Abstract
Proper activation of the Ras/MAPK pathway is broadly required during development, and in many cases, signal transduction downstream of the receptor is linear. Thus, different mechanisms exist to properly regulate the large number of specific developmental outputs that are required by the activation of this pathway. Previously, we have reported a regulated cytoplasmic sequestration of phosphorylated MAPK (pMAPK) in developing Drosophila compound eyes and wings “called MAPK Cytoplasmic Hold”. In the developing wing, we have shown that cytoplasmic hold promotes the differentiation of wing vein tissue, while pMAPK nuclear translocation regulates growth and division. We had also suggested that the Ras pathway signals for inducing cell growth and cell division split upstream of the nuclear translocation of MAPK itself. Here, we further refine the role of MAPK in Drosophila. We report evidence that suggests, for the first time, that the phosphorylation of MAPK is itself another step in the regulation of cell growth and division in both Drosophila wing and eye cells. We show that inhibition of MAPK phosphorylation, or pMAPK nuclear translocation, is sufficient to block cell growth, but not cell division. These data suggest that non-phosphorylated MAPK is sufficient to induce cell division, but not cell growth, once inside the nucleus of the cell.
Introduction
Activation of Mitogen Activated Protein Kinase (MAPK, also called Extracellular Signal Regulated Kinase, ERK) is the final cytoplasmic event in the Ras/MAPK signal transduction pathway. Signaling from this pathway begins at the plasma membrane with the activation of a transmembrane Receptor Tyrosine Kinase (RTK, such as the Epidermal Growth Factor Receptor, Egfr) in response to a variety of physiological stimuli. This activation leads to RTK dimerization and autophosphorylation, resulting in the activation of the small GTPase protein Ras.Citation1 Ras then transduces the signal through a series of phosphorylation events, ultimately leading to the dual phosphorylation and activation of MAPK (MAPK to di-phospho MAPK, or pMAPK) in the cytoplasm.Citation2-Citation4 Upon activation by phosphorylation, MAPK proteins dimerize,Citation5 and can then phosphorylate targets in the cytoplasm, and/or rapidly translocate to the nucleus where they phosphorylate and regulate nuclear target proteins and regulate gene expression.Citation6-Citation9
Proper activation and regulation of Ras/MAPK is required for a number of different developmental processes including cell division, growth, shape, survival, adhesion, migration, differentiation and death.Citation2,Citation4,Citation10,Citation11 Even though this one pathway is broadly required throughout development, in the majority of cases, transduction of the signal downstream of the RTK is linear.Citation12 Thus, a variety of different mechanisms exist in order to properly regulate the large number of specific developmental outputs that are required by the activation of this single pathway. Differences in signal strength and/or duration of the signal, activating or inhibitory ligands, and the subcellular compartmentalization of specific pathway components are all methods by which signal specificity is achieved in vivo.Citation3,Citation12,Citation13 Determining how each method of pathway regulation correlates to each downstream cellular effect is of critical importance to fully understand how this pathway properly functions throughout development in different tissues.
Previously, we have reported a regulated cytoplasmic -sequestration of pMAPK antigen in the developing Drosophila compound eye and wing “MAPK cytoplasmic hold”.Citation14-Citation16 We have further shown that this MAPK cytoplasmic hold is functionally regulated in the developing eye by the apical sequestration (and functional inactivation) of the MAPK import cofactor Moleskin (Msk), which is controlled by both the Decepentaplegic (Dpp) and Hedgehog (Hh) signal transduction pathways.Citation17 In the developing wing, we have shown that pMAPK cytoplasmic hold promotes the differentiation of wing vein tissue, while pMAPK nuclear translocation regulates growth and divisionCitation16 (). This study also suggested that the Ras pathway signals for inducing growth and -division split upstream of the nuclear translocation of the MAPK protein itself.Citation16 Thus, when we expressed a constitutively nuclear form of MAPK in wing tissue, the overall size of the wing did not change, while the number of cells within this wing increased, suggesting an increase in cell division without a subsequent increase in cell growth.Citation16 However, when we -specifically increased the nuclear accumulation of the phosphorylated form of MAPK (done by expressing the Msk protein, a nuclear import cofactor which specifically imports the phosphorylated form of MAPKCitation18) we found that both cell division and cell growth were increased.Citation16
Figure 6. Model of MAPK signaling. (A) Previous literature suggested that the subcellular localization of MAPK regulates the difference between growth and division and differentiation in developing fly wing and eye cells. (B) Modified model to incorporate additional regulation of growth and division by the phosphorylation status of the MAPK protein.
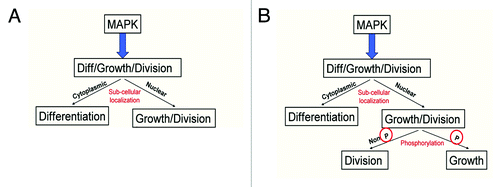
Here, we further refine the role of MAP Kinase in Drosophila wing and eye development. We report evidence that suggests, for the first time, that the phosphorylation state of the MAP Kinase protein itself is another step in the regulation of cell growth and division processes in both Drosophila wing and eye cells. We show that inhibition of MAPK phosphorylation in cells either through overexpression of the negative MAPK regulator Argos, or through mutation in the MAP Kinase Kinase MEK (encoded by the Drosophila dSor1 gene) is sufficient to block cell growth, but not cell division when we express a constitutively nuclear form of MAPK in these cells. These data suggest that non-phosphorylated MAPK is sufficient to induce cell division, but not cell growth, once inside the nucleus of the cell.
