Abstract
Commensal bacteria that colonize mammalian mucosal surfaces are reported to influence T helper type 2 (TH2) cytokine-dependent inflammation and susceptibility to allergic disease. However, the mechanisms that underlie these observations are only beginning to be understood. We recently utilized studies of murine model systems and atopic patient populations to elucidate a mechanism by which commensal bacteria-derived signals limit serum immunoglobulin E levels, influence basophil development and steady-state circulating basophil populations and regulate basophil-associated TH2 cell responses and allergic inflammation. In this addendum, we summarize the findings of our recent work and other developments in the field, discuss the broader implications of these findings and generate new hypotheses regarding our understanding of host-commensal relationships. These areas of investigation may be applicable to the development of new preventative or therapeutic approaches to reduce the burden of allergic disease.
Introduction
Allergic diseases have reached pandemic levelsCitation1 and represent a significant source of morbidity, mortality and healthcare cost.Citation2 These chronic inflammatory diseases are characterized by interleukin (IL)-4, IL-5, IL-9 and IL-13 production by CD4+ T helper type 2 (TH2) cells, immunoglobulin E (IgE) production by B cells and the recruitment of effector cells to sites of tissue inflammation.Citation3,Citation4 It is thought that susceptibility to TH2 cytokine-dependent allergic inflammation is influenced by both polymorphisms in mammalian genesCitation5 as well as environmental factors including host diet and exposure to pollutants or infectious agents.Citation6-Citation8 However, the specific genetic and environmental stimuli that influence allergy susceptibility, and how these factors contribute to the development of allergic disease, are ongoing fields of research.
The human intestine is colonized by 100 trillion microorganisms belonging to each of the three domains of life.Citation9,Citation10 Of these, bacteria are the most abundant; the colon is home to a population of trillions of commensal bacteriaCitation11 that is estimated to be composed of thousands of different species.Citation12 Epidemiologic studies have identified associations between alterations in the composition of commensal bacterial communities and the development of allergic disease. For example, infants who develop allergies display altered intestinal bacterial populations early in life,Citation13 and children who have undergone treatment with broad-spectrum antibiotics are at an increased risk of developing allergic disease.Citation14,Citation15 Together, these and other observations provoke the hypothesis that commensal bacteria influence the development or severity of allergic disease. However, the molecular and cellular mechanisms by which commensal bacteria-derived signals regulate allergic inflammation are not well understood.Citation16,Citation17
Studies of animal model systems have identified correlations between alterations in commensal bacteria-derived signals and exaggerated allergic inflammatory responses.Citation18-Citation20 For example, work by Noverr et al. indicated that disruption of normal murine commensal bacterial populations via oral antibiotic treatment, followed by subsequent oral inoculation of the gastrointestinal tract with Candida albicans, results in exaggerated inflammatory responses in an IL-13-dependent model of allergic airway inflammation.Citation21,Citation22 Similarly, work by Bashir et al. indicated that oral antibiotic treatment potentiates the development of IgE-mediated allergy to food antigens in mice, a phenomenon that is mediated in part by TLR-dependent signaling.Citation23 More recent studies have focused on identifying the specific effects of antibiotic treatment on commensal bacterial populationsCitation24,Citation25 and have correlated allergic propensity with alterations in specific innate or adaptive immune cell populations.Citation18,Citation26 For example, work by Olszak et al. indicated that germ-free mice display exaggerated lung inflammation in a model of asthma that is concurrent with increased pulmonary expression of the chemokine ligand CXCL16 and accumulation of invariant natural killer T cells in lung tissue.Citation27 Despite these advances, the specific cellular and molecular mechanisms responsible for recognition of commensal bacteria-derived signals and the initiation and propagation of subsequent Th2 cytokine-dependent inflammatory responses are poorly understood.
