the extensive and coordinated interactions among cells and tissues, conversely the in vivo research conducted to date targeting PBDE effects on male fish reproduction has only been in two species, targeting largely transcriptional changes in immature animals for just a limited number of genes. In addition, very little is known about the potential reproductive effects of BDE-209 in fishes but limited evidence suggests alterations to some reproductive endpoints in male fish. Specifically declines in spermatogenesis were observed in male Chinese rare minnows (G. rarus) exposed aqueously to BDE-209,Citation155 and reductions in the gonadal somatic index (GSI) have been measured in adult male fathead minnows exposed to BDE-209Citation62,Citation205 with evidence of declining sperm counts in some of this research.Citation205 Taken together, more research is necessary to understand whether PBDEs play a role in eliciting potential feminization and reproductive impairments in male animals.
Toxicity Mechanisms and Thyroid Interactions
Fish have evolved diverse reproductive strategies that are closely integrated with gonadal differentiation and functioning and demonstrate sensitivity to external environmental cues, such as photoperiod and temperature. While there are important differences between reproduction in mammalian and non-mammalian vertebrates, reproduction in jawed vertebrates is controlled by the HPG axis and the structure of this endocrine system is highly conserved. It is possible that there could be shared pathways of PBDE impacts on mammalian and non-mammalian vertebrate sex steroid synthesis and gonadal development and functioning. It is also possible that there may be important species- and sex-specific differences in reproductive responses to PBDEs as current studies are limited by teleost species and focused largely on altered reproduction in males with less known about reproductive effects in female fish.
For instance, in female fish, declines in ovarian aromatase (CYP19) activity were measured in European flounder (P. flesus) exposed to the PentaBDE mixture purified of PBDDs/PBDFs, suggesting that PBDEs may be altering pathways of steroidogenesis in female fish.Citation96 Somewhat consistent with these findings, in vitro testing also has shown potential inhibitory effects of OH-PBDEs on CYP19 and CYP17 in human placental microsomesCitation227 and human adrenocortical carcinoma cells.Citation228,Citation229 Several OH-BDEs have also been found to bind with the estrogen receptor (ER) with the general trend that para-OH metabolites displayed the highest affinity for ERs with the lower OH-BDEs (1–4 bromines) tending to act as weak agonists while higher OH-BDEs (4 bromines or more) had antagonistic properties.Citation189,Citation230,Citation231In addition, rodent studiesCitation232-Citation236 and cell-based assaysCitation187,Citation237 have shown that some PBDEs (PentaBDE, BDE-47, -99, -100, and -209) may be estrogenic and/or induce feminization in male animals by anti-androgenic pathways. In humans, epidemiology studies have measured PBDE associations with: cryptorchidismCitation238; early onset of menarcheCitation239; decreased testosterone, luteinizing hormone (LH), and follicle stimulating hormone (FSH) in adult menCitation151; increased E2 in 3-mo old boys (BDE-154)Citation240; and decreased sperm counts and testis size in young adults (BDE-153).Citation241 Taken together, there appear to be important common mechanistic pathways of PBDE effects on vertebrate reproduction that are not well understood at this time.
While some evidence points to PBDEs affecting the reproductive health of fish and other vertebrates, few studies have examined interactions between thyroid and reproductive functioning. Thus, questions remain as to whether PBDE effects on reproduction are being mediated directly and/or indirectly by altered TH regulation. In mammals, both hypothyroidism and hyperthyroidism have been shown to impair reproductive physiology and lower fertility.Citation242 An early review described important interactions between TH regulation and reproductive physiology in fishes.Citation243 More recently, studies in goldfish (C. auratus) and zebrafish suggest that THs may have important inhibitory effects on teleost reproductive functioning at different levels of the HPG axis, including by: inhibiting pituitary LH and FSH; and reducing steroidogenesis and gonadal aromatase expression.Citation244,Citation245 Recent evidence, in some contrast, also supports a stimulatory role for THs in the proliferation of sertolli cells and spermatogonia in zebrafish testes.Citation246
Little is known about the role of PBDE-induced TH disruption in potential reproductive toxicity in fishes or other vertebrates. One hypothesis is that PBDEs could be mimicking THs that is in turn leading to altered steroidogenesis and steroidal hormone regulation among exposed animals, although this has not been examined. However, in data collected in male fathead minnows exposed orally to BDE-209, the model goitrogen 6-propyl-2-thiouracil (PTU), which was used as a positive control, reduced TH levels as predicted but had no effect on the GSI, unlike BDE-209 which caused substantial reductions in the GSI. These results suggest that BDE-209 reproductive effects in male fish could be acting through thyroid-independent pathways.Citation62 Still though, it remains unclear from the limited data whether PBDE effects on reproduction are mediated by directly impairing HPG functioning or whether these effects are also mediated in cross-talk with perturbations of the thyroid.
Research Needs and Conclusions
A substantial body of evidence suggests that PBDE metabolism in teleost fishes proceeds through reductive debromination pathways. Studies report both the presence and absence of OH-BDE metabolites forming in fish, and given their bioactivity, it would be informative to investigate further whether these metabolites form in vivo in fish. It might be expected that the OH-BDEs, if they are being produced by fish, would be found at higher concentrations in the blood and highly perfused tissues like the liver where they are formed mostly. Related to this, questions remain as to the identity and kinetics of the enzymatic biotransformation pathways of PBDE metabolism in fish with some indirect evidence implicating Dio enzymes. In mammals, PBDEs appear to operate predominantly through AhR-independent toxicity pathways. However, the extent to which these pathways (e.g., CAR, PXR) are operational in fish exposed to PBDEs is less clear, but potentially relevant to the hypothyroidism observed given their additional role in TH metabolism and elimination.
PBDEs have been shown now to disrupt TH regulation and signaling in several teleost species with mechanisms of action that proceed through multiple pathways depending on the congener, fish life stage, and tissue type, including by: enhancing the metabolism and elimination of THs; binding competitively with plasma and membrane bound transporter proteins; altering interactions of T3 ligand with nuclear TRs; disrupting Dio activity; and altering the transcription of genes involved in TH production, transport, and genomic signaling. Despite expanding knowledge of these thyroid disruption mechanisms, there continues to be a limited understanding of PBDE impacts on thyroid-response gene expression and downstream apical endpoints of concern, including neurodevelopment and reproduction. PBDE effects on neurological, developmental, and reproductive physiology have been indicated across fish and other vertebrates exposed to PBDEs, suggestive of common toxicity pathways that remain unclear. Related to this, additional work is needed to understand whether there exist localized compensatory or adaptive physiological responses of the thyroid system to TH insufficiency caused by PBDE exposures. Continued study of these apical endpoints of concern in fish would be helpful for not only understanding potential toxicities in free ranging fishes, but also would contribute to understanding PBDE mechanisms of toxicity in humans.
The thyroid endocrine systems of fish and mammals are well conserved and similar in structure and function, supporting the relevance of fish as models for understanding PBDE effects more broadly across vertebrate taxa. However, there are differences between mammalian and non-mammalian thyroid signaling that could have implications for using fish as models for evaluating PBDE thyroid toxicity in higher level animals. One important difference is that unlike in mammals, the fish thyroid may not to be centrally directed through the HPT but rather appears to rely strongly on localized peripheral tissues for T3 production and regulation. There is evidence for this dominant peripheral signaling in mammalian models as well, but nonetheless demonstrates the need to continually evaluate the choice of animal model when studying chemically induced thyroid disruption. For some PBDE congeners, evidence in fish demonstrates thyroid dysregulation at low doses (i.e., ~ppb levels) that roughly compares to levels detected in the environment and tends to be lower than doses typically administered in rodent studies. Additional low dose studies are needed to determine whether non-monotonic dose responses are occurring in fish and other vertebrates exposed to PBDEs.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
This review was supported by National Institute of Environmental Health Sciences research grants (R01-ES016099 and T32ES007060) and US EPA STAR graduate fellowship (FP-917145010). Findings and conclusions in this article are those of the authors and do not necessarily represent the views of the NIEHS or EPA. The authors would also like to thank Dr. Linda Birnbaum, Dr. Rich Di Giulio, Dr. David Hinton, Dr. Sean Lema, and Dr. Joel Meyer for their insightful comments as committee members on the Ph.D. dissertation that served as the basis for this review.
Citation: Noyes PD, Stapleton HM. PBDE flame retardants: Toxicokinetics and thyroid hormone endocrine disruption in fish. Endocrine Disruptors 2014; 2:e29430; 10.4161/endo.29430
References
- Hale RC, La Guardia MJ, Harvey E, Gaylor MO, Mainor TM. Brominated flame retardant concentrations and trends in abiotic media. Chemosphere 2006; 64:181 - 6; http://dx.doi.org/10.1016/j.chemosphere.2005.12.006; PMID: 16434082
- Hites RA. Polybrominated diphenyl ethers in the environment and in people: a meta-analysis of concentrations. Environ Sci Technol 2004; 38:945 - 56; http://dx.doi.org/10.1021/es035082g; PMID: 14998004
- Gauthier LT, Hebert CE, Weseloh DVC, Letcher RJ. Dramatic changes in the temporal trends of polybrominated diphenyl ethers (PBDEs) in herring gull eggs from the Laurentian Great Lakes: 1982-2006. Environ Sci Technol 2008; 42:1524 - 30; http://dx.doi.org/10.1021/es702382k; PMID: 18441798
- Klosterhaus SL, Stapleton HM, La Guardia MJ, Greig DJ. Brominated and chlorinated flame retardants in San Francisco Bay sediments and wildlife. Environ Int 2012; 47:56 - 65; http://dx.doi.org/10.1016/j.envint.2012.06.005; PMID: 22766500
- Law RJ, Herzke D, Harrad S, Morris S, Bersuder P, Allchin CR. Levels and trends of HBCD and BDEs in the European and Asian environments, with some information for other BFRs. Chemosphere 2008; 73:223 - 41; http://dx.doi.org/10.1016/j.chemosphere.2008.02.066; PMID: 18472134
- Garcia-Reyero N, Escalon BL, Prats E, Stanley JK, Thienpont B, Melby NL, Barón E, Eljarrat E, Barceló D, Mestres J, et al. Effects of BDE-209 contaminated sediments on zebrafish development and potential implications to human health. Environ Int 2014; 63:216 - 23; http://dx.doi.org/10.1016/j.envint.2013.11.012; PMID: 24317228
- Schecter A, Harris TR, Brummitt S, Shah N, Paepke O. PBDE and HBCD Brominated Flame Retardants in the USA, Update 2008: Levels in Human Milk and Blood, Food, and Environmental Samples. Epidemiology 2008; 19:S76
- Shaw SD, Kannan K. Polybrominated diphenyl ethers in marine ecosystems of the American continents: foresight from current knowledge. Rev Environ Health 2009; 24:157 - 229; http://dx.doi.org/10.1515/REVEH.2009.24.3.157; PMID: 19891120
- Voorspoels S, Covaci A, Lepom P, Escutenaire S, Schepens P. Remarkable findings concerning PBDEs in the terrestrial top-predator red fox (Vulpes vulpes). Environ Sci Technol 2006; 40:2937 - 43; http://dx.doi.org/10.1021/es060081k; PMID: 16719094
- Hale RC, La Guardia MJ, Harvey E, Mainor TM. Potential role of fire retardant-treated polyurethane foam as a source of brominated diphenyl ethers to the US environment. Chemosphere 2002; 46:729 - 35; http://dx.doi.org/10.1016/S0045-6535(01)00237-5; PMID: 11999796
- UNEP. Listing of commercial pentabromodiphenyl ether and commercial octabromodiphenyl ether. United Nations Environment Programme; Stockholm Convention. UNEP-POPS-COP.4-SC-4-18. Available on-line at: http://chm.pops.int/Implementation/NewPOPs/TheNewPOPs/tabid/672/Default.aspx. 2009.