Results
Expression of Argos leads to increased cell density with decreased compartment size
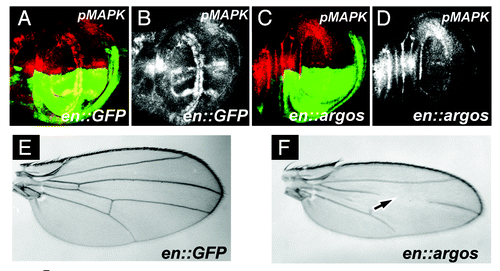
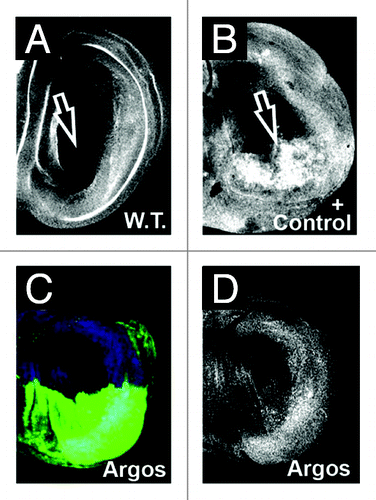
The Drosophila Argos protein is a secreted Egfr ligand that functions to repress pathway activation by sequestering the Egfr activating ligand sSpi.Citation30,Citation31 Expression of Argos in various tissues in Drosophila has been a well established, often used, and reproducible means to downregulate pathway activity, and leads to a decrease in the levels of phosphorylated MAPK (pMAPK) in vivo. To determine the effect on cell division and cell growth after downregulation of pMAPK expression, we initially utilized the Gal4/UAS systemCitation25 to overexpress Argos specifically in the posterior compartment of the developing Drosophila wing. pMAPK is normally expressed in both the developing wing veins, as well as in two columns of cells on both sides of the Dorsal/Ventral compartment boundary of the wing margin ().Citation32 Expression of Gal4 under the engrailed enhancer limits expression of target responder sequences to the posterior compartment of the developing wing disc only (green expression in ). Expression of Argos in the posterior compartment of wing cells significantly decreases the levels of pMAPK in vivo, compared to the anterior control compartment (). Note that this leads to loss of wing vein structures in the adult wing (), as previously described.Citation33
Figure 1A–F. Argos expression increases cell density while decreasing cell growth. (A-D) Third instar larval wing discs, anterior up, dorsal left, genotypes indicated bottom right, stain listed top right. (A) engrailed-Gal4; UAS:GFP (en::GFP) wing disc showing GFP expression (green) in the posterior compartment, and phosphorylated MAPK (pMAPK, red) throughout the wing pouch. (B) pMAPK staining (white) from (A). (C) engrailed-Gal4; UAS:GFP/UAS:Argos (en::argos) wing disc showing GFP expression (green) in the posterior compartment, and pMAPK (red) throughout the wing pouch. Note decreased pMAPK staining in the posterior compartment compared to the anterior compartment. (D) pMAPK staining (white) from (C). (E and F) Adult wings. (E) Normal wing venation pattern in engrailed-Gal4; UAS:GFP wing. (F) Phenotype of engrailed-Gal4; UAS:GFP/UAS:Argos adult wing. Note missing and decreased wing venation (arrow).
To determine relative cell number compared to cell size, we utilized a well established technique to quantify the number of wing hairs in a defined area of the wing (a measure of cell density, or cells per unit area), as each wing cell is known to secrete one hair.Citation34 We then compared this to the measured surface area of the compartments analyzed in the adult wing.Citation16 We again utilized the engrailed Gal4 (en::Gal4) reagent to restrict the expression of proteins to the posterior compartment of the developing wing disc, allowing the use of the anterior compartment to serve as an internal control among the various samples. In order to compare the effect of cell density and compartment size upon perturbation of Egfr/Ras/MAPK signaling in the developing wing we expressed both activating and inhibitory ligands (UAS:Argos and UAS:secreted Spitz [sSpi] respectively). These ligands will have their effect at the level of the Egfr receptor within these cells. To analyze the effect of increased pathway activation downstream of the receptor, we expressed the activated (phosphorylated) form of MAP Kinase (RlSEM) in the posterior compartment of these wings, as well as a constitutively nuclear form of MAPK (Rl.nls). As a control for the Gal4/UAS system employed in these assays, we expressed GFP within the posterior compartment.
Expression of GFP in the posterior compartment of wing discs shows no difference in the cell density between the posterior and anterior compartments, suggesting that the number of cells per unit area is largely the same between these regions in normal adult wings (). Because the number of cells per unit area is equivalent between these two compartments, the relative size of cells in the wing must be similar between the posterior and anterior compartments. However, the posterior compartment in normal wings is significantly larger than the anterior compartment ().
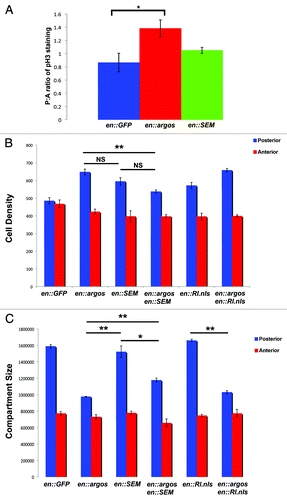
Figure 1G and H. Argos expression increases cell density while decreasing cell growth. (G) Cell Density in the posterior and anterior compartments of adult wings that express different proteins in their posterior compartment. Average cell density of the same area in the posterior and anterior compartments of each genotype is shown with Standard Error. All genotypes show engrailed:Gal4 expressing different transgenes, as indicated. (H) Surface area (expressed in pixel number) in the posterior and anterior compartments of adult wings that express different proteins in their posterior compartment. Average pixel number (representative of wing surface area) of the entire posterior and anterior compartments of each genotype is shown with Standard Error. All genotypes show engrailed:Gal4 expressing different transgenes, as indicated. In both graphs * indicates p < 0.05, while ** indicates p < 0.01.