Granulocytes, including mast cells, eosinophils and basophils can be activated by an array of stimuli including antibodies, cytokines and proteases,Citation28,Citation29 have been shown to express MHC class II,Citation30-Citation32 to be potent sources of IL-4 and, depending on the model, contribute to the development of optimal TH2 cytokine responses.Citation33-Citation35 Given the proposed role for commensal bacteria in influencing allergy susceptibility, and the identification of granulocytes as innate cells that promote TH2 cytokine-mediated inflammation, our recent publication examined whether commensal bacteria-derived signals influence granulocyte homeostasis or allergic inflammation.Citation77 We found that depletion or the absence of commensal bacteria, achieved via treatment with broad-spectrum oral antibiotics or utilization of a germ-free mouse model, is associated with elevations in serum IgE levels, increased frequencies and numbers of circulating basophil populations and exaggerated TH2 cytokine-dependent inflammation in a model of allergic airway inflammation. Exaggerated TH2 cell responses were reduced upon depletion of basophils, implicating this cell type in contributing to the exaggerated allergic inflammation observed in commensal-depleted hosts. Furthermore, IgE was found to be a critical regulator of steady-state basophil responses in antibiotic-treated or germ-free mice, and B cell-intrinsic expression of MyD88 was found to limit serum IgE levels and circulating basophil populations in vivo. Commensal-derived signals influenced circulating basophil populations by regulating the proliferative capacity of bone marrow-resident basophil progenitor populations in an IL-3 receptor (IL-3R)-dependent manner, providing one mechanism by which commensal bacteria limit basophil populations in the steady-state. Finally, subjects with hyperimmunoglobulinemia E syndrome were found to have elevated frequencies of circulating basophils compared with control subjects, an observation that is consistent with IgE influencing circulating basophil responses in human patients. Here, we discuss these and other relevant findings that suggest commensal bacteria-derived signals act via IgE and basophils to influence the development of TH2 cytokine-dependent inflammation and allergic disease.
The Commensal Bacteria-IgE-Basophil Axis of Allergic Inflammation
Commensal-deficient mice display elevated serum IgE levels compared with commensal-sufficient mice.Citation36 Distinct from allergen-specific IgE that mediates anaphylactic reactions, IgE found in the serum of germ-free mice is composed of germ-line encoded proteins with natural specificities, implicating this molecule in functions other than antigen detection.Citation37 Consistent with this hypothesis, IgE has recently been shown to influence multiple aspects of granulocyte homeostasis in an antigen-independent manner including surface protein expression, cell survival and cell adhesion.Citation38-Citation40 Building upon these findings, we hypothesized that antibiotic-induced elevations in serum IgE levels might be associated with quantitative or qualitative alterations in systemic granulocyte populations. Consistent with this hypothesis, increases in steady-state serum IgE levels that occurred upon antibiotic treatment of mice correlated with elevated blood basophil populations. A similar correlation was observed in germ-free miceCitation41 indicating that the elevations in circulating basophils were the result of a reduction in commensal-derived signals. To determine whether the correlation between serum IgE levels and blood basophil populations represented a causal relationship, an IgE-deficient mouse strain (Igh-7–/–) was utilized. Antibiotic treatment of IgE-deficient mice did not influence blood basophil populations, indicating that IgE contributes to the elevations in circulating basophils observed in antibiotic-treated mice. Additionally, injection of IgE-deficient mice with monoclonal IgE resulted in antigen-independent elevations in blood basophils. Together, these findings identified commensal bacteria and IgE as regulators of circulating basophil populations in mice.