- Weil ED, Levchik SV. Flame retardants for polystyrenes in commercial use or development. J Fire Sci 2007; 25:241 - 65; http://dx.doi.org/10.1177/0734904107071607
- Fink U, Hajduk F, Wei Y, Mori H. Flame Retardants. SRI Consulting, Englewood, CO. Available on-line at: http://www.ihs.com/products/chemical/planning/scup/flame-retardants.aspx?pu=1&rd=chemihs and http://www.flameretardants-online.com/web/en/106/114.htm. 2008.
- Posner S, Roos S, Olsson E. Exploration of Management Options for HBCD. Swerea IVF, Swerea Group, Molindal, Sweden. Swerea IVF Project Report 10/11. 2011
- Ni K, Lu Y, Wang T, Shi Y, Kannan K, Xu L, Li Q, Liu S. Polybrominated diphenyl ethers (PBDEs) in China: policies and recommendations for sound management of plastics from electronic wastes. J Environ Manage 2013; 115:114 - 23; http://dx.doi.org/10.1016/j.jenvman.2012.09.031; PMID: 23246772
- Law RJ, Alaee M, Allchin CR, Boon JP, Lebeuf M, Lepom P, Stern GA. Levels and trends of polybrominated diphenylethers and other brominated flame retardants in wildlife. Environ Int 2003; 29:757 - 70; http://dx.doi.org/10.1016/S0160-4120(03)00110-7; PMID: 12850094
- Shaw SD, Berger ML, Brenner D, Kannan K, Lohmann N, Päpke O. Bioaccumulation of polybrominated diphenyl ethers and hexabromocyclododecane in the northwest Atlantic marine food web. Sci Total Environ 2009; 407:3323 - 9; http://dx.doi.org/10.1016/j.scitotenv.2009.02.018; PMID: 19269019
- Stapleton HM, Eagle S, Sjödin A, Webster TF. Serum PBDEs in a North Carolina toddler cohort: associations with handwipes, house dust, and socioeconomic variables. Environ Health Perspect 2012; 120:1049 - 54; http://dx.doi.org/10.1289/ehp.1104802; PMID: 22763040
- Wu N, Herrmann T, Paepke O, Tickner J, Hale R, Harvey LE, La Guardia M, McClean MD, Webster TF. Human exposure to PBDEs: associations of PBDE body burdens with food consumption and house dust concentrations. Environ Sci Technol 2007; 41:1584 - 9; http://dx.doi.org/10.1021/es0620282; PMID: 17396645
- Law RJ, Herzke D. Current levels and trends of brominated flame retardants in the environment. In: Barcelo D, Kostianoy AG, eds. The Handbook of Environmental Chemistry; Brominated Flame Retardants. Heidelberg, Germany: Springer Publishing Services, 2011:123-41.
- Trudel D, Scheringer M, von Goetz N, Hungerbühler K. Total consumer exposure to polybrominated diphenyl ethers in North America and Europe. Environ Sci Technol 2011; 45:2391 - 7; http://dx.doi.org/10.1021/es1035046; PMID: 21348481
- de Wit CA, Herzke D, Vorkamp K. Brominated flame retardants in the Arctic environment--trends and new candidates. Sci Total Environ 2010; 408:2885 - 918; http://dx.doi.org/10.1016/j.scitotenv.2009.08.037; PMID: 19815253
- Stapleton HM, Dodder NG. Photodegradation of decabromodiphenyl ether in house dust by natural sunlight. Environ Toxicol Chem 2008; 27:306 - 12; http://dx.doi.org/10.1897/07-301R.1; PMID: 18348638
- Gerecke AC, Hartmann PC, Heeb NV, Kohler HPE, Giger W, Schmid P, Zennegg M, Kohler M. Anaerobic degradation of decabromodiphenyl ether. Environ Sci Technol 2005; 39:1078 - 83; http://dx.doi.org/10.1021/es048634j; PMID: 15773480
- Stapleton HM, Alaee M, Letcher RJ, Baker JE. Debromination of the flame retardant decabromodiphenyl ether by juvenile carp (Cyprinus carpio) following dietary exposure. Environ Sci Technol 2004; 38:112 - 9; http://dx.doi.org/10.1021/es034746j; PMID: 14740725
- Dodson RE, Perovich LJ, Covaci A, Van den Eede N, Ionas AC, Dirtu AC, Brody JG, Rudel RA. After the PBDE phase-out: a broad suite of flame retardants in repeat house dust samples from California. Environ Sci Technol 2012; 46:13056 - 66; http://dx.doi.org/10.1021/es303879n; PMID: 23185960
- Dinn PM, Johannessen SC, Ross PS, Macdonald RW, Whiticar MJ, Lowe CJ, van Roodselaar A. PBDE and PCB accumulation in benthos near marine wastewater outfalls: the role of sediment organic carbon. Environ Pollut 2012; 171:241 - 8; http://dx.doi.org/10.1016/j.envpol.2012.07.023; PMID: 22960365
- Kohler M, Zennegg M, Bogdal C, Gerecke AC, Schmid P, Heeb NV, Sturm M, Vonmont H, Kohler HP, Giger W. Temporal trends, congener patterns, and sources of octa-, nona-, and decabromodiphenyl ethers (PBDE) and hexabromocyclododecanes (HBCD) in Swiss lake sediments. Environ Sci Technol 2008; 42:6378 - 84; http://dx.doi.org/10.1021/es702586r; PMID: 18800504
- Marvin C, Waltho J, Jia J, Burniston D. Spatial distributions and temporal trends in polybrominated diphenyl ethers in Detroit River suspended sediments. Chemosphere 2013; 91:778 - 83; http://dx.doi.org/10.1016/j.chemosphere.2013.02.009; PMID: 23478126
- Hale RC, Alaee M, Manchester-Neesvig JB, Stapleton HM, Ikonomou MG. Polybrominated diphenyl ether flame retardants in the North American environment. Environ Int 2003; 29:771 - 9; http://dx.doi.org/10.1016/S0160-4120(03)00113-2; PMID: 12850095
- Peng X, Tang C, Yu Y, Tan J, Huang Q, Wu J, Chen S, Mai B. Concentrations, transport, fate, and releases of polybrominated diphenyl ethers in sewage treatment plants in the Pearl River Delta, South China. Environ Int 2009; 35:303 - 9; http://dx.doi.org/10.1016/j.envint.2008.07.021; PMID: 18774173
- Huang K, Lin K, Guo J, Zhou X, Wang J, Zhao J, Zhou P, Xu F, Liu L, Zhang W. Polybrominated diphenyl ethers in birds from Chongming Island, Yangtze estuary, China: insight into migratory behavior. Chemosphere 2013; 91:1416 - 25; http://dx.doi.org/10.1016/j.chemosphere.2013.01.042; PMID: 23411092
- Chen D, Hale RC. A global review of polybrominated diphenyl ether flame retardant contamination in birds. Environ Int 2010; 36:800 - 11; http://dx.doi.org/10.1016/j.envint.2010.05.013; PMID: 20557935
- Mizukawa H, Nomiyama K, Nakatsu S, Yachimori S, Hayashi T, Tashiro Y, Nagano Y, Tanabe S. Species-specific differences in the accumulation features of organohalogen contaminants and their metabolites in the blood of Japanese terrestrial mammals. Environ Pollut 2013; 174:28 - 37; http://dx.doi.org/10.1016/j.envpol.2012.11.004; PMID: 23246744
- Shaw SD, Berger ML, Weijs L, Covaci A. Tissue-specific accumulation of polybrominated diphenyl ethers (PBDEs) including Deca-BDE and hexabromocyclododecanes (HBCDs) in harbor seals from the northwest Atlantic. Environ Int 2012; 44:1 - 6; http://dx.doi.org/10.1016/j.envint.2012.01.001; PMID: 22321537
- Yu L, Luo X, Zheng X, Zeng Y, Chen D, Wu J, Mai B. Occurrence and biomagnification of organohalogen pollutants in two terrestrial predatory food chains. Chemosphere 2013; 93:506 - 11; http://dx.doi.org/10.1016/j.chemosphere.2013.06.023; PMID: 23830888
- La Guardia MJ, Hale RC, Harvey E, Mainor TM, Ciparis S. In situ accumulation of HBCD, PBDEs, and several alternative flame-retardants in the bivalve (Corbicula fluminea) and gastropod (Elimia proxima). Environ Sci Technol 2012; 46:5798 - 805; http://dx.doi.org/10.1021/es3004238; PMID: 22571713
- Bi X, Thomas GO, Jones KC, Qu W, Sheng G, Martin FL, Fu J. Exposure of electronics dismantling workers to polybrominated diphenyl ethers, polychlorinated biphenyls, and organochlorine pesticides in South China. Environ Sci Technol 2007; 41:5647 - 53; http://dx.doi.org/10.1021/es070346a; PMID: 17874768
- He S, Li M, Jin J, Wang Y, Bu Y, Xu M, Yang X, Liu A. Concentrations and trends of halogenated flame retardants in the pooled serum of residents of Laizhou Bay, China. Environ Toxicol Chem 2013; 32:1242 - 7; http://dx.doi.org/10.1002/etc.2172; PMID: 23408421
- Lunder S, Hovander L, Athanassiadis I, Bergman A. Significantly higher polybrominated diphenyl ether levels in young U.S. children than in their mothers. Environ Sci Technol 2010; 44:5256 - 62; http://dx.doi.org/10.1021/es1009357; PMID: 20540541
- La Guardia MJ, Hale RC, Harvey E. Evidence of debromination of decabromodiphenyl ether (BDE-209) in biota from a wastewater receiving stream. Environ Sci Technol 2007; 41:6663 - 70; http://dx.doi.org/10.1021/es070728g; PMID: 17969678
- Arkoosh MR, Boylen D, Dietrich J, Anulacion BF, Ginaylitalo, Bravo CF, Johnson LL, Loge FJ, Collier TK. Disease susceptibility of salmon exposed to polybrominated diphenyl ethers (PBDEs). Aquat Toxicol 2010; 98:51 - 9; http://dx.doi.org/10.1016/j.aquatox.2010.01.013; PMID: 20207027
- Birchmeier KL, Smith KA, Passino-Reader DR, Sweet LI, Chernyak SM, Adams JV, Omann GM. Effects of selected polybrominated diphenyl ether flame retardants on lake trout (Salvelinus namaycush). Environ Toxicol Chem 2005; 24:1518 - 22; http://dx.doi.org/10.1897/04-347R.1; PMID: 16117131
- Shao J, Eckert ML, Lee LEJ, Gallagher EP. Comparative oxygen radical formation and toxicity of BDE 47 in rainbow trout cell lines. Mar Environ Res 2008; 66:7 - 8; http://dx.doi.org/10.1016/j.marenvres.2008.02.007; PMID: 18400291
- van Boxtel AL, Kamstra JH, Cenijn PH, Pieterse B, Wagner JM, Antink M, Krab K, van der Burg B, Marsh G, Brouwer A, et al. Microarray analysis reveals a mechanism of phenolic polybrominated diphenylether toxicity in zebrafish. Environ Sci Technol 2008; 42:1773 - 9; http://dx.doi.org/10.1021/es0720863; PMID: 18441834
- Zhao A, Liu H, Zhang A, Wang X, Zhang H, Wang H. Effect of BDE-209 on glutathione system in Carassius auratus. Environ Toxicol Pharmacol 2011; 32:35 - 9; http://dx.doi.org/10.1016/j.etap.2011.03.004; PMID: 21787727
- NTP. Toxicology and carcinogenesis studies of decabromodiphenyl oxide (CAS no. 1163-19-5) in F344/N and B 6c3F1 mice (fed studies). National Toxicology Program, National Instititute of Environmental Health and Safety. Available on-line at: ntp.niehs.nih.gov/ntp/htdocs/lt_rpts/tr309.pdf. NTP: National Toxicology Program. NTP TR 309, 1986.