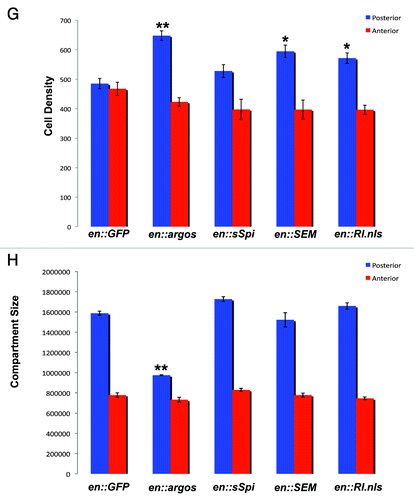
Expression of Argos in the posterior compartment of wings leads to a statistically significant increase in cell density (), with a concomitant decrease in overall compartment size (). Because cell density is increased in these wings, these data suggest that the posterior compartment of wings that overexpress Argos have more cells per unit area than wild-type wings. This suggests that the cells in the posterior compartment are smaller in wings that express Argos compared to cells in the posterior compartment of wild-type wings.
Expression of the activating ligand sSpi in the posterior -compartment of these wings has been well established to lead to an increase in the pool of endogenous phosphorylated MAPK (pMPAK) protein, which in turn leads to extra vein formation,Citation16,Citation35 something that we also see when we overexpress sSpi in the posterior compartment of wings (). We have shown that the phosphorylated MAPK protein induced upon -activation of Egfr signaling in this fashion remains cytoplasmic when Rhomboid (the protease that activates Spitz) is overexpressed in the wing.Citation16 We therefore analyzed the subcellular localization of phosphorylated MAPK in the posterior compartment of wings that overexpress sSpi, and find that the pMAPK in this compartment is also predominantly cytoplasmic (). We found that expression of sSpi in this fashion did not significantly alter either cell density or posterior compartment size compared to controls (). These data suggest that though increased cytoplasmic pMAPK can induce alterations in cell differentiation by increasing vein formation, it does not affect either cell density or compartment size in this tissue, presumably because this pMAPK remains cytoplasmic.
Figure 2. Increased pMAPK expression by sSpi expression is predominantly cytoplasmic. All panels show late third instar wing discs, anterior up, dorsal left, genotypes indicated bottom right, stain listed top right. (A) Wild-type wing discs shows GFP expression (green) in posterior compartment driven by the engrailed:Gal4 driver (en::GFP). Normal pMAPK expression (red) is indicated. (B) Expression of pMAPK (white) in engrailed-Gal4; UAS:RlSEM wing discs. Note increased expression of pMAPK in normal expression domains in posterior compartment. (C) Expression of pMAPK (white) in engrailed-Gal4; UAS:Rl.nls wing discs. Note no significant alteration of pMAPK expression compared to wild-type. (D) Expression of pMAPK (white) in engrailed-Gal4; UAS:sSpi wing discs. Note increased and expanded expression of pMAPK in posterior compartment. (E) Expression of pMAPK (red) and nuclear GFP (green) from red box in (D). Note that pMAPK is predominantly cytoplasmic. Scale bar indicates 5 μm. (F) pMAPK expression (white) from (E).
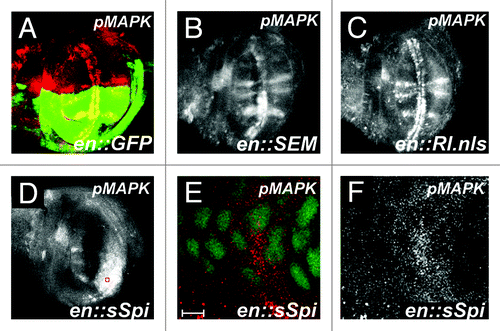
We next analyzed the effect of increasing MAPK -downstream of Egfr within developing wing cells. Expression of exogenous pMAPK protein (RlSEM) in the posterior compartment of wings leads to a significant increase in cell density (). However, it does not significantly alter posterior compartment size (). Similarly, expression of a constitutively nuclear form of MAPK (MAPK.nls) also leads to an increase in cell density compared to controls (), with no significant effect on posterior compartment size (). To determine the effect of this overexpression on MAPK phosphorylation in the wing, we analyzed the expression of pMAPK in both of these genetic backgrounds. In contrast to overexpression of sSpi in this tissue, neither RlSEM or Rl.nls expression significantly altered pMAPK expression within intervein cells (). Expression of pMAPK in wing discs overexpressing RlSEM appeared more intense in the posterior compartment compared to anterior -controls (). However, expression of Rl.nls did not significantly alter the expression pattern in either compartment. Taken together, these data suggest that increasing the total amount of MAPK downstream of the EGF receptor, either phosphorylated or nuclear, can increase total cell density without affecting -compartment size.
Argos dependent increases in cell density are due to increased cell division.
Increased cell density could be due to a decrease in cellular apoptosis, an increase in cell division, or both. To determine the nature of the increased cell density we observe in posterior compartments of wings that express Argos, we first analyzed levels of apoptosis in the posterior compartment of these discs by examining the expression of the activated version of Caspase 3, which marks cells undergoing apoptosis. We did not observe a decrease in apoptosis in posterior compartments, compared to anterior controls, in this tissue (), suggesting that the increase in cell density we observe is not due to a general decrease in apoptosis.
Figure 3. Argos expression does not decrease apoptosis in developing wings. All panels show wild-type third instar wing discs stained for activated Caspase 3. Anterior up, dorsal left. (A) Wild-type wing disc. (B) Positive control showing increased Caspase 3 activation (arrow) in the posterior compartment. Genotype is engrailed-Gal4/ UAS:DIM-7 (Drosophila Importin 7 homolog). (C) Caspase 3 staining (blue) in engrailed-Gal4; UAS:GFP/UAS:Argos discs. GFP is colored green. (D) Caspase 3 staining (white) from (C). Note there is no decrease in Caspase 3 activation upon Argos expression in the posterior compartment of wing discs.