In addition to their roles as late phase effector cells that infiltrate inflamed tissues, basophils are also recruited to draining lymph nodes early in the response to some infectiousCitation42 or allergicCitation29,Citation43 stimuli. While the development of TH2 cell responses can occur independently of basophils in some models of allergy or helminth infection,Citation28,Citation44-Citation46 basophils can promote optimal TH2 cell responses in others.Citation33-Citation35,Citation42,Citation43,Citation47,Citation48-Citation50 In some circumstances, basophils have been reported to cooperate with dendritic cells (DCs) to promote optimal TH2 cell responses.Citation42,Citation43,Citation49 Based on these findings, we hypothesized that elevated circulating basophil populations in antibiotic-treated mice might contribute to the well-established phenotype of exaggerated allergic inflammation in this model system.Citation22,Citation23 To test this hypothesis, we utilized two models in which basophils are thought to contribute to the development of optimal TH2 cytokine-mediated allergic inflammation; the papain protease model of TH2 cell induction and the house dust mite model of allergic airway inflammation.Citation28,Citation29 Compared with conventionally-reared mice, antibiotic-treated mice displayed exaggerated TH2 cell development and allergic airway inflammation using these models. Depletion of basophils, using a monoclonal antibody to the FcεRIα receptor,Citation29 resulted in reduced TH2 cell responses upon allergen exposure. However, mast cells and some dendritic cells can express FcεRIα so caution must be exercised in interpreting findings using this approach. As an alternative strategy, we employed a diphtheria toxin-dependent basophil depletion mouse system.Citation51 Efficient and selective depletion of basophils using this system also resulted in reduced TH2 cell responses. Together, these results indicate that commensal bacteria-derived signals limit basophil-mediated TH2 cell responses and allergic inflammation.
The steady-state changes in basophil populations observed in antibiotic-treated or germ-free mice were primarily quantitative. Other than an increase in surface bound IgE, significant alterations in surface marker phenotype were not observed in our experimental systems when comparing basophils from commensal-sufficient or commensal-deficient hosts. However, these findings do not exclude a role for commensal-derived signals in regulating qualitative measures of basophil function such as inflammatory cytokine production. One mechanism by which commensal-derived signals could influence qualitative measures of basophil function is by altering expression of thymic stromal lymphopoietin (TSLP). TSLP, a cytokine derived from epithelial cells as well as other immune cell populations, has been found to be regulated by IgE and is elevated in the intestines of germ-free mice.Citation52,Citation53 Compared with the basophil growth factor IL-3, TSLP can elicit a functionally distinct population of basophils from bone marrow progenitor populations.Citation51 While TSLP was not critical for the quantitative expansion of steady-state circulating basophil populations in our experimental systems, commensal bacteria-derived signals may influence TSLP-TSLP receptor interactions and basophil populations in ways not yet appreciated. As such, further analysis of the effects of selective manipulation of commensal bacteria on TSLP expression and TSLP-dependent basophil responses is warranted.
As discussed above, basophils cooperate with DCs to promote optimal TH2 cell responses in some cases.Citation42,Citation43,Citation49 Non-basophil antigen presenting cell populations were not significantly influenced by commensal bacteria-derived signals in our experimental systems. However, while basophils were shown to contribute to the development of exaggerated allergen-induced TH2 cell responses using two methods of basophil depletion, TH2 cell frequencies were slightly higher in antibiotic-treated mice depleted of basophils compared with conventionally-reared mice depleted of basophils. This observation suggests that other immune cell types or molecules contribute to the exaggerated TH2 cell responses observed in antibiotic-treated or germ-free mice. Further examination of DCs, regulatory T cells and other immune cell populations known to influence TH2 cytokine-mediated inflammatory responses should therefore be undertaken. When undertaking such studies, it is important to note that DCs, basophils, eosinophils and mast cells can share expression of certain cell surface markers such as FcεR1 and CD123.Citation54-Citation58 As such, gating strategies based on multiple cell surface markers, utilization of cell sorting and subsequent cytological and transcription analyses as well as cell-specific depletion methods must be utilized to ensure that resulting phenotypes are accurately attributed to the cell population in question.