- Dingemans MML, van den Berg M, Westerink RHS. Neurotoxicity of brominated flame retardants: (in)direct effects of parent and hydroxylated polybrominated diphenyl ethers on the (developing) nervous system. Environ Health Perspect 2011; 119:900 - 7; http://dx.doi.org/10.1289/ehp.1003035; PMID: 21245014
- Costa LG, Giordano G. Is decabromodiphenyl ether (BDE-209) a developmental neurotoxicant?. Neurotoxicology 2011; 32:9 - 24; http://dx.doi.org/10.1016/j.neuro.2010.12.010; PMID: 21182867
- Staskal D, Birnbaum L. Human health effects of brominated flame retardants. In: Barcelo D, Kostianoy AG, eds. The Handbook of Environmental Chemistry; Brominated Flame Retardants. Heidelberg, Germany: Springer Publishing Services, 2011:19-54.
- Burreau S, Axelman J, Broman D, Jakobsson E. Dietary uptake in pike (Esox lucius) of some polychlorinated biphenyls, polychlorinated naphthalenes and polybrominated diphenyl ethers administered in natural diet. Environ Toxicol Chem 1997; 16:2508 - 13; http://dx.doi.org/10.1897/1551-5028(1997)016<2508:DUIPEL>2.3.CO;2
- Burreau S, Broman D, Orn U. Tissue distribution of 2,2′,4,4′-tetrabromo[14C]diphenyl ether ([14C]-PBDE 47) in pike (Esox lucius) after dietary exposure--a time series study using whole body autoradiography. Chemosphere 2000; 40:977 - 85; http://dx.doi.org/10.1016/S0045-6535(99)00342-2; PMID: 10739035
- Chen LJ, Lebetkin EH, Sanders JM, Burka LT. Metabolism and disposition of 2,2′,4,4′,5-pentabromodiphenyl ether (BDE99) following a single or repeated administration to rats or mice. Xenobiotica 2006; 36:515 - 34; http://dx.doi.org/10.1080/00498250600674477; PMID: 16769647
- Sanders JM, Lebetkin EH, Chen LJ, Burka LT. Disposition of 2,2′,4,4′,5,5′-hexabromodiphenyl ether (BDE153) and its interaction with other polybrominated diphenyl ethers (PBDEs) in rodents. Xenobiotica 2006; 36:824 - 37; http://dx.doi.org/10.1080/00498250600815906; PMID: 16971346
- Staskal DF, Diliberto JJ, DeVito MJ, Birnbaum LS. Toxicokinetics of BDE 47 in female mice: effect of dose, route of exposure, and time. Toxicol Sci 2005; 83:215 - 23; http://dx.doi.org/10.1093/toxsci/kfi018; PMID: 15509665
- Munschy C, Héas-Moisan K, Tixier C, Olivier N, Gastineau O, Le Bayon N, Buchet V. Dietary exposure of juvenile common sole (Solea solea L.) to polybrominated diphenyl ethers (PBDEs): Part 1. Bioaccumulation and elimination kinetics of individual congeners and their debrominated metabolites. Environ Pollut 2011; 159:229 - 37; http://dx.doi.org/10.1016/j.envpol.2010.09.001; PMID: 20888677
- Nyholm JR, Norman A, Norrgren L, Haglund P, Andersson PL. Uptake and biotransformation of structurally diverse brominated flame retardants in zebrafish (Danio rerio) after dietary exposure. Environ Toxicol Chem 2009; 28:1035 - 42; http://dx.doi.org/10.1897/08-302.1; PMID: 19049262
- Stapleton HM, Letcher RJ, Baker JE. Debromination of polybrominated diphenyl ether congeners BDE 99 and BDE 183 in the intestinal tract of the common carp (Cyprinus carpio). Environ Sci Technol 2004; 38:1054 - 61; http://dx.doi.org/10.1021/es0348804; PMID: 14998018
- Kierkegaard A, Balk L, Tjarnlund U, De Wit CA, Jansson B. Dietary uptake and biological effects of decabromodiphenyl ether in rainbow trout (Oncorhynchus mykiss). Environ Sci Technol 1999; 33:1612 - 7; http://dx.doi.org/10.1021/es9807082
- Stapleton HM, Brazil B, Holbrook RD, Mitchelmore CL, Benedict R, Konstantinov A, Potter D. In vivo and in vitro debromination of decabromodiphenyl ether (BDE 209) by juvenile rainbow trout and common carp. Environ Sci Technol 2006; 40:4653 - 8; http://dx.doi.org/10.1021/es060573x; PMID: 16913120
- Noyes PD, Hinton DE, Stapleton HM. Accumulation and debromination of decabromodiphenyl ether (BDE-209) in juvenile fathead minnows (Pimephales promelas) induces thyroid disruption and liver alterations. Toxicol Sci 2011; 122:265 - 74; http://dx.doi.org/10.1093/toxsci/kfr105; PMID: 21546348
- Noyes PD, Lema SC, Macaulay LJ, Douglas NK, Stapleton HM. Low level exposure to the flame retardant BDE-209 reduces thyroid hormone levels and disrupts thyroid signaling in fathead minnows. Environ Sci Technol 2013; 47:10012 - 21; http://dx.doi.org/10.1021/es402650x; PMID: 23899252
- Wan Y, Zhang K, Dong Z, Hu J. Distribution is a major factor affecting bioaccumulation of decabrominated diphenyl ether: Chinese sturgeon (Acipenser sinensis) as an example. Environ Sci Technol 2013; 47:2279 - 86; http://dx.doi.org/10.1021/es304926r; PMID: 23387833
- Eskenazi B, Chevrier J, Rauch SA, Kogut K, Harley KG, Johnson C, Trujillo C, Sjödin A, Bradman A. In utero and childhood polybrominated diphenyl ether (PBDE) exposures and neurodevelopment in the CHAMACOS study. Environ Health Perspect 2013; 121:257 - 62; PMID: 23154064
- Petreas M, Nelson D, Brown FR, Goldberg D, Hurley S, Reynolds P. High concentrations of polybrominated diphenylethers (PBDEs) in breast adipose tissue of California women. Environ Int 2011; 37:190 - 7; http://dx.doi.org/10.1016/j.envint.2010.09.001; PMID: 20951435
- Dominguez AA, Law RJ, Herzke D, de Boer J. Bioaccumulation of brominated flame retardants. In: Barcelo D, Kostianoy AG, eds. The Handbook of Environmental Chemistry; Brominated Flame Retardants. Heidelberg, Germany: Springer Publishing Services, 2011:141-87.
- Xia C, Lam JCW, Wu X, Sun L, Xie Z, Lam PKS. Levels and distribution of polybrominated diphenyl ethers (PBDEs) in marine fishes from Chinese coastal waters. Chemosphere 2011; 82:18 - 24; http://dx.doi.org/10.1016/j.chemosphere.2010.10.037; PMID: 21051072
- Kodavanti PRS, Coburn CG, Moser VC, MacPhail RC, Fenton SE, Stoker TE, Rayner JL, Kannan K, Birnbaum LS. Developmental exposure to a commercial PBDE mixture, DE-71: neurobehavioral, hormonal, and reproductive effects. Toxicol Sci 2010; 116:297 - 312; http://dx.doi.org/10.1093/toxsci/kfq105; PMID: 20375078
- Naert C, Van Peteghem C, Kupper J, Jenni L, Naegeli H. Distribution of polychlorinated biphenyls and polybrominated diphenyl ethers in birds of prey from Switzerland. Chemosphere 2007; 68:977 - 87; http://dx.doi.org/10.1016/j.chemosphere.2007.01.009; PMID: 17307228
- Dunnick JK, Nyska A. Characterization of liver toxicity in F344/N rats and B6C3F1 mice after exposure to a flame retardant containing lower molecular weight polybrominated diphenyl ethers. Exp Toxicol Pathol 2009; 61:1 - 12; http://dx.doi.org/10.1016/j.etp.2008.06.008; PMID: 18774282
- Hardy ML. The toxicology of the three commercial polybrominated diphenyl oxide (ether) flame retardants. Chemosphere 2002; 46:757 - 77; http://dx.doi.org/10.1016/S0045-6535(01)00240-5; PMID: 11999799
- Huwe JK, Hakk H, Birnbaum LS. Tissue distribution of polybrominated diphenyl ethers in male rats and implications for biomonitoring. Environ Sci Technol 2008; 42:7018 - 24; http://dx.doi.org/10.1021/es801344a; PMID: 18853825
- Morck A, Hakk H, Orn U, Klasson Wehler E. Decabromodiphenyl ether in the rat: absorption, distribution, metabolism, and excretion. Drug Metab Dispos 2003; 31:900 - 7; http://dx.doi.org/10.1124/dmd.31.7.900; PMID: 12814967
- Benedict RT, Stapleton HM, Letcher RJ, Mitchelmore CL. Debromination of polybrominated diphenyl ether-99 (BDE-99) in carp (Cyprinus carpio) microflora and microsomes. Chemosphere 2007; 69:987 - 93; http://dx.doi.org/10.1016/j.chemosphere.2007.05.010; PMID: 17640709
- Boon JP, van Zanden JJ, Lewis WE, Zegers BN, Goksøyr A, Arukwe A. The expression of CYP1A, vitellogenin and zona radiata proteins in Atlantic salmon (Salmo salar) after oral dosing with two commercial PBDE flame retardant mixtures: absence of short-term responses. Mar Environ Res 2002; 54:719 - 24; http://dx.doi.org/10.1016/S0141-1136(02)00127-7; PMID: 12408642
- Browne EP, Stapleton HM, Kelly SM, Tilton SC, Gallagher EP. In vitro hepatic metabolism of 2,2′,4,4′,5-pentabromodiphenyl ether (BDE 99) in Chinook salmon (Onchorhynchus tshawytscha). Aquat Toxicol 2009; 92:281 - 7; http://dx.doi.org/10.1016/j.aquatox.2009.02.017; PMID: 19346012
- Cheng J, Mao L, Zhao Z, Shen M, Zhang S, Huang Q, Gao S. Bioaccumulation, depuration and biotransformation of 4,4′-dibromodiphenyl ether in crucian carp (Carassius auratus). Chemosphere 2012; 86:446 - 53; http://dx.doi.org/10.1016/j.chemosphere.2011.09.038; PMID: 22036552
- Kuiper RV, Murk AJ, Leonards PEG, Grinwis GCM, van den Berg M, Vos JG. In vivo and in vitro Ah-receptor activation by commercial and fractionated pentabromodiphenylether using zebrafish (Danio rerio) and the DR-CALUX assay. Aquat Toxicol 2006; 79:366 - 75; http://dx.doi.org/10.1016/j.aquatox.2006.07.005; PMID: 16919340