To verify if there was an increase in cell division in the posterior compartment of wings that express Argos, we stained these third instar wing discs with an antibody against the -phosphorylated version of Histone H3 (pH3), which specifically marks the cells that are undergoing mitosis. We expressed Argos within the posterior compartment of wing discs with the engrailed Gal4 driver, while simultaneously expressing GFP within this compartment, and counted the number of pH3 positive nuclei within the -posterior compartment (GFP) compared to the anterior compartment (No GFP). In control wing discs expressing only GFP, the ratio of pH3 nuclei in the posterior compartment compared to the anterior compartment was 0.865 ± 0.141 (). However, in wing discs expressing Argos in their posterior compartment, we observed a significant increase in the ratio of pH3 nuclei in the posterior compartment compared to the anterior compartment, 1.383 ± 0.127, (, p = 0.029). To determine whether an increase in the phosphorylated form MAPK could also increase this ratio, we analyzed this posterior to anterior ratio in wings expressing RlSEM. While the posterior to anterior ratio of these wings were slightly larger, 1.05 ± 0.045, we observed no significant increase in the ratio of pH3 nuclei in the compartments of these wings compared to controls (, p = 0.657). Taken together, these data suggest that the increased cell density observed in the posterior compartment of adult wings that express Argos is due to an increase in cell division, and not a decrease in apoptosis within these compartments. Further, because an increase in the amount of phosphorylated MAPK does not significantly increase the number of mitoses observed in these wings when compared to controls, these results are consistent with a role for increased unphosphorylated MAPK in promoting the observed increase in cell division in wings that overexpress Argos.
Figure 4. Phosphorylated MAPK, but not nuclear MAPK rescues Argos phenotype. (A) Shows ratio of posterior to anterior compartment of phosphorylated Histone H3 (pH3) staining in engrailed-Gal4; UAS:GFP third instar wing discs as compared to engrailed-Gal4; UAS:GFP/UAS:Argos and engrailed-Gal4; UAS:GFP/UAS:RlSEM wing discs. Note increased pH3 staining in engrailed-Gal4; UAS:GFP/UAS:Argos, indicative of increased mitotic events in the posterior compartment of these discs. (B) Cell Density in the posterior and anterior compartments of adult wings that express different proteins in their posterior compartment. Average cell density of the same area in the posterior and anterior compartments of each genotype is shown with Standard Error. Genotypes are indicated. (C) Surface area (expressed in pixel number) in the posterior and anterior compartments of adult wings that express different proteins in their posterior compartment. Average pixel number (representative of wing surface area) of the entire posterior and anterior compartments of each genotype is shown with Standard Error. Genotypes are indicated. In both graphs * indicates p < 0.05, while ** indicates p < 0.01.
Argos dependent increases in cell density are due to increased nuclear, unphosphorylated MAPK
MAPK functions downstream of Egfr. Because we are suppressing the MAPK pathway upstream at the level of receptor activation, we wanted to -determine the effect of expressing different downstream MAPKs on the Argos phenotypes. To accomplish this, we expressed both phosphorylated MAPK (RlSEM), and nuclear MAPK (Rl.nls) while simultaneously expressing Argos in the posterior -compartment of wing discs. Expression of phosphorylated MAPK (RlSEM) within an Argos background showed no significant difference in cell density compared to flies expressing only RlSEM alone (). These flies did, however, show a significant decrease in cell density compared to flies that only express Argos within their posterior compartments (). These data, while not significant, do suggest a trend towards a smaller cell density (larger cells) in flies that express both Argos and RlSEM. In contrast to these results, increasing the amount of nuclear MAPK in wings that simultaneously express Argos did not show a significant increase or decrease in cell density (), suggesting that the cell density phenotype observed in Argos wings may be saturated, and unable to be enhanced. Taken together, these data suggest that the trend towards decreased cell density in wings that overexpress Argos is specific for expression of the -phosphorylated form of MAPK.
We next analyzed posterior compartment size in these flies. Expression of phosphorylated MAPK (RlSEM) showed a significant increase in the posterior compartment size of wings expressing both Argos and RlSEM (). However, expression of nuclear MAPK (Rl.nls) did not significantly increase posterior compartment size when co-expressed with Argos (). Taken together, these data suggest that by specifically increasing the phosphorylated form of MAPK within the posterior compartment of these wings (as opposed to nuclear MAPK), we are partially rescuing the defective cell growth and division phenotypes normally observed when we express Argos.
The results illustrated in the fly wing suggest that a decrease in the phosphorylated form of MAPK (due to increased expression of the inhibitory ligand Argos) leads to an increase in cell density with a concomitant decrease in cell and organ size. We suggest the data presented in the fly wing is consistent with the hypothesis that it is an increase in the nuclear non-phosphorylated form of MAPK that leads to the observed increase in cell density and decrease in posterior compartment size in these wings. These results are consistent with previous results, suggesting that the forced nuclear translocation of MAPK could also lead to an increase in cell -division with a concomitant decrease in cell growth.Citation16
Nuclear, unphosphorylated MAPK can induce cell division in eye tissue
To test whether increased nuclear MAPK could induce cell division in tissues outside the Drosophila wing, we turned to the developing Drosophila retina. We used the eyeless-Flip reagent to create clones of cells mutant for the Drosophila MAP Kinase Kinase (MEK, encoded by the dSor1 gene in flies)Citation36,Citation37 in the developing Drosophila retina. Clones of dSor1 mutants have been previously shown to have severe defects in the rate of cell proliferation, showing only one or two doubling events compared to their wild-type twin clones.Citation38 When we used the null mutant dSor1LH110 to create clones in the developing retina, we also observed very small clones (data not shown). However, to generate clones of sufficient size in which to analyze cell proliferation, we utilized the loss-of-function mutation dSor1S-1221,Citation23 which generated clones large enough for analysis of individual markers of cell growth and division within these clones (). Loss of dSor1 function within these mutant clones is expected to generate a loss of pMAPK staining, with a concomitant decrease in photoreceptor differentiation, cell growth and cell division. In order to determine decreased cell growth, we analyzed the expression of the Capicua protein, which has been shown to restrict cell growth, and whose expression is decreased in response to Ras/MAPK signaling.Citation39 In clones of both Egfr and Ras in the developing retina, Capicua protein levels are increased, indicating a decrease in cell growth in these cells.Citation39 Similarly, Capicua levels are increased in clones of dSor1S-1221, confirming a decrease in cell growth within these clones (). Photoreceptor differentiation is decreased in these clones (), and the phosphorylated form of MAPK (pMAPK) is absent within these clones as well (). Cell division, as measured by counting the number of mitotic events stained with pH3 within clones was also decreased (, clones within the second mitotic wave in the developing eye), and averaged roughly 0.03 mitoses per clone ().