Finally, while we did not observe significant differences between the germ-free or antibiotic-treated models in our study, these model systems are not identical.Citation10 One of the best characterized differences between these two models is the significantly reduced lymphoid compartment of germ-free compared with antibiotic-treated mice.Citation59-Citation61 Due to such limitations, it is essential that observations made in germ-free mice be confirmed in an antibiotic treatment system. However, there are also considerable benefits to germ-free systems such as the ability to perform monocolonization studies with defined microbial species. Gnotobiotic studies, in which the intestine is selectively colonized with a single bacterial species or a cocktail of known bacteria, have led to the identification of specific commensal populations with distinct immunomodulatory functions. For example, Clostridium species have been shown to induce intestinal regulatory T cell populations and promote resistance to colitisCitation62 while segmented filamentous bacteria have been shown to induce TH17 cell populations in the small intestine and mediate protective responses to the intestinal pathogen Citrobacter rodentium.Citation63 Because of their various strengths and weaknesses, complementary use of both the germ-free and the antibiotic-treated model systems will be essential to furthering our understanding of mammalian host-commensal mutualistic relationships.
CpG and MyD88 as Regulators of Circulating Basophil Populations
To further elucidate the molecular and cellular mechanisms by which commensal bacteria-derived signals influence serum IgE levels and circulating basophil populations, we next sought to identify specific commensal bacteria-derived signals and immune cell types that were mediators of the commensal bacteria-IgE-basophil axis. Unmethylated CpG motifs in bacterial DNA are recognized by Toll-like receptor 9 and inhibit IgE class switching by B cells in vitro in a MyD88-dependent manner.Citation64 Additionally, administration of CpG has been shown to reduce the severity of food allergy in a TLR4-deficient mouse model.Citation23 Consistent with CpG regulating IgE levels and circulating basophils in vivo, treatment of germ-free mice with CpG motifs resulted in reductions in both serum IgE levels and blood basophil populations. We next sought to expand upon these observations by examining the role of MyD88-dependent signaling in B cells on serum IgE levels and circulating basophils in vivo. To do so, we adoptively transferred MyD88-sufficient or deficient B cells into B cell-deficient mice. We found that mice reconstituted with MyD88-deficient B cells displayed elevated serum IgE levels and blood basophil populations compared to controls. Together, these findings indicate that that B cell-intrinsic MyD88-dependent signaling limits serum IgE levels and circulating basophil populations in vivo and that CpG is sufficient to limit serum IgE levels and circulating basophil populations in the absence of other commensal bacteria-derived signals.
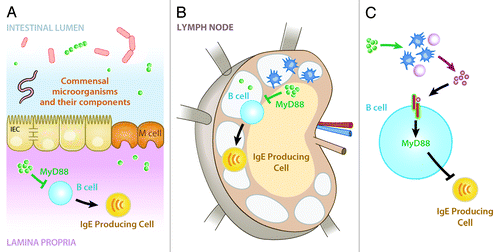
While extending the molecular and cellular understanding of how commensal bacteria influence serum IgE levels in the steady-state, the observation that B cell-intrinsic MyD88-dependent signaling limits serum IgE levels and circulating basophils in vivo raises further questions. The simplest explanation for our observation is direct detection of commensal bacteria-derived signals by B cells resulting in reduced IgE class switching and production. However, this raises the question as to where in the body B cells interact with commensal-derived signals. One possibility is that B cells circulate through intestinal-associated compartments such as the lamina propria where they are exposed to commensal bacteria-derived signals (). Alternatively, B cells may encounter commensal bacteria-derived signals in mesenteric lymph nodesCitation65 or commensal bacteria-derived signals may be present systemically at high enough levels to impart effects on systemic B cell populations (). Finally, commensal-derived signals may act on one or more intermediary cell populations to influence production of a signaling molecule that is detected by B cells in a MyD88-dependent manner (). Elucidating these mechanisms may lead to the development of specific therapeutics designed to manipulate production of IgE by B cells.