- Kuo Y-M, Sepúlveda MS, Sutton TM, Ochoa-Acuña HG, Muir AM, Miller B, Hua I. Bioaccumulation and biotransformation of decabromodiphenyl ether and effects on daily growth in juvenile lake whitefish (Coregonus clupeaformis). Ecotoxicology 2010; 19:751 - 60; http://dx.doi.org/10.1007/s10646-009-0451-x; PMID: 20033485
- Munschy C, Héas-Moisan K, Tixier C, Pacepavicius G, Alaee M. Dietary exposure of juvenile common sole (Solea solea L.) to polybrominated diphenyl ethers (PBDEs): Part 2. Formation, bioaccumulation and elimination of hydroxylated metabolites. Environ Pollut 2010; 158:3527 - 33; http://dx.doi.org/10.1016/j.envpol.2010.08.021; PMID: 20864231
- Noyes PD, Kelly SM, Mitchelmore CL, Stapleton HM. Characterizing the in vitro hepatic biotransformation of the flame retardant BDE 99 by common carp. Aquat Toxicol 2010; 97:142 - 50; http://dx.doi.org/10.1016/j.aquatox.2009.12.013; PMID: 20080306
- Olsvik PA, Lie KK, Sturve J, Hasselberg L, Andersen OK. Transcriptional effects of nonylphenol, bisphenol A and PBDE-47 in liver of juvenile Atlantic cod (Gadus morhua). Chemosphere 2009; 75:360 - 7; http://dx.doi.org/10.1016/j.chemosphere.2008.12.039; PMID: 19167021
- Roberts SC, Noyes PD, Gallagher EP, Stapleton HM. Species-specific differences and structure-activity relationships in the debromination of PBDE congeners in three fish species. Environ Sci Technol 2011; 45:1999 - 2005; http://dx.doi.org/10.1021/es103934x; PMID: 21291240
- Stapleton HM, Letcher RJ, Li J, Baker JE. Dietary accumulation and metabolism of polybrominated diphenyl ethers by juvenile carp (Cyprinus carpio). Environ Toxicol Chem 2004; 23:1939 - 46; http://dx.doi.org/10.1897/03-462; PMID: 15352483
- Wan Y, Liu F, Wiseman S, Zhang X, Chang H, Hecker M, Jones PD, Lam MH, Giesy JP. Interconversion of hydroxylated and methoxylated polybrominated diphenyl ethers in Japanese medaka. Environ Sci Technol 2010; 44:8729 - 35; http://dx.doi.org/10.1021/es102287q; PMID: 20973477
- Zeng YH, Luo XJ, Chen HS, Yu LH, Chen SJ, Mai BX. Gastrointestinal absorption, metabolic debromination, and hydroxylation of three commercial polybrominated diphenyl ether mixtures by common carp. Environ Toxicol Chem 2012; 31:731 - 8; http://dx.doi.org/10.1002/etc.1716; PMID: 22170638
- Letcher RJ, Klaassen-Wehler E, Bergman A. Methyl Sulfone and Hydroxylated Metabolites of Polychlorinated Biphenyls. In: Paasivirta J, ed. The Handbook of Environmental Chemistry. Berlin: Springer-Verlag, 2001:315-59.
- Staskal DF, Hakk H, Bauer D, Diliberto JJ, Birnbaum LS. Toxicokinetics of polybrominated diphenyl ether congeners 47, 99, 100, and 153 in mice. Toxicol Sci 2006; 94:28 - 37; http://dx.doi.org/10.1093/toxsci/kfl091; PMID: 16936226
- Tomy GT, Palace VP, Halldorson T, Braekevelt E, Danell R, Wautier K, Evans B, Brinkworth L, Fisk AT. Bioaccumulation, biotransformation, and biochemical effects of brominated diphenyl ethers in juvenile lake trout (Salvelinus namaycush). Environ Sci Technol 2004; 38:1496 - 504; http://dx.doi.org/10.1021/es035070v; PMID: 15046352
- Blanton ML, Specker JL. The hypothalamic-pituitary-thyroid (HPT) axis in fish and its role in fish development and reproduction. Crit Rev Toxicol 2007; 37:97 - 115; http://dx.doi.org/10.1080/10408440601123529; PMID: 17364706
- Hakk H, Larsen G, Klasson-Wehler E. Tissue disposition, excretion and metabolism of 2,2′,4,4′,5-pentabromodiphenyl ether (BDE-99) in the male Sprague-Dawley rat. Xenobiotica 2002; 32:369 - 82; http://dx.doi.org/10.1080/00498250110119117; PMID: 12065060
- Fernie KJ, Shutt JL, Mayne G, Hoffman D, Letcher RJ, Drouillard KG, Ritchie IJ. Exposure to polybrominated diphenyl ethers (PBDEs): changes in thyroid, vitamin A, glutathione homeostasis, and oxidative stress in American kestrels (Falco sparverius). Toxicol Sci 2005; 88:375 - 83; http://dx.doi.org/10.1093/toxsci/kfi295; PMID: 16120752
- Feng C, Xu Y, Zhao G, Zha J, Wu F, Wang Z. Relationship between BDE 209 metabolites and thyroid hormone levels in rainbow trout (Oncorhynchus mykiss). Aquat Toxicol 2012; 122-123:28 - 35; http://dx.doi.org/10.1016/j.aquatox.2012.05.008; PMID: 22721785
- Stapleton HM. Instrumental methods and challenges in quantifying polybrominated diphenyl ethers in environmental extracts: a review. Anal Bioanal Chem 2006; 386:807 - 17; http://dx.doi.org/10.1007/s00216-006-0400-y; PMID: 17165211
- Chen TH, Cheng YM, Cheng JO, Chou CT, Hsiao YC, Ko FC. Growth and transcriptional effect of dietary 2,2′,4,4′-tetrabromodiphenyl ether (PBDE-47) exposure in developing zebrafish (Danio rerio). Ecotoxicol Environ Saf 2010; 73:377 - 83; http://dx.doi.org/10.1016/j.ecoenv.2009.12.033; PMID: 20074802
- Kuiper RV, Vethaak AD, Cantón RF, Anselmo H, Dubbeldam M, van den Brandhof EJ, Leonards PE, Wester PW, van den Berg M. Toxicity of analytically cleaned pentabromodiphenylether after prolonged exposure in estuarine European flounder (Platichthys flesus), and partial life-cycle exposure in fresh water zebrafish (Danio rerio). Chemosphere 2008; 73:195 - 202; http://dx.doi.org/10.1016/j.chemosphere.2008.04.079; PMID: 18556046
- Kuiper RV, Bergman A, Vos JG, van den Berg M. Some polybrominated diphenyl ether (PBDE) flame retardants with wide environmental distribution inhibit TCDD-induced EROD activity in primary cultured carp (Cyprinus carpio) hepatocytes. Aquat Toxicol 2004; 68:129 - 39; http://dx.doi.org/10.1016/j.aquatox.2004.03.005; PMID: 15145223
- Timme-Laragy AR, Levin ED, Di Giulio RT. Developmental and behavioral effects of embryonic exposure to the polybrominated diphenylether mixture DE-71 in the killifish (Fundulus heteroclitus). Chemosphere 2006; 62:1097 - 104; http://dx.doi.org/10.1016/j.chemosphere.2005.05.037; PMID: 16045967
- Kawamoto T, Sueyoshi T, Zelko I, Moore R, Washburn K, Negishi M. Phenobarbital-responsive nuclear translocation of the receptor CAR in induction of the CYP2B gene. Mol Cell Biol 1999; 19:6318 - 22; PMID: 10454578
- Schlenk D, Celander M, Gallagher EP, George SC, James M, Kullman SW, et al. Biotransformation in Fishes. In: Di Giulio RT, Hinton DE, eds. The Toxicology of Fishes. New York: CRC Press, 2008:153-234.
- McArthur AG, Hegelund T, Cox RL, Stegeman JJ, Liljenberg M, Olsson U, Sundberg P, Celander MC. Phylogenetic analysis of the cytochrome P450 3 (CYP3) gene family. J Mol Evol 2003; 57:200 - 11; http://dx.doi.org/10.1007/s00239-003-2466-x; PMID: 14562963
- Leaver MJ, Wright J, Hodgson P, Boukouvala E, George SG. Piscine UDP-glucuronosyltransferase 1B. Aquat Toxicol 2007; 84:356 - 65; http://dx.doi.org/10.1016/j.aquatox.2007.06.015; PMID: 17686537
- Liu TA, Bhuiyan S, Liu MY, Sugahara T, Sakakibara Y, Suiko M, Yasuda S, Kakuta Y, Kimura M, Williams FE, et al. Zebrafish as a model for the study of the phase II cytosolic sulfotransferases. Curr Drug Metab 2010; 11:538 - 46; http://dx.doi.org/10.2174/138920010791636158; PMID: 20545621
- Sugahara T, Liu CC, Pai TG, Collodi P, Suiko M, Sakakibara Y, Nishiyama K, Liu MC. Sulfation of hydroxychlorobiphenyls. Molecular cloning, expression, and functional characterization of zebrafish SULT1 sulfotransferases. Eur J Biochem 2003; 270:2404 - 11; http://dx.doi.org/10.1046/j.1432-1033.2003.03608.x; PMID: 12755695
- George SG, Taylor B. Molecular evidence for multiple UDP-glucuronosyltransferase gene familes in fish. Mar Environ Res 2002; 54:253 - 7; http://dx.doi.org/10.1016/S0141-1136(02)00186-1; PMID: 12408571
- Chen Q, Yu L, Yang L, Zhou B. Bioconcentration and metabolism of decabromodiphenyl ether (BDE-209) result in thyroid endocrine disruption in zebrafish larvae. Aquat Toxicol 2012; 110-111:141 - 8; http://dx.doi.org/10.1016/j.aquatox.2012.01.008; PMID: 22307006
- Hakk H, Huwe JK, Larsen GL. Absorption, distribution, metabolism and excretion (ADME) study with 2,2′,4,4′,5,6′-hexabromodiphenyl ether (BDE-154) in male Sprague-Dawley rats. Xenobiotica 2009; 39:46 - 56; http://dx.doi.org/10.1080/00498250802546853; PMID: 19219747
- Muirhead EK, Skillman AD, Hook SE, Schultz IR. Oral exposure of PBDE-47 in fish: toxicokinetics and reproductive effects in Japanese Medaka (Oryzias latipes) and fathead minnows (Pimephales promelas). Environ Sci Technol 2006; 40:523 - 8; http://dx.doi.org/10.1021/es0513178; PMID: 16468398
- Geyer HJ, Schramm KW, Darnerud PO, Aune M, Feight A, Fried KW, et al. Terminal elimination half-lives of the brominated flame retardants TBBPA, HBCD, and lower brominated PBDEs in humans. Organohal Comp 2004; 66.