Figure 5. Nuclear, unphosphorylated MAPK increases cell division in Drosophila retinas. (A–H) Show third instar retinas, anterior up (A–D), anterior right (E–H). All retinas show clones of dSor1S-1221 marked by the absence of GFP (green). (A) Shows Capicua staining (blue) is increased in dSor1S-1221 clones. (B) Shows Capicua (white) from (A). (C) Shows ELAV (red, a marker of photoreceptor differentiation) is decreased in dSor1S-1221 clones. (D) Shows ELAV (white) from (C). (E) Shows phosphorylated MAPK (pMAPK, red) is absent in dSor1S-1221 clones. (F) Shows pMAPK (white) from (D). (G) Shows phosphorylated Histone H3 (pH3, blue) is absent from dSor1S-1221 clones in the second mitotic wave. (H) Shows pH3 (white) from (G). (I) Shows the average number of pH3 positive nuclei in dSor1S-1221 clones alone (first column), in dSor1S-1221 clones that also express full length MAPK within the clone (hs-Rl, second column), and in dSor1S-1221 clones that also express full length MAPK with a strong nuclear localization signal attached to it (hs-Rl.nls, third column). Error bars represent Standard Error. ** Indicates p < 0.01.
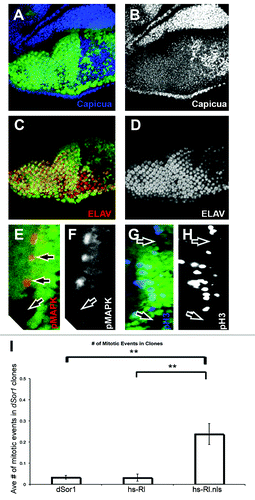
In order to determine if increased nuclear MAPK could -activate cell division even in the absence (or severe diminishment) of its phosphorylation by MEK, we generated dSor1S-1221 mutant clones in the developing retina, and expressed both the normal form of the MAPK protein, as well as a constitutively nuclear form of the MAPK protein within these clones (hs-MAPK and hs-MAPK.nls respectivelyCitation15,Citation16). We found that though the markers of cell growth, differentiation and phosphorylated MAPK were unchanged in these clones, clones in which we expressed the nuclear form of MAPK showed a significant increase in the number of mitoses (pH3 nuclei) as compared to clones in which we expressed normal MAPK protein, or clones in which we did not express either (). When taken together with both our previous results,Citation16 and the results described above in the fly wing, these results suggest that the phosphorylation state of the MAPK protein is dispensable for cell division in developing Drosophila retina and wing tissue, but is required for cell growth and -differentiation in these tissues.
Discussion
Understanding how the same canonical pathway is capable of eliciting multiple distinct cellular processes in both a tissue specific and temporally distinct manner remains an important problem in biology. The results we present in this study reveal a novel method of regulating the Ras/MAPK pathway, and suggest that the non-phosphorylated form of MAPK is sufficient to induce cell division, but not cell growth, once inside the nucleus of the cell ().
What might be the mechanism for nuclear import of -non-phosphorylated MAPK? Monomers of MAPK have been previously shown to passively diffuse into the nucleus in Xenopus.Citation40 Further, literature points out that the non-phosphorylated form of MAPK can enter the nucleus in an energy-independent fashion by directly interacting with nuclear pore complex proteins.Citation41,Citation42 Though phosphorylation of MAPK was inhibited successfully in our model system in both the eye and the wing, if the normal synthesis and degradation of MAPK is unperturbed in these cells, this might cause an abnormally high concentration of non-phosphorylated MAPK in the cytoplasm. This accumulation may, in turn, cause an increase in the concentration gradient of non-phosphorylated MAPK across the nucleus, facilitating the passive nuclear diffusion of -non-phosphorylated MAPK in these cells.
Perhaps the most interesting results of our present study are that once the non-phosphorylated MAPK does move into the nucleus, it appears to have a critical cellular function in promoting cell division there. How could this non-phosphorylated MAPK cause the activation of cell division in Drosophila -tissues? Results in both flies and mammals suggest that both cell division and cell growth are regulated by MAPK activation and nuclear localization.Citation6,Citation16,Citation43,Citation44 Our results show that cell growth was impaired in both wings and eyes on inhibition of MAPK phosphorylation, consistent with the idea that MAPK phosphorylation is necessary to activate cell growth-related processes in developing wings and eyes of Drosophila. However, our results also suggest that phosphorylation of MAPK is not required to activate cell division. Once in the nucleus, MAPK is normally thought to phosphorylate transcription factors that subsequently promote cell division and growth. Non-phosphorylated MAPK could not facilitate transcription factor activation through phosphorylation however. Thus, this form of MAPK must be able to activate cell division by another mechanism. Recent studies have shown that ERK2 can directly bind to DNA and function as a transcriptional repressor for interferon gamma induced genes.Citation45 Thus, it may be that Drosophila MAPK, once inside the nucleus, is able to directly bind to DNA and regulate the expression of gene(s) critical for cell division.