Figure 1. Potential mechanisms by which commensal-derived signals may act in a B cell-intrinsic, MyD88-dependent manner to influence IgE production. (A) Commensal-derived signals (green spheres) may act in a MyD88-dependent manner on B cells in the lamina propria or other intestinal-associated tissues to limit production of IgE. (B) Alternatively, commensal bacteria or their signals may be transported to mesenteric lymph nodes where they could act in a MyD88-dependent manner on B cells to limit production of IgE. (C) Finally, commensal-derived signals may act via one or more intermediate cell types or signaling molecules to influence B cell production of IgE in a MyD88-dependent manner. Figure adapted from reference Citation10.
Regulation of Basophil Development by Commensal Bacteria and IgE
We next sought to determine the mechanism by which commensal-derived signals and IgE regulate circulating basophil populations. Initially, based on previous studies of other granulocyte populations, we hypothesized that commensal bacteria-derived signals would act via IgE to influence basophil survival.Citation38-Citation40 However, our initial studies suggested that basophil survival is not significantly dysregulated in antibiotic-treated or germ-free mice. These findings provoked the hypothesis that commensal bacteria-derived signals may influence basophil development. Consistent with this hypothesis, we found that bone marrow cells from antibiotic-treated or germ-free mice exhibited increased potential for basophil development compared with an equal number of bone marrow cells from conventionally-reared mice. These findings indicate that bone marrow from antibiotic-treated or germ-free mice has an increased hematopoietic potential for the development of mature basophils.
To further examine the mechanisms by which commensal bacteria influence basophil development, we first examined bone marrow-resident basophil precursor (BaP) populations. We found that BaPs from antibiotic-treated or germ-free mice displayed elevated surface expression of IL-3R compared with BaPs from conventionally-reared mice. To determine whether the increased IL-3R expression on BaPs from antibiotic-treated mice conferred increased cell responsiveness to IL-3, we sort-purified BaPs from conventionally-reared or antibiotic-treated mice and cultured them in the presence of IL-3. Cultures of BaPs from antibiotic-treated mice displayed increased basophil development in the presence of IL-3 compared with cultures of BaPs from conventionally-reared mice, indicating that BaPs from antibiotic-treated mice are more responsive to IL-3 stimulation. Finally, to determine whether IgE was required for commensal bacterial effects on BaP populations, we examined the influence of IgE depletion on both bone marrow BaP IL-3R expression and total bone marrow BaP populations. Consistent with IgE mediating the alterations to BaP populations observed in germ-free mice, BaP expression of IL-3R, as well as total BaP frequencies, were reduced in germ-free mice upon depletion of IgE. Together, these results indicate that commensal bacteria-derived signals limit basophil development from the bone marrow by reducing circulating IgE and expression of IL-3R on BaPs.
The finding that commensal bacteria-derived signals influence development of an immune cell progenitor population in the bone marrow has broad implications for understanding both normal mammalian physiology and the pathophysiology of multiple inflammatory diseases. By influencing bone marrow hematopoietic programs, relatively small alterations in commensal populations could dramatically influence the propensity of their host to develop protective or pathogenic TH2 cell responses to helminth parasites, environmental allergens or other stimuli that provoke this module of the immune response. Recently, several innate progenitor cell populations have been identified that can give rise to multiple mature immune cell populations depending on immunologic stimuli to which they are exposed.Citation66,Citation67 Commensal bacteria-derived signals may influence these or other hematopoietic progenitor populations to tip the immunologic balance toward or away from the development of adaptive immune responses. Finally, given that alterations in commensal bacteria have been shown to influence TH17 and Treg responses,Citation62,Citation63,Citation68,Citation69 commensal regulation of hematopoietic programs may have implications beyond TH2 cell-mediated immunity and allergic inflammation to other inflammatory and autoimmune disease states.