- von Meyerinck L, Hufnagel B, Schmoldt A, Benthe HF. Induction of rat liver microsomal cytochrome P-450 by the pentabromo diphenyl ether Bromkal 70 and half-lives of its components in the adipose tissue. Toxicology 1990; 61:259 - 74; http://dx.doi.org/10.1016/0300-483X(90)90176-H; PMID: 2330598
- Thuresson K, Höglund P, Hagmar L, Sjödin A, Bergman A, Jakobsson K. Apparent half-lives of hepta- to decabrominated diphenyl ethers in human serum as determined in occupationally exposed workers. Environ Health Perspect 2006; 114:176 - 81; http://dx.doi.org/10.1289/ehp.8350; PMID: 16451851
- Sandholm A, Emanuelsson BM, Wehler EK. Bioavailability and half-life of decabromodiphenyl ether (BDE-209) in rat. Xenobiotica 2003; 33:1149 - 58; http://dx.doi.org/10.1080/00498250310001609156; PMID: 14660178
- Huwe JK, Smith DJ. Accumulation, whole-body depletion, and debromination of decabromodiphenyl ether in male sprague-dawley rats following dietary exposure. Environ Sci Technol 2007; 41:2371 - 7; http://dx.doi.org/10.1021/es061954d; PMID: 17438789
- Anderson GW. Thyroid hormone and cerebellar development. Cerebellum 2008; 7:60 - 74; http://dx.doi.org/10.1007/s12311-008-0021-4; PMID: 18418681
- Kapoor R, Desouza LA, Nanavaty IN, Kernie SG, Vaidya VA. Thyroid hormone accelerates the differentiation of adult hippocampal progenitors. J Neuroendocrinol 2012; 24:1259 - 71; http://dx.doi.org/10.1111/j.1365-2826.2012.02329.x; PMID: 22497336
- Shiao JC, Hwang PP. Thyroid hormones are necessary for the metamorphosis of tarpon Megalops cyprinoides leptocephali. J Exp Mar Biol Ecol 2006; 331:121 - 32; http://dx.doi.org/10.1016/j.jembe.2005.10.014
- Schreiber AM, Specker JL. Metamorphosis in the summer flounder (Paralichthys dentatus): stage-specific developmental response to altered thyroid status. Gen Comp Endocrinol 1998; 111:156 - 66; http://dx.doi.org/10.1006/gcen.1998.7095; PMID: 9679087
- Klaren PHM, Guzman JM, Mancera JM, Geven EJW, Flik G. The involvement of thyroid hormone metabolism in Gilthead sea bream. (Sparus auratus) osmoregulation. In: Vaudry H, Roubos E, Schoofs L, Fiik G, Larhammar D, eds. Trends in Comparative Endocrinology and Neurobiology, 2005:360-2.
- Lema SC, Nevitt GA. Evidence that thyroid hormone induces olfactory cellular proliferation in salmon during a sensitive period for imprinting. J Exp Biol 2004; 207:3317 - 27; http://dx.doi.org/10.1242/jeb.01143; PMID: 15326208
- Peter MCS. The role of thyroid hormones in stress response of fish. Gen Comp Endocrinol 2011; 172:198 - 210; http://dx.doi.org/10.1016/j.ygcen.2011.02.023; PMID: 21362420
- Coffin AB, Raine JC, Hawryshyn CW. Exposure to thyroid hormone in ovo affects otolith crystallization in rainbow trout Oncorhynchus mykiss. Exp Biol Fishes 2012; 95:347 - 54; http://dx.doi.org/10.1007/s10641-012-0007-4
- Nelson ER, Allan ERO, Pang FY, Habibi HR. Thyroid hormone and reproduction: regulation of estrogen receptors in goldfish gonads. Mol Reprod Dev 2010; 77:784 - 94; http://dx.doi.org/10.1002/mrd.21219; PMID: 20722048
- Dickhoff WW, Folmar LC, Mighell JL, Mahnken CVW. Plasma thyroid hormones during smoltification of yearling and underyearling Coho salmon and yearling Chinook salmon and Steelhead trout. Aquaculture 1982; 28:39 - 48; http://dx.doi.org/10.1016/0044-8486(82)90006-0
- Larsen DA, Swanson P, Dickhoff WW. The pituitary-thyroid axis during the parr-smolt transformation of Coho salmon, Oncorhynchus kisutch: quantification of TSH β mRNA, TSH, and thyroid hormones. Gen Comp Endocrinol 2011; 171:367 - 72; http://dx.doi.org/10.1016/j.ygcen.2011.03.003; PMID: 21377468
- Eales JG, Brown SB. Measurement and regulation of thyroidal status in teleost fish. Rev Fish Biol Fish 1993; 3:299 - 347; http://dx.doi.org/10.1007/BF00043383
- Schussler GC. The thyroxine-binding proteins. Thyroid 2000; 10:141 - 9; http://dx.doi.org/10.1089/thy.2000.10.141; PMID: 10718550
- Kawakami Y, Seoka M, Miyashita S, Kumai H, Ohta H. Characterization of transthyretin in the Pacific bluefin tuna, Thunnus orientalis. Zoolog Sci 2006; 23:443 - 8; http://dx.doi.org/10.2108/zsj.23.443; PMID: 16766863
- Santos CRA, Power DM. Identification of transthyretin in fish (Sparus aurata): cDNA cloning and characterisation. Endocrinology 1999; 140:2430 - 3; http://dx.doi.org/10.1210/endo.140.5.6898; PMID: 10218999
- Arjona FJ, de Vrieze E, Visser TJ, Flik G, Klaren PHM. Identification and functional characterization of zebrafish solute carrier Slc16a2 (Mct8) as a thyroid hormone membrane transporter. Endocrinology 2011; 152:5065 - 73; http://dx.doi.org/10.1210/en.2011-1166; PMID: 21952246
- Muzzio AM, Noyes PD, Stapleton HM, Lema SC. The organic anion transporting protein (OATP) family in a teleost fish model. Integr Comp Biol 2013; 53:Suppl. 1 E340
- Popovic M, Zaja R, Smital T. Organic anion transporting polypeptides (OATP) in zebrafish (Danio rerio): Phylogenetic analysis and tissue distribution. Comp Biochem Physiol A Mol Integr Physiol 2010; 155:327 - 35; http://dx.doi.org/10.1016/j.cbpa.2009.11.011; PMID: 19931635
- Visser WE, Friesema ECH, Visser TJ. Minireview: thyroid hormone transporters: the knowns and the unknowns. Mol Endocrinol 2011; 25:1 - 14; http://dx.doi.org/10.1210/me.2010-0095; PMID: 20660303
- Nelson ER, Habibi HR. Thyroid receptor subtypes: structure and function in fish. Gen Comp Endocrinol 2009; 161:90 - 6; http://dx.doi.org/10.1016/j.ygcen.2008.09.006; PMID: 18840444
- Hiroi Y, Kim HH, Ying H, Furuya F, Huang Z, Simoncini T, Noma K, Ueki K, Nguyen NH, Scanlan TS, et al. Rapid nongenomic actions of thyroid hormone. Proc Natl Acad Sci U S A 2006; 103:14104 - 9; http://dx.doi.org/10.1073/pnas.0601600103; PMID: 16966610
- Yonkers MA, Ribera AB. Molecular components underlying nongenomic thyroid hormone signaling in embryonic zebrafish neurons. Neural Dev 2009; 4:20; http://dx.doi.org/10.1186/1749-8104-4-20; PMID: 19505305
- Gereben B, Zeöld A, Dentice M, Salvatore D, Bianco AC. Activation and inactivation of thyroid hormone by deiodinases: local action with general consequences. Cell Mol Life Sci 2008; 65:570 - 90; http://dx.doi.org/10.1007/s00018-007-7396-0; PMID: 17989921
- Orozco A, Valverde-R C, Olvera A, García-G C. Iodothyronine deiodinases: a functional and evolutionary perspective. J Endocrinol 2012; 215:207 - 19; http://dx.doi.org/10.1530/JOE-12-0258; PMID: 22872760
- Orozco A, Villalobos P, Jeziorski MC, Valverde-R C. The liver of Fundulus heteroclitus expresses deiodinase type 1 mRNA. Gen Comp Endocrinol 2003; 130:84 - 91; http://dx.doi.org/10.1016/S0016-6480(02)00570-1; PMID: 12535629
- Orozco A, Valverde-R C. Thyroid hormone deiodination in fish. Thyroid 2005; 15:799 - 813; http://dx.doi.org/10.1089/thy.2005.15.799; PMID: 16131323
- Frith SD, Eales JG. Thyroid hormone deiodination pathways in brain and liver of rainbow trout, Oncorhynchus mykiss. Gen Comp Endocrinol 1996; 101:323 - 32; http://dx.doi.org/10.1006/gcen.1996.0035; PMID: 8729942
- Johnson KM, Lema SC. Tissue-specific thyroid hormone regulation of gene transcripts encoding iodothyronine deiodinases and thyroid hormone receptors in striped parrotfish (Scarus iseri). Gen Comp Endocrinol 2011; 172:505 - 17; http://dx.doi.org/10.1016/j.ygcen.2011.04.022; PMID: 21549118
- Wambiji N, Park Y-J, Kim S-J, Hur S-P, Takeuchi Y, Takemura A. Expression of type II iodothyronine deiodinase gene in the brain of a tropical spinefoot, Siganus guttatus. Comp Biochem Physiol A Mol Integr Physiol 2011; 160:447 - 52; http://dx.doi.org/10.1016/j.cbpa.2011.03.023; PMID: 21463701
- St Germain DL. The effects and interactions of substrates, inhibitors, and the cellular thiol-disulfide balance on the regulation of type II iodothyronine 5′-deiodinase. Endocrinology 1988; 122:1860 - 8; http://dx.doi.org/10.1210/endo-122-5-1860; PMID: 3359966
- Lee E, Kim TH, Choi JS, Nabanata P, Kim NY, Ahn MY, Jung KK, Kang IH, Kim TS, Kwack SJ, et al. Evaluation of liver and thyroid toxicity in Sprague-Dawley rats after exposure to polybrominated diphenyl ether BDE-209. J Toxicol Sci 2010; 35:535 - 45; http://dx.doi.org/10.2131/jts.35.535; PMID: 20686340
- Richardson VM, Staskal DF, Ross DG, Diliberto JJ, DeVito MJ, Birnbaum LS. Possible mechanisms of thyroid hormone disruption in mice by BDE 47, a major polybrominated diphenyl ether congener. Toxicol Appl Pharmacol 2008; 226:244 - 50; http://dx.doi.org/10.1016/j.taap.2007.09.015; PMID: 17964624
- Tseng LH, Li MH, Tsai SS, Lee CW, Pan MH, Yao WJ, Hsu PC. Developmental exposure to decabromodiphenyl ether (PBDE 209): effects on thyroid hormone and hepatic enzyme activity in male mouse offspring. Chemosphere 2008; 70:640 - 7; http://dx.doi.org/10.1016/j.chemosphere.2007.06.078; PMID: 17698168
- Butt CM, Wang D, Stapleton HM. Halogenated phenolic contaminants inhibit the in vitro activity of the thyroid-regulating deiodinases in human liver. Toxicol Sci 2011; 124:339 - 47; http://dx.doi.org/10.1093/toxsci/kfr117; PMID: 21565810
- Szabo DT, Richardson VM, Ross DG, Diliberto JJ, Kodavanti PRS, Birnbaum LS. Effects of perinatal PBDE exposure on hepatic phase I, phase II, phase III, and deiodinase 1 gene expression involved in thyroid hormone metabolism in male rat pups. Toxicol Sci 2009; 107:27 - 39; http://dx.doi.org/10.1093/toxsci/kfn230; PMID: 18978342