Targeted inactivation of the Egf Receptor is an effective and promising therapy for many cancers,Citation46 but their efficacy in some solid tumors, and in brain tumor glioblastoma multiforme (the most prevalent tumor in the central nervous system of human adults) has been low.Citation46,Citation47 Previous research has shown that co-activation of multiple different RTKs within some of these tumors is responsible for some of the inefficacy of single RTK inhibitor treatment in these tumors.Citation47 Our results shown here suggest an additional level of regulation, whereby simply inactivating the Ras/MAPK pathway is not sufficient to limit MAPK‑mediated cell division.
Materials and Methods
Drosophila stocks and genetics
Unless otherwise noted, all crosses were carried out at 25°C on standard cornmeal-molasses-agar medium. BL# refers to Bloomington Stock Center stock number. Stocks used are described: en:Gal4; UAS:GFP,Citation19 UAS:Argos,Citation20 UAS:sSpi,Citation21 UAS:Rl-SEM;Citation22 dSor1S-1221,Citation23 -eyeless-FLP (ey:FLP),Citation24 UAS:Rl.nls (MAPK overexpression under -control of the Gal4 system), hs-Rl (MAPK overexpression under heat shock control) and hs-Rl.nls (nuclear MAPK expression under heat shock control).Citation15,Citation16
The Gal4/UAS system was used for the overexpression of Argos, sSpi and Rl-SEM as described.Citation25 Mosaic clones were generated using ey:FLP,Citation24 as described.Citation26 Flip stock y-, w-, Ubi-GFP, P{neoFRT}19A; ey:FLP (on 2nd Chromosome) were crossed to the following stocks as appropriate to generate mutant clones:
(1) dSor1S-1221, P{neoFRT}19A/FM7C
(2) dSor1S-1221, P{neoFRT}19A/FM7C; hs-Rl/hs-Rl
(3) dSor1S-1221, P{neoFRT}19A/FM7C; hs-Rl.nls/hs-Rl.nls.
In order to express both MAPK and nuclear MAPK within dSor1 clones, late third instar larvae were heat shocked at 37°C for 1 hour, were then allowed to recover for 1 hour, and eye imaginal discs were then dissected and immediately fixed and stained for mitotic markers. A minimum of 10 larval retinas were examined for each genotype, and a minimum of 50 different clones were examined total for each genotype.
Immunohistochemistry
Wing and eye imaginal discs were dissected, fixed and prepared as described earlier.Citation27 The wing discs were mounted in vectashield (Vector Labs, H-1000). Immunohistochemistry imaging was done using a Nikon TE-2000 laser scanning confocal microscope. The primary antibodies used were mouse pMAPK (Sigma #M8159, 1:200 for wing imaginal discs and 1:250 for eye imaginal discs), rabbit anti-phosophorylated histone-3 (pH3, Cell Signaling Technologies # 9701, 1:1,000), Rabbit anti-activated caspase3 (BD Biosciences #551150, 1:200), Rat anti-ELAV (Developmental Studies Hybridoma Bank #7E8A10, 1:1,000), and guinea pig anti-capicua (a gift from Iswar Hariharan, University of California, Berkeley, 1:300, as describedCitation28).
Secondary antibodies used were goat anti-mouse TRITC (for wing imaginal discs, # 115-116-072, 1:150), goat anti-rabbit TRITC (for eye imaginal discs, # 111-116-144, 1:250), goat -anti-rabbit Cy5 (for wing imaginal discs, #111-176-144, 1:1,000), goat anti-rat Cy5 (for eye imaginal discs, # 712-176-150, 1:200), goat anti-mouse Cy5 (eye imaginal discs, # 115-176-072, 1:500). All above mentioned secondary antibodies were from Jackson ImmunoResearch. HRP antibodies used were Goat anti-rabbit HRP (General Bioscience #  GR2201, 1:100), and goat anti-mouse HRP (General Bioscience #  GM5201, 1:40).
Adult wing analysis
Adult wings were dissected and dehydration was done in 70% ethanol for one minute. Mounting was done using DPX mountant (Fluka corporation, Sigma-Aldrich # 44581). Cell count assays were performed using phase contrast microscopy, and wing surface area was measured using pixel counts through ImageJ software, as previously described.Citation29 For each genotype a minimum of five wings were independently analyzed. For surface area compartment measurements, posterior compartments were defined as the area below wing vein L3, while anterior was the area above for all genotypes. For cell density, wing hair counts were analyzed in an area distal to posterior cross-vein and below vein L5, while anterior measurements were above wing vein L2 in all genotypes.
Statistical analysis
All statistical analyses were performed on SPSS version 17. To determine significance between multiple different genotypes, a one-way ANOVA analysis was performed with Tukey posthoc analysis. Genotype is the independent variable while actual wing measurements or hair counts were the dependent variable. To determine significance between two different genotypes, a two-tailed Student’s t-test was performed between the two groups. Significance was determined at the 95% confidence interval.
Acknowledgements
We would like to thank Iswar Hariharan for the Capicua antibody, the Bloomington Stock Center, and the Iowa Hybridoma Bank for reagents. We would additionally like to thank members of the Marenda lab, and two anonymous reviewers for helpful suggestions. This work was supported by a grant from the NIH, R15EY018431-01A1 (to Daniel R. Marenda).