Relevance to Atopic Patient Populations
Given the considerable public health and economic burden of allergic diseases, the need for the development of new preventative and therapeutic strategies has never been greater.Citation1,Citation2 As such, we sought to correlate our findings in animal models with observations in human subjects with atopic disease. Subjects with loss-of-function polymorphisms in the dedicator of cytokinesis 8 gene exhibit an autosomal recessive form of hyperimmunoglobulin IgE syndromeCitation70,Citation71 which is characterized by recurrent infections, increased susceptibility to atopic eczema and average serum IgE levels 10 times higher than those found in control subjects.Citation72 Consistent with our observations in mice, we found that subjects with hyperimmunoglobulin E syndrome display elevations in circulating basophil populations compared with controls suggesting that IgE-mediated regulation of basophil homeostasis may be relevant to the pathogenesis of this human disease. Additionally, Herbst et al. recently reported a role for isotype-switched antibodies in promoting basophil expansion and effector function following animal models of helminth infection.Citation73 Further investigation is need to determine whether IgE-mediated regulation of basophil homeostasis contributes to the inflammation observed in patients with hyperimmunoglobulin E syndrome or other conditions in which serum IgE levels are elevated such as atopy or infection.
Omalizumab is a monoclonal, humanized mouse anti-human IgE-specific antibody and FDA approved anti-allergy drug.Citation74,Citation75 We hypothesized that omalizumab treatment could be a therapeutic option to influence antigen-independent, IgE-mediated regulation of systemic basophil populations. To test whether omalizumab treatment influenced circulating basophils, conventionally-reared or germ-free mice were treated with omalizumab or control antibody and serum IgE levels and blood basophils were examined. Omalizumab treatment of germ-free mice resulted in reductions in serum IgE levels and circulating basophil populations suggesting a potential role for this drug in therapeutically manipulating IgE-dependent basophil responses. Analysis of the effects of omalizumab on human basophil populations has also been undertaken. Depletion of IgE in human subjects results in downregulation of basophil surface FcεR1α receptor expression and impaired FcεR1α-mediated basophil activation indicating that omalizumab therapy influences some qualitative aspects of basophil physiology.Citation76 Despite these advances, further investigation is necessary to determine whether omalizumab treatment influences basophil progenitor populations, circulating basophil numbers, TH2 cell responses or basophil recruitment to sites of tissue inflammation in human subjects. An appropriately conducted clinical study would be required to test these hypotheses in humans. With further investigation, omalizumab therapy may be found to be an important intervention to influence antigen-independent effects of IgE on granulocyte populations.
Conclusion
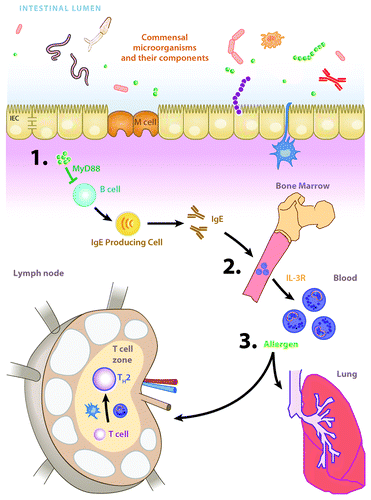
Here we discuss our recent report of a cellular and molecular mechanism by which commensal bacteria-derived signals influence basophil development and basophil-associated allergic inflammation (). We also propose some hypotheses to be tested in future studies. Further investigation of the commensal bacteria-IgE-basophil axis may allow for the design of new strategies to prevent or treat allergic disease in atopic patients.