- Meerts IA, van Zanden JJ, Luijks EAC, van Leeuwen-Bol I, Marsh G, Jakobsson E, Bergman A, Brouwer A. Potent competitive interactions of some brominated flame retardants and related compounds with human transthyretin in vitro. Toxicol Sci 2000; 56:95 - 104; http://dx.doi.org/10.1093/toxsci/56.1.95; PMID: 10869457
- Bloom M, Spliethoff H, Vena J, Shaver S, Addink R, Eadon G. Environmental exposure to PBDEs and thyroid function among New York anglers. Environ Toxicol Pharmacol 2008; 25:386 - 92; http://dx.doi.org/10.1016/j.etap.2007.12.004; PMID: 21783878
- Meeker JD, Johnson PI, Camann D, Hauser R. Polybrominated diphenyl ether (PBDE) concentrations in house dust are related to hormone levels in men. Sci Total Environ 2009; 407:3425 - 9; http://dx.doi.org/10.1016/j.scitotenv.2009.01.030; PMID: 19211133
- Stapleton HM, Eagle S, Anthopolos R, Wolkin A, Miranda ML. Associations between polybrominated diphenyl ether (PBDE) flame retardants, phenolic metabolites, and thyroid hormones during pregnancy. Environ Health Perspect 2011; 119:1454 - 9; http://dx.doi.org/10.1289/ehp.1003235; PMID: 21715241
- Dong W, Macaulay LJ, Kwok KWH, Hinton DE, Stapleton HM. Using whole mount in situ hybridization to examine thyroid hormone deiodinase expression in embryonic and larval zebrafish: a tool for examining OH-BDE toxicity to early life stages. Aquat Toxicol 2013; 132-133:190 - 9; http://dx.doi.org/10.1016/j.aquatox.2013.02.008; PMID: 23531416
- Lema SC, Dickey JT, Schultz IR, Swanson P. Dietary exposure to 2,2′,4,4′-tetrabromodiphenyl ether (PBDE-47) alters thyroid status and thyroid hormone-regulated gene transcription in the pituitary and brain. Environ Health Perspect 2008; 116:1694 - 9; http://dx.doi.org/10.1289/ehp.11570; PMID: 19079722
- Li W, Zhu L, Zha J, Wang Z. Effects of decabromodiphenyl ether (BDE-209) on mRNA transcription of thyroid hormone pathway and spermatogenesis associated genes in Chinese rare minnow (Gobiocypris rarus). Environ Toxicol 2014; 29:1 - 9; PMID: 21901812
- Morgado I, Hamers T, Van der Ven L, Power DM. Disruption of thyroid hormone binding to sea bream recombinant transthyretin by ioxinyl and polybrominated diphenyl ethers. Chemosphere 2007; 69:155 - 63; http://dx.doi.org/10.1016/j.chemosphere.2007.04.010; PMID: 17553549
- Yu L, Deng J, Shi X, Liu C, Yu K, Zhou B. Exposure to DE-71 alters thyroid hormone levels and gene transcription in the hypothalamic-pituitary-thyroid axis of zebrafish larvae. Aquat Toxicol 2010; 97:226 - 33; http://dx.doi.org/10.1016/j.aquatox.2009.10.022; PMID: 19945756
- Yu L, Lam JCW, Guo Y, Wu RSS, Lam PKS, Zhou B. Parental transfer of polybrominated diphenyl ethers (PBDEs) and thyroid endocrine disruption in zebrafish. Environ Sci Technol 2011; 45:10652 - 9; http://dx.doi.org/10.1021/es2026592; PMID: 22039834
- Bernal J. Thyroid hormones and brain development. In: Litwack G, ed. Vitamins and Hormones - Advances in Research and Applications, Vol 71, 2005:95-+.
- Gilbert ME, Lasley SM. Developmental thyroid hormone insufficiency and brain development: a role for brain-derived neurotrophic factor (BDNF)?. Neuroscience 2013; 239:253 - 70; http://dx.doi.org/10.1016/j.neuroscience.2012.11.022; PMID: 23201250
- Gilbert ME, Rovet J, Chen Z, Koibuchi N. Developmental thyroid hormone disruption: prevalence, environmental contaminants and neurodevelopmental consequences. Neurotoxicology 2012; 33:842 - 52; http://dx.doi.org/10.1016/j.neuro.2011.11.005; PMID: 22138353
- Sharlin DS, Gilbert ME, Taylor MA, Ferguson DC, Zoeller RT. The nature of the compensatory response to low thyroid hormone in the developing brain. J Neuroendocrinol 2010; 22:153 - 65; http://dx.doi.org/10.1111/j.1365-2826.2009.01947.x; PMID: 20041985
- Zhou T, Taylor MM, DeVito MJ, Crofton KM. Developmental exposure to brominated diphenyl ethers results in thyroid hormone disruption. Toxicol Sci 2002; 66:105 - 16; http://dx.doi.org/10.1093/toxsci/66.1.105; PMID: 11861977
- Zhou T, Ross DG, DeVito MJ, Crofton KM. Effects of short-term in vivo exposure to polybrominated diphenyl ethers on thyroid hormones and hepatic enzyme activities in weanling rats. Toxicol Sci 2001; 61:76 - 82; http://dx.doi.org/10.1093/toxsci/61.1.76; PMID: 11294977
- Hallgren S, Sinjari T, Håkansson H, Darnerud PO. Effects of polybrominated diphenyl ethers (PBDEs) and polychlorinated biphenyls (PCBs) on thyroid hormone and vitamin A levels in rats and mice. Arch Toxicol 2001; 75:200 - 8; http://dx.doi.org/10.1007/s002040000208; PMID: 11482517
- Marchesini GR, Meimaridou A, Haasnoot W, Meulenberg E, Albertus F, Mizuguchi M, Takeuchi M, Irth H, Murk AJ. Biosensor discovery of thyroxine transport disrupting chemicals. Toxicol Appl Pharmacol 2008; 232:150 - 60; http://dx.doi.org/10.1016/j.taap.2008.06.014; PMID: 18647617
- Hamers T, Kamstra JH, Sonneveld E, Murk AJ, Visser TJ, Van Velzen MJM, Brouwer A, Bergman A. Biotransformation of brominated flame retardants into potentially endocrine-disrupting metabolites, with special attention to 2,2′,4,4′-tetrabromodiphenyl ether (BDE-47). Mol Nutr Food Res 2008; 52:284 - 98; http://dx.doi.org/10.1002/mnfr.200700104; PMID: 18161906
- Ren XM, Guo LH. Assessment of the binding of hydroxylated polybrominated diphenyl ethers to thyroid hormone transport proteins using a site-specific fluorescence probe. Environ Sci Technol 2012; 46:4633 - 40; http://dx.doi.org/10.1021/es2046074; PMID: 22482873
- Muzzio AM, Noyes PD, Stapleton HM, Lema SC. Tissue distribution and thyroid hormone effects on mRNA abundance for membrane transporters Mct8, Mct10, and organic anion-transporting polypeptides (Oatps) in a teleost fish. Comp Biochem Physiol A Mol Integr Physiol 2014; 167:77 - 89; http://dx.doi.org/10.1016/j.cbpa.2013.09.019; PMID: 24113777
- Friesema ECH, Ganguly S, Abdalla A, Manning Fox JE, Halestrap AP, Visser TJ. Identification of monocarboxylate transporter 8 as a specific thyroid hormone transporter. J Biol Chem 2003; 278:40128 - 35; http://dx.doi.org/10.1074/jbc.M300909200; PMID: 12871948
- van der Deure WM, Hansen PS, Peeters RP, Kyvik KO, Friesema ECH, Hegedüs L, Visser TJ. Thyroid hormone transport and metabolism by organic anion transporter 1C1 and consequences of genetic variation. Endocrinology 2008; 149:5307 - 14; http://dx.doi.org/10.1210/en.2008-0430; PMID: 18566113
- Pacyniak E, Roth M, Hagenbuch B, Guo GL. Mechanism of polybrominated diphenyl ether uptake into the liver: PBDE congeners are substrates of human hepatic OATP transporters. Toxicol Sci 2010; 115:344 - 53; http://dx.doi.org/10.1093/toxsci/kfq059; PMID: 20176623
- Pacyniak E, Hagenbuch B, Klaassen CD, Lehman-McKeeman L, Guo GL. Organic anion transporting polypeptides in the hepatic uptake of PBDE congeners in mice. Toxicol Appl Pharmacol 2011; 257:23 - 31; http://dx.doi.org/10.1016/j.taap.2011.08.014; PMID: 21884716
- Essner JJ, Breuer JJ, Essner RD, Fahrenkrug SC, Hackett PB Jr.. The zebrafish thyroid hormone receptor alpha 1 is expressed during early embryogenesis and can function in transcriptional repression. Differentiation 1997; 62:107 - 17; http://dx.doi.org/10.1046/j.1432-0436.1997.6230107.x; PMID: 9447705
- Liu YW, Lo LJ, Chan WK. Temporal expression and T3 induction of thyroid hormone receptors alpha1 and beta1 during early embryonic and larval development in zebrafish, Danio rerio. Mol Cell Endocrinol 2000; 159:187 - 95; http://dx.doi.org/10.1016/S0303-7207(99)00193-8; PMID: 10687864
- Yamano K, Miwa S. Differential gene expression of thyroid hormone receptor alpha and beta in fish development. Gen Comp Endocrinol 1998; 109:75 - 85; http://dx.doi.org/10.1006/gcen.1997.7011; PMID: 9446725
- Nelson ER, Habibi HR. Molecular characterization and sex-related seasonal expression of thyroid receptor subtypes in goldfish. Mol Cell Endocrinol 2006; 253:83 - 95; http://dx.doi.org/10.1016/j.mce.2006.05.003; PMID: 16777315
- Filby AL, Tyler CR. Cloning and characterization of cDNAs for hormones and/or receptors of growth hormone, insulin-like growth factor-I, thyroid hormone, and corticosteroid and the gender-, tissue-, and developmental-specific expression of their mRNA transcripts in fathead minnow (Pimephales promelas). Gen Comp Endocrinol 2007; 150:151 - 63; http://dx.doi.org/10.1016/j.ygcen.2006.07.014; PMID: 16970945
- Lema SC, Dickey JT, Schultz IR, Swanson P. Thyroid hormone regulation of mRNAs encoding thyrotropin beta-subunit, glycoprotein alpha-subunit, and thyroid hormone receptors alpha and beta in brain, pituitary gland, liver, and gonads of an adult teleost, Pimephales promelas. J Endocrinol 2009; 202:43 - 54; http://dx.doi.org/10.1677/JOE-08-0472; PMID: 19380459
- Marchand O, Safi R, Escriva H, Van Rompaey E, Prunet P, Laudet V. Molecular cloning and characterization of thyroid hormone receptors in teleost fish. J Mol Endocrinol 2001; 26:51 - 65; http://dx.doi.org/10.1677/jme.0.0260051; PMID: 11174854