References
- Mitin N, Rossman KL, Der CJ. Signaling interplay in Ras superfamily function. Curr Biol 2005; 15:R563 - 74; http://dx.doi.org/10.1016/j.cub.2005.07.010; PMID: 16051167
- Schaeffer HJ, Weber MJ. Mitogen-activated protein kinases: specific messages from ubiquitous messengers. Mol Cell Biol 1999; 19:2435 - 44; PMID: 10082509
- Simon MA. Receptor tyrosine kinases: specific outcomes from general signals. Cell 2000; 103:13 - 5; http://dx.doi.org/10.1016/S0092-8674(00)00100-8; PMID: 11051543
- Shilo BZ. Signaling by the Drosophila epidermal growth factor receptor pathway during development. Exp Cell Res 2003; 284:140 - 9; http://dx.doi.org/10.1016/S0014-4827(02)00094-0; PMID: 12648473
- Cobb MH, Goldsmith EJ. Dimerization in MAP-kinase signaling. Trends Biochem Sci 2000; 25:7 - 9; http://dx.doi.org/10.1016/S0968-0004(99)01508-X; PMID: 10637602
- Brunet A, Roux D, Lenormand P, Dowd S, Keyse S, Pouysségur J. Nuclear translocation of p42/p44 mitogen-activated protein kinase is required for growth factor-induced gene expression and cell cycle entry. EMBO J 1999; 18:664 - 74; http://dx.doi.org/10.1093/emboj/18.3.664; PMID: 9927426
- Chen R-H, Sarnecki C, Blenis J. Nuclear localization and regulation of erk- and rsk-encoded protein kinases. Mol Cell Biol 1992; 12:915 - 27; PMID: 1545823
- Khokhlatchev AV, Canagarajah B, Wilsbacher J, Robinson M, Atkinson M, Goldsmith E, et al. Phosphorylation of the MAP kinase ERK2 promotes its homodimerization and nuclear translocation. Cell 1998; 93:605 - 15; http://dx.doi.org/10.1016/S0092-8674(00)81189-7; PMID: 9604935
- Lenormand P, Sardet C, Pagès G, L’Allemain G, Brunet A, Pouysségur J. Growth factors induce nuclear translocation of MAP kinases (p42mapk and p44mapk) but not of their activator MAP kinase kinase (p45mapkk) in fibroblasts. J Cell Biol 1993; 122:1079 - 88; http://dx.doi.org/10.1083/jcb.122.5.1079; PMID: 8394845
- Bonni A, Brunet A, West AE, Datta SR, Takasu MA, Greenberg ME. Cell survival promoted by the Ras-MAPK signaling pathway by transcription-dependent and -independent mechanisms. Science 1999; 286:1358 - 62; http://dx.doi.org/10.1126/science.286.5443.1358; PMID: 10558990
- Huang C, Jacobson K, Schaller MD. MAP kinases and cell migration. J Cell Sci 2004; 117:4619 - 28; http://dx.doi.org/10.1242/jcs.01481; PMID: 15371522
- Shilo BZ. Regulating the dynamics of EGF receptor signaling in space and time. Development 2005; 132:4017 - 27; http://dx.doi.org/10.1242/dev.02006; PMID: 16123311
- Ebisuya M, Kondoh K, Nishida E. The duration, magnitude and compartmentalization of ERK MAP kinase activity: mechanisms for providing signaling specificity. J Cell Sci 2005; 118:2997 - 3002; http://dx.doi.org/10.1242/jcs.02505; PMID: 16014377
- Kumar JP, Tio M, Hsiung F, Akopyan S, Gabay L, Seger R, et al. Dissecting the roles of the Drosophila EGF receptor in eye development and MAP kinase activation. Development 1998; 125:3875 - 85; PMID: 9729495
- Kumar JP, Hsiung F, Powers MA, Moses K. Nuclear translocation of activated MAP kinase is developmentally regulated in the developing Drosophila eye. Development 2003; 130:3703 - 14; http://dx.doi.org/10.1242/dev.00556; PMID: 12835387
- Marenda DR, Vrailas AD, Rodrigues AB, Cook S, Powers MA, Lorenzen JA, et al. MAP kinase subcellular localization controls both pattern and proliferation in the developing Drosophila wing. Development 2006; 133:43 - 51; http://dx.doi.org/10.1242/dev.02168; PMID: 16308331
- Vrailas AD, Marenda DR, Cook SE, Powers MA, Lorenzen JA, Perkins LA, et al. smoothened and thickveins regulate Moleskin/Importin 7-mediated MAP kinase signaling in the developing Drosophila eye. Development 2006; 133:1485 - 94; http://dx.doi.org/10.1242/dev.02334; PMID: 16540506
- Lorenzen JA, Baker SE, Denhez F, Melnick MB, Brower DL, Perkins LA. Nuclear import of activated D-ERK by DIM-7, an importin family member encoded by the gene moleskin.. Development 2001; 128:1403 - 14; PMID: 11262240
- Morata G, Ripoll P. Minutes: mutants of drosophila autonomously affecting cell division rate. Dev Biol 1975; 42:211 - 21; http://dx.doi.org/10.1016/0012-1606(75)90330-9; PMID: 1116643
- Freeman M. Misexpression of the Drosophila argos gene, a secreted regulator of cell determination. Development 1994; 120:2297 - 304; PMID: 7925030
- Freeman M. Reiterative use of the EGF receptor triggers differentiation of all cell types in the Drosophila eye. Cell 1996; 87:651 - 60; http://dx.doi.org/10.1016/S0092-8674(00)81385-9; PMID: 8929534
- Martín-Blanco E. Regulatory control of signal transduction during morphogenesis in Drosophila. Int J Dev Biol 1998; 42:363 - 8; PMID: 9654020
- Karim FD, Chang HC, Therrien M, Wassarman DA, Laverty T, Rubin GM. A screen for genes that function downstream of Ras1 during Drosophila eye development. Genetics 1996; 143:315 - 29; PMID: 8722784
- Newsome TP, Asling B, Dickson BJ. Analysis of Drosophila photoreceptor axon guidance in eye-specific mosaics. Development 2000; 127:851 - 60; PMID: 10648243
- Brand AH, Perrimon N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development 1993; 118:401 - 15; PMID: 8223268