Figure 2. The commensal bacteria-IgE-basophil axis of allergic inflammation. (A) Commensal bacteria-derived signals (green spheres) act via B cell-intrinsic, MyD88-dependent signaling pathways to limit circulating levels of IgE. (B) IgE acts on bone marrow-resident basophil precursor populations to increase surface expression of the IL-3 receptor and development of mature circulating basophils. © Upon allergen exposure, basophils are recruited to draining lymph nodes where they potentiate the development of TH2 cell responses and TH2 cytokine-dependent allergic inflammation. Figure adapted from reference Citation10.
| Abbreviations: | ||
| IL | = | Interleukin |
| TH2 | = | T Helper Type 2 |
| IgE | = | Immunoglobulin E |
| IL-3R | = | IL-3 receptor |
| DC | = | Dendritic cell |
| BaP | = | Basophil precursor |
| TSLP | = | Thymic stromal lymphopoietin |
Acknowledgments
We thank members of the Artis laboratory for discussions and critical reading of the manuscript. Research in the Artis lab is supported by the National Institutes of Health (AI061570, AI087990, AI074878, AI083480, AI095466, AI095608 and AI097333 to D.A.) and the Burroughs Wellcome Fund Investigator in Pathogenesis of Infectious Disease Award (D.A.). We also thank the Matthew J. Ryan Veterinary Hospital Pathology Lab, the Penn Microarray Facility and the Mucosal Immunology Studies Team of the NIAID for expertise and resources. The authors would also like to thank the Abramson Cancer Center Flow Cytometry and Cell Sorting Resource Laboratory for technical advice and support. The ACC Flow Cytometry and Cell Sorting Shared Resource is partially supported by NCI Comprehensive Cancer Center Support Grant (#2-P30 CA016520). This work was supported by the NIH/NIDDK P30 Center for Molecular Studies in Digestive and Liver Diseases (P30-DK050306), its pilot grant program and scientific core facilities (Molecular Pathology and Imaging, Molecular Biology, Cell Culture and Mouse), as well as the Joint CHOP-Penn Center in Digestive, Liver and Pancreatic Medicine and its pilot grant program.
References
- Eder W, Ege MJ, von Mutius E. The asthma epidemic. N Engl J Med 2006; 355:2226 - 35; http://dx.doi.org/10.1056/NEJMra054308; PMID: 17124020
- Bahadori K, Doyle-Waters MM, Marra C, Lynd L, Alasaly K, Swiston J, et al. Economic burden of asthma: a systematic review. BMC Pulm Med 2009; 9:24; http://dx.doi.org/10.1186/1471-2466-9-24; PMID: 19454036
- Mowen KA, Glimcher LH. Signaling pathways in Th2 development. Immunol Rev 2004; 202:203 - 22; http://dx.doi.org/10.1111/j.0105-2896.2004.00209.x; PMID: 15546395
- Holgate ST. Pathogenesis of asthma. Clin Exp Allergy 2008; 38:872 - 97; http://dx.doi.org/10.1111/j.1365-2222.2008.02971.x; PMID: 18498538
- Vercelli D. Discovering susceptibility genes for asthma and allergy. Nat Rev Immunol 2008; 8:169 - 82; http://dx.doi.org/10.1038/nri2257; PMID: 18301422
- Zeiger RS. Food allergen avoidance in the prevention of food allergy in infants and children. Pediatrics 2003; 111:1662 - 71; PMID: 12777607
- Gilliland FD. Outdoor air pollution, genetic susceptibility, and asthma management: opportunities for intervention to reduce the burden of asthma. Pediatrics 2009; 123:Suppl 3 S168 - 73; http://dx.doi.org/10.1542/peds.2008-2233G; PMID: 19221160
- Ege MJ, Mayer M, Normand AC, Genuneit J, Cookson WO, Braun-Fahrländer C, et al, GABRIELA Transregio 22 Study Group. Exposure to environmental microorganisms and childhood asthma. N Engl J Med 2011; 364:701 - 9; http://dx.doi.org/10.1056/NEJMoa1007302; PMID: 21345099
- Eckburg PB, Lepp PW, Relman DA. Archaea and their potential role in human disease. Infect Immun 2003; 71:591 - 6; http://dx.doi.org/10.1128/IAI.71.2.591-596.2003; PMID: 12540534
- Hill DA, Artis D. Intestinal bacteria and the regulation of immune cell homeostasis. Annu Rev Immunol