- Bertrand S, Thisse B, Tavares R, Sachs L, Chaumot A, Bardet PL, Escrivà H, Duffraisse M, Marchand O, Safi R, et al. Unexpected novel relational links uncovered by extensive developmental profiling of nuclear receptor expression. PLoS Genet 2007; 3:e188; http://dx.doi.org/10.1371/journal.pgen.0030188; PMID: 17997606
- Takayama S, Hostick U, Haendel M, Eisen J, Darimont B. An F-domain introduced by alternative splicing regulates activity of the zebrafish thyroid hormone receptor alpha. Gen Comp Endocrinol 2008; 155:176 - 89; http://dx.doi.org/10.1016/j.ygcen.2007.04.012; PMID: 17583703
- Darras VM, Van Herck SL, Heijlen M, De Groef B. Thyroid hormone receptors in two model species for vertebrate embryonic development: chicken and zebrafish. J Thyroid Res 2011; 2011:402320; http://dx.doi.org/10.4061/2011/402320; PMID: 21760979
- Ren X-M, Guo L-H. Molecular toxicology of polybrominated diphenyl ethers: nuclear hormone receptor mediated pathways. Environ Sci Process Impacts 2013; 15:702 - 8; http://dx.doi.org/10.1039/c3em00023k; PMID: 23467608
- Zoeller RT. Environmental chemicals as thyroid hormone analogues: new studies indicate that thyroid hormone receptors are targets of industrial chemicals?. Mol Cell Endocrinol 2005; 242:10 - 5; http://dx.doi.org/10.1016/j.mce.2005.07.006; PMID: 16150534
- Suvorov A, Bissonnette C, Takser L, Langlois MF. Does 2,2′,4,4′-tetrabromodiphenyl ether interact directly with thyroid receptor?. J Appl Toxicol 2011; 31:179 - 84; PMID: 20737425
- Hamers T, Kamstra JH, Sonneveld E, Murk AJ, Kester MHA, Andersson PL, Legler J, Brouwer A. In vitro profiling of the endocrine-disrupting potency of brominated flame retardants. Toxicol Sci 2006; 92:157 - 73; http://dx.doi.org/10.1093/toxsci/kfj187; PMID: 16601080
- Schriks M, Vrabie CM, Gutleb AC, Faassen EJ, Rietjens IM, Murk AJ. T-screen to quantify functional potentiating, antagonistic and thyroid hormone-like activities of poly halogenated aromatic hydrocarbons (PHAHs). Toxicol In Vitro 2006; 20:490 - 8; http://dx.doi.org/10.1016/j.tiv.2005.09.001; PMID: 16219445
- Kitamura S, Shinohara S, Iwase E, Sugihara K, Uramaru N, Shigematsu H, et al. Affinity for thyroid hormone and estrogen receptors of hydroxylated polybrominated diphenyl ethers. J Health Sci 2008; 54:607 - 14; http://dx.doi.org/10.1248/jhs.54.607
- Kojima H, Takeuchi S, Uramaru N, Sugihara K, Yoshida T, Kitamura S. Nuclear hormone receptor activity of polybrominated diphenyl ethers and their hydroxylated and methoxylated metabolites in transactivation assays using Chinese hamster ovary cells. Environ Health Perspect 2009; 117:1210 - 8; http://dx.doi.org/10.1289/ehp.0900753; PMID: 19672399
- Ibhazehiebo K, Iwasaki T, Kimura-Kuroda J, Miyazaki W, Shimokawa N, Koibuchi N. Disruption of thyroid hormone receptor-mediated transcription and thyroid hormone-induced Purkinje cell dendrite arborization by polybrominated diphenyl ethers. Environ Health Perspect 2011; 119:168 - 75; http://dx.doi.org/10.1289/ehp.1002065; PMID: 20870570
- Freitas J, Cano P, Craig-Veit C, Goodson ML, Furlow JD, Murk AJ. Detection of thyroid hormone receptor disruptors by a novel stable in vitro reporter gene assay. Toxicol In Vitro 2011; 25:257 - 66; http://dx.doi.org/10.1016/j.tiv.2010.08.013; PMID: 20732405
- Li F, Xie Q, Li X, Li N, Chi P, Chen J, Wang Z, Hao C. Hormone activity of hydroxylated polybrominated diphenyl ethers on human thyroid receptor-beta: in vitro and in silico investigations. Environ Health Perspect 2010; 118:602 - 6; http://dx.doi.org/10.1289/ehp.0901457; PMID: 20439171
- Ren XM, Guo LH, Gao Y, Zhang BT, Wan B. Hydroxylated polybrominated diphenyl ethers exhibit different activities on thyroid hormone receptors depending on their degree of bromination. Toxicol Appl Pharmacol 2013; 268:256 - 63; http://dx.doi.org/10.1016/j.taap.2013.01.026; PMID: 23402801
- Blanco J, Mulero M, López M, Domingo JL, Sánchez DJ. BDE-99 deregulates BDNF, Bcl-2 and the mRNA expression of thyroid receptor isoforms in rat cerebellar granular neurons. Toxicology 2011; 290:305 - 11; http://dx.doi.org/10.1016/j.tox.2011.10.010; PMID: 22024335
- Souza PCT, Puhl AC, Martínez L, Aparício R, Nascimento AS, Figueira ACM, Nguyen P, Webb P, Skaf MS, Polikarpov I. Identification of a new hormone-binding site on the surface of thyroid hormone receptor. Mol Endocrinol 2014; 28:534 - 45; http://dx.doi.org/10.1210/me.2013-1359; PMID: 24552590
- Chen L, Huang C, Hu C, Yu K, Yang L, Zhou B. Acute exposure to DE-71: effects on locomotor behavior and developmental neurotoxicity in zebrafish larvae. Environ Toxicol Chem 2012; 31:2338 - 44; http://dx.doi.org/10.1002/etc.1958; PMID: 22833361
- Chen L, Yu K, Huang C, Yu L, Zhu B, Lam PKS, Lam JC, Zhou B. Prenatal transfer of polybrominated diphenyl ethers (PBDEs) results in developmental neurotoxicity in zebrafish larvae. Environ Sci Technol 2012; 46:9727 - 34; http://dx.doi.org/10.1021/es302119g; PMID: 22866812
- Usenko CY, Robinson EM, Usenko S, Brooks BW, Bruce ED. PBDE developmental effects on embryonic zebrafish. Environ Toxicol Chem 2011; 30:1865 - 72; http://dx.doi.org/10.1002/etc.570; PMID: 21560146
- Chen X, Huang C, Wang X, Chen J, Bai C, Chen Y, Chen X, Dong Q, Yang D. BDE-47 disrupts axonal growth and motor behavior in developing zebrafish. Aquat Toxicol 2012; 120-121:35 - 44; http://dx.doi.org/10.1016/j.aquatox.2012.04.014; PMID: 22609740
- Chou CT, Hsiao YC, Ko FC, Cheng JO, Cheng YM, Chen TH. Chronic exposure of 2,2′,4,4′-tetrabromodiphenyl ether (PBDE-47) alters locomotion behavior in juvenile zebrafish (Danio rerio). Aquat Toxicol 2010; 98:388 - 95; http://dx.doi.org/10.1016/j.aquatox.2010.03.012; PMID: 20416957
- Lema SC, Schultz IR, Scholz NL, Incardona JP, Swanson P. Neural defects and cardiac arrhythmia in fish larvae following embryonic exposure to 2,2′,4,4′-tetrabromodiphenyl ether (PBDE 47). Aquat Toxicol 2007; 82:296 - 307; http://dx.doi.org/10.1016/j.aquatox.2007.03.002; PMID: 17412433
- Zhao J, Xu T, Yin DQ. Locomotor activity changes on zebrafish larvae with different 2,2′,4,4′-tetrabromodiphenyl ether (PBDE-47) embryonic exposure modes. Chemosphere 2014; 94:53 - 61; http://dx.doi.org/10.1016/j.chemosphere.2013.09.010; PMID: 24080000
- McClain V, Stapleton HM, Tilton F, Gallagher EP. BDE 49 and developmental toxicity in zebrafish. Comp Biochem Physiol C Toxicol Pharmacol 2012; 155:253 - 8; http://dx.doi.org/10.1016/j.cbpc.2011.09.004; PMID: 21951712
- He J, Yang D, Wang C, Liu W, Liao J, Xu T, Bai C, Chen J, Lin K, Huang C, et al. Chronic zebrafish low dose decabrominated diphenyl ether (BDE-209) exposure affected parental gonad development and locomotion in F1 offspring. Ecotoxicology 2011; 20:1813 - 22; http://dx.doi.org/10.1007/s10646-011-0720-3; PMID: 21695510
- Desouza LA, Sathanoori M, Kapoor R, Rajadhyaksha N, Gonzalez LE, Kottmann AH, Tole S, Vaidya VA. Thyroid hormone regulates the expression of the sonic hedgehog signaling pathway in the embryonic and adult Mammalian brain. Endocrinology 2011; 152:1989 - 2000; http://dx.doi.org/10.1210/en.2010-1396; PMID: 21363934
- Herbstman JB, Sjödin A, Kurzon M, Lederman SA, Jones RS, Rauh V, Needham LL, Tang D, Niedzwiecki M, Wang RY, et al. Prenatal exposure to PBDEs and neurodevelopment. Environ Health Perspect 2010; 118:712 - 9; http://dx.doi.org/10.1289/ehp.0901340; PMID: 20056561
- Branchi I, Alleva E, Costa LG. Effects of perinatal exposure to a polybrominated diphenyl ether (PBDE 99) on mouse neurobehavioural development. Neurotoxicology 2002; 23:375 - 84; http://dx.doi.org/10.1016/S0161-813X(02)00078-5; PMID: 12387364
- Eriksson P, Jakobsson E, Fredriksson A. Brominated flame retardants: a novel class of developmental neurotoxicants in our environment?. Environ Health Perspect 2001; 109:903 - 8; http://dx.doi.org/10.1289/ehp.01109903; PMID: 11673118
- Viberg H, Fredriksson A, Eriksson P. Neonatal exposure to the brominated flame retardant 2,2′,4,4′,5-pentabromodiphenyl ether causes altered susceptibility in the cholinergic transmitter system in the adult mouse. Toxicol Sci 2002; 67:104 - 7; http://dx.doi.org/10.1093/toxsci/67.1.104; PMID: 11961222
- Kuriyama SN, Wanner A, Fidalgo-Neto AA, Talsness CE, Koerner W, Chahoud I. Developmental exposure to low-dose PBDE-99: tissue distribution and thyroid hormone levels. Toxicology 2007; 242:80 - 90; http://dx.doi.org/10.1016/j.tox.2007.09.011; PMID: 17964054
- Rice DC, Reeve EA, Herlihy A, Zoeller RT, Thompson WD, Markowski VP. Developmental delays and locomotor activity in the C57BL6/J mouse following neonatal exposure to the fully-brominated PBDE, decabromodiphenyl ether. Neurotoxicol Teratol 2007; 29:511 - 20; http://dx.doi.org/10.1016/j.ntt.2007.03.061; PMID: 17482428
- Li T, Wang W, Pan YW, Xu L, Xia Z. A hydroxylated metabolite of flame-retardant PBDE-47 decreases the survival, proliferation, and neuronal differentiation of primary cultured adult neural stem cells and interferes with signaling of ERK5 MAP kinase and neurotrophin 3. Toxicol Sci 2013; 134:111 - 24; http://dx.doi.org/10.1093/toxsci/kft083; PMID: 23564643