- Xu T, Rubin GM. Analysis of genetic mosaics in developing and adult Drosophila tissues. Development 1993; 117:1223 - 37; PMID: 8404527
- Tio M, Moses K. The Drosophila TGF alpha homolog Spitz acts in photoreceptor recruitment in the developing retina. Development 1997; 124:343 - 51; PMID: 9053310
- Tseng AS, Tapon N, Kanda H, Cigizoglu S, Edelmann L, Pellock B, et al. Capicua regulates cell proliferation downstream of the receptor tyrosine kinase/ras signaling pathway. Curr Biol 2007; 17:728 - 33; http://dx.doi.org/10.1016/j.cub.2007.03.023; PMID: 17398096
- Marenda DR, Zraly CB, Feng Y, Egan S, Dingwall AK. The Drosophila SNR1 (SNF5/INI1) subunit directs essential developmental functions of the Brahma chromatin remodeling complex. Mol Cell Biol 2003; 23:289 - 305; http://dx.doi.org/10.1128/MCB.23.1.289-305.2003; PMID: 12482982
- Klein DE, Nappi VM, Reeves GT, Shvartsman SY, Lemmon MA. Argos inhibits epidermal growth factor receptor signalling by ligand sequestration. Nature 2004; 430:1040 - 4; http://dx.doi.org/10.1038/nature02840; PMID: 15329724
- Klein DE, Stayrook SE, Shi F, Narayan K, Lemmon MA. Structural basis for EGFR ligand sequestration by Argos. Nature 2008; 453:1271 - 5; http://dx.doi.org/10.1038/nature06978; PMID: 18500331
- Gabay L, Seger R, Shilo B-Z. In situ activation pattern of Drosophila EGF receptor pathway during development. Science 1997; 277:1103 - 6; http://dx.doi.org/10.1126/science.277.5329.1103; PMID: 9262480
- Sawamoto K, Okano H, Kobayakawa Y, Hayashi S, Mikoshiba K, Tanimura T. The function of argos in regulating cell fate decisions during Drosophila eye and wing vein development. Dev Biol 1994; 164:267 - 76; http://dx.doi.org/10.1006/dbio.1994.1197; PMID: 8026629
- Meyer CA, Jacobs HW, Datar SA, Du W, Edgar BA, Lehner CF. Drosophila Cdk4 is required for normal growth and is dispensable for cell cycle progression. EMBO J 2000; 19:4533 - 42; http://dx.doi.org/10.1093/emboj/19.17.4533; PMID: 10970847
- Sturtevant MA, Roark M, Bier E. The Drosophila rhomboid gene mediates the localized formation of wing veins and interacts genetically with components of the EGF-R signaling pathway. Genes Dev 1993; 7:961 - 73; http://dx.doi.org/10.1101/gad.7.6.961; PMID: 8504935
- Hsu JC, Perrimon N. A temperature-sensitive MEK mutation demonstrates the conservation of the signaling pathways activated by receptor tyrosine kinases. Genes Dev 1994; 8:2176 - 87; http://dx.doi.org/10.1101/gad.8.18.2176; PMID: 7958887
- Tsuda L, Inoue YH, Yoo M-A, Mizuno M, Hata M, Lim Y-M, et al. A protein kinase similar to MAP kinase activator acts downstream of the raf kinase in Drosophila. Cell 1993; 72:407 - 14; http://dx.doi.org/10.1016/0092-8674(93)90117-9; PMID: 8381718
- Lim YM, Nishizawa K, Nishi Y, Tsuda L, Inoue YH, Nishida Y. Genetic analysis of rolled, which encodes a Drosophila mitogen-activated protein kinase. Genetics 1999; 153:763 - 71; PMID: 10511556
- Tseng AS, Tapon N, Kanda H, Cigizoglu S, Edelmann L, Pellock B, et al. Capicua regulates cell proliferation downstream of the receptor tyrosine kinase/ras signaling pathway. Curr Biol 2007; 17:728 - 33; http://dx.doi.org/10.1016/j.cub.2007.03.023; PMID: 17398096
- Adachi M, Fukuda M, Nishida E. Two co-existing mechanisms for nuclear import of MAP kinase: passive diffusion of a monomer and active transport of a dimer. EMBO J 1999; 18:5347 - 58; http://dx.doi.org/10.1093/emboj/18.19.5347; PMID: 10508167
- Yazicioglu MN, Goad DL, Ranganathan A, Whitehurst AW, Goldsmith EJ, Cobb MH. Mutations in ERK2 binding sites affect nuclear entry. J Biol Chem 2007; 282:28759 - 67; http://dx.doi.org/10.1074/jbc.M703460200; PMID: 17656361
- Matsubayashi Y, Fukuda M, Nishida E. Evidence for existence of a nuclear pore complex-mediated, cytosol-independent pathway of nuclear translocation of ERK MAP kinase in permeabilized cells. J Biol Chem 2001; 276:41755 - 60; http://dx.doi.org/10.1074/jbc.M106012200; PMID: 11546808
- Neufeld TP, de la Cruz AF, Johnston LA, Edgar BA. Coordination of growth and cell division in the Drosophila wing. Cell 1998; 93:1183 - 93; http://dx.doi.org/10.1016/S0092-8674(00)81462-2; PMID: 9657151
- Prober DA, Edgar BA. Ras1 promotes cellular growth in the Drosophila wing. Cell 2000; 100:435 - 46; http://dx.doi.org/10.1016/S0092-8674(00)80679-0; PMID: 10693760
- Hu S, Xie Z, Onishi A, Yu X, Jiang L, Lin J, et al. Profiling the human protein-DNA interactome reveals ERK2 as a transcriptional repressor of interferon signaling. Cell 2009; 139:610 - 22; http://dx.doi.org/10.1016/j.cell.2009.08.037; PMID: 19879846
- Bianco R, Gelardi T, Damiano V, Ciardiello F, Tortora G. Rational bases for the development of EGFR inhibitors for cancer treatment. Int J Biochem Cell Biol 2007; 39:1416 - 31; http://dx.doi.org/10.1016/j.biocel.2007.05.008; PMID: 17596994
- Stommel JM, Kimmelman AC, Ying H, Nabioullin R, Ponugoti AH, Wiedemeyer R, et al. Coactivation of receptor tyrosine kinases affects the response of tumor cells to targeted therapies. Science 2007; 318:287 - 90; http://dx.doi.org/10.1126/science.1142946; PMID: 17872411