- Dingemans MML, Heusinkveld HJ, Bergman A, van den Berg M, Westerink RHS. Bromination pattern of hydroxylated metabolites of BDE-47 affects their potency to release calcium from intracellular stores in PC12 cells. Environ Health Perspect 2010; 118:519 - 25; http://dx.doi.org/10.1289/ehp.0901339; PMID: 20368133
- Dufault C, Poles G, Driscoll LL. Brief postnatal PBDE exposure alters learning and the cholinergic modulation of attention in rats. Toxicol Sci 2005; 88:172 - 80; http://dx.doi.org/10.1093/toxsci/kfi285; PMID: 16107551
- Johansson N, Viberg H, Fredriksson A, Eriksson P. Neonatal exposure to deca-brominated diphenyl ether (PBDE 209) causes dose-response changes in spontaneous behaviour and cholinergic susceptibility in adult mice. Neurotoxicology 2008; 29:911 - 9; http://dx.doi.org/10.1016/j.neuro.2008.09.008; PMID: 18930763
- Xing T, Chen L, Tao Y, Wang M, Chen J, Ruan DY. Effects of decabrominated diphenyl ether (PBDE 209) exposure at different developmental periods on synaptic plasticity in the dentate gyrus of adult rats In vivo. Toxicol Sci 2009; 110:401 - 10; http://dx.doi.org/10.1093/toxsci/kfp114; PMID: 19535737
- Huang SC, Giordano G, Costa LG. Comparative cytotoxicity and intracellular accumulation of five polybrominated diphenyl ether congeners in mouse cerebellar granule neurons. Toxicol Sci 2010; 114:124 - 32; http://dx.doi.org/10.1093/toxsci/kfp296; PMID: 19969594
- Tagliaferri S, Caglieri A, Goldoni M, Pinelli S, Alinovi R, Poli D, Pellacani C, Giordano G, Mutti A, Costa LG. Low concentrations of the brominated flame retardants BDE-47 and BDE-99 induce synergistic oxidative stress-mediated neurotoxicity in human neuroblastoma cells. Toxicol In Vitro 2010; 24:116 - 22; http://dx.doi.org/10.1016/j.tiv.2009.08.020; PMID: 19720130
- Chen L, Hu C, Huang C, Wang Q, Wang X, Yang L, Zhou B. Alterations in retinoid status after long-term exposure to PBDEs in zebrafish (Danio rerio). Aquat Toxicol 2012; 120-121:11 - 8; http://dx.doi.org/10.1016/j.aquatox.2012.04.010; PMID: 22580571
- Nyholm JR, Norman A, Norrgren L, Haglund P, Andersson PL. Maternal transfer of brominated flame retardants in zebrafish (Danio rerio). Chemosphere 2008; 73:203 - 8; http://dx.doi.org/10.1016/j.chemosphere.2008.04.033; PMID: 18514256
- van de Merwe JP, Chan AKY, Lei ENY, Yau MS, Lam MHW, Wu RSS. Bioaccumulation and maternal transfer of PBDE 47 in the marine medaka (Oryzias melastigma) following dietary exposure. Aquat Toxicol 2011; 103:199 - 204; http://dx.doi.org/10.1016/j.aquatox.2011.02.021; PMID: 21481818
- Han XB, Lei ENY, Lam MHW, Wu RSS. A whole life cycle assessment on effects of waterborne PBDEs on gene expression profile along the brain-pituitary-gonad axis and in the liver of zebrafish. Mar Pollut Bull 2011; 63:160 - 5; http://dx.doi.org/10.1016/j.marpolbul.2011.04.001; PMID: 21549400
- Han XB, Yuen KWY, Wu RSS. Polybrominated diphenyl ethers affect the reproduction and development, and alter the sex ratio of zebrafish (Danio rerio). Environ Pollut 2013; 182:120 - 6; http://dx.doi.org/10.1016/j.envpol.2013.06.045; PMID: 23906559
- Schultz I, Brown KH, Nagler JJ. Effect of parental exposure to trenbolone and the brominated flame retardant BDE-47 on fertility in rainbow trout (Oncorhynchus mykiss). Mar Environ Res 2008; 66:47 - 9; http://dx.doi.org/10.1016/j.marenvres.2008.02.018; PMID: 18397801
- Søfteland L, Petersen K, Stavrum AK, Wu T, Olsvik PA. Hepatic in vitro toxicity assessment of PBDE congeners BDE47, BDE153 and BDE154 in Atlantic salmon (Salmo salar L.). Aquat Toxicol 2011; 105:246 - 63; http://dx.doi.org/10.1016/j.aquatox.2011.03.012; PMID: 21767471
- Cantón RF, Scholten DEA, Marsh G, de Jong PC, van den Berg M. Inhibition of human placental aromatase activity by hydroxylated polybrominated diphenyl ethers (OH-PBDEs). Toxicol Appl Pharmacol 2008; 227:68 - 75; http://dx.doi.org/10.1016/j.taap.2007.09.025; PMID: 18022659
- Cantón RF, Sanderson JT, Letcher RJ, Bergman A, van den Berg M. Inhibition and induction of aromatase (CYP19) activity by brominated flame retardants in H295R human adrenocortical carcinoma cells. Toxicol Sci 2005; 88:447 - 55; http://dx.doi.org/10.1093/toxsci/kfi325; PMID: 16177243
- Cantón RF, Sanderson JT, Nijmeijer S, Bergman A, Letcher RJ, van den Berg M. In vitro effects of brominated flame retardants and metabolites on CYP17 catalytic activity: a novel mechanism of action?. Toxicol Appl Pharmacol 2006; 216:274 - 81; http://dx.doi.org/10.1016/j.taap.2006.05.007; PMID: 16828825
- Li X, Gao Y, Guo LH, Jiang G. Structure-dependent activities of hydroxylated polybrominated diphenyl ethers on human estrogen receptor. Toxicology 2013; 309:15 - 22; http://dx.doi.org/10.1016/j.tox.2013.04.001; PMID: 23603053
- Mercado-Feliciano M, Bigsby RM. Hydroxylated metabolites of the polybrominated diphenyl ether mixture DE-71 are weak estrogen receptor-alpha ligands. Environ Health Perspect 2008; 116:1315 - 21; http://dx.doi.org/10.1289/ehp.11343; PMID: 18941571
- Kuriyama SN, Talsness CE, Grote K, Chahoud I. Developmental exposure to low dose PBDE 99: effects on male fertility and neurobehavior in rat offspring. Environ Health Perspect 2005; 113:149 - 54; http://dx.doi.org/10.1289/ehp.7421; PMID: 15687051
- Lilienthal H, Hack A, Roth-Härer A, Grande SW, Talsness CE. Effects of developmental exposure to 2,2, 4,4, 5-pentabromodiphenyl ether (PBDE-99) on sex steroids, sexual development, and sexually dimorphic behavior in rats. Environ Health Perspect 2006; 114:194 - 201; http://dx.doi.org/10.1289/ehp.8391; PMID: 16451854
- Stoker TE, Cooper RL, Lambright CS, Wilson VS, Furr J, Gray LE. In vivo and in vitro anti-androgenic effects of DE-71, a commercial polybrominated diphenyl ether (PBDE) mixture. Toxicol Appl Pharmacol 2005; 207:78 - 88; http://dx.doi.org/10.1016/j.taap.2005.05.010; PMID: 16005038
- Tseng LH, Lee CW, Pan MH, Tsai SS, Li MH, Chen JR, Lay JJ, Hsu PC. Postnatal exposure of the male mouse to 2,2′,3,3′,4,4′,5,5′,6,6′-decabrominated diphenyl ether: decreased epididymal sperm functions without alterations in DNA content and histology in testis. Toxicology 2006; 224:33 - 43; http://dx.doi.org/10.1016/j.tox.2006.04.003; PMID: 16713668
- van der Ven LTM, van de Kuil T, Verhoef A, Leonards PEG, Slob W, Cantón RF, Germer S, Hamers T, Visser TJ, Litens S, et al. A 28-day oral dose toxicity study enhanced to detect endocrine effects of a purified technical pentabromodiphenyl ether (pentaBDE) mixture in Wistar rats. Toxicology 2008; 245:109 - 22; http://dx.doi.org/10.1016/j.tox.2007.12.016; PMID: 18243468
- Meerts IA, Letcher RJ, Hoving S, Marsh G, Bergman A, Lemmen JG, van der Burg B, Brouwer A. In vitro estrogenicity of polybrominated diphenyl ethers, hydroxylated PDBEs, and polybrominated bisphenol A compounds. Environ Health Perspect 2001; 109:399 - 407; http://dx.doi.org/10.1289/ehp.01109399; PMID: 11335189
- Main KM, Kiviranta H, Virtanen HE, Sundqvist E, Tuomisto JT, Tuomisto J, Vartiainen T, Skakkebaek NE, Toppari J. Flame retardants in placenta and breast milk and cryptorchidism in newborn boys. Environ Health Perspect 2007; 115:1519 - 26; PMID: 17938745
- Chen A, Chung E, DeFranco EA, Pinney SM, Dietrich KN. Serum PBDEs and age at menarche in adolescent girls: analysis of the National Health and Nutrition Examination Survey 2003-2004. Environ Res 2011; 111:831 - 7; http://dx.doi.org/10.1016/j.envres.2011.05.016; PMID: 21663902
- Meijer L, Martijn A, Melessen J, Brouwer A, Weiss J, de Jong FH, Sauer PJ. Influence of prenatal organohalogen levels on infant male sexual development: sex hormone levels, testes volume and penile length. Hum Reprod 2012; 27:867 - 72; http://dx.doi.org/10.1093/humrep/der426; PMID: 22215630
- Akutsu K, Takatori S, Nozawa S, Yoshiike M, Nakazawa H, Hayakawa K, Makino T, Iwamoto T. Polybrominated diphenyl ethers in human serum and sperm quality. Bull Environ Contam Toxicol 2008; 80:345 - 50; http://dx.doi.org/10.1007/s00128-008-9370-4; PMID: 18320132
- Krassas GE, Pontikides N, Deligianni V, Miras K. A prospective controlled study of the impact of hyperthyroidism on reproductive function in males. J Clin Endocrinol Metab 2002; 87:3667 - 71; http://dx.doi.org/10.1210/jcem.87.8.8714; PMID: 12161493
- Cyr DG, Eales JG. Interrelationships between thyroidal and reproductive endocrine systems in fish. Rev Fish Biol Fish 1996; 6:165 - 200; http://dx.doi.org/10.1007/BF00182342
- Habibi HR, Nelson ER, Allan ERO. New insights into thyroid hormone function and modulation of reproduction in goldfish. Gen Comp Endocrinol 2012; 175:19 - 26; http://dx.doi.org/10.1016/j.ygcen.2011.11.003; PMID: 22100124
- Liu C, Zhang X, Deng J, Hecker M, Al-Khedhairy A, Giesy JP, Zhou B. Effects of prochloraz or propylthiouracil on the cross-talk between the HPG, HPA, and HPT axes in zebrafish. Environ Sci Technol 2011; 45:769 - 75; http://dx.doi.org/10.1021/es102659p; PMID: 21158436
- Morais RD, Nóbrega RH, Gómez-González NE, Schmidt R, Bogerd J, França LR, Schulz RW. Thyroid hormone stimulates the proliferation of Sertoli cells and single type A spermatogonia in adult zebrafish (Danio rerio) testis. Endocrinology 2013; 154:4365 - 76; http://dx.doi.org/10.1210/en.2013-1308; PMID: 24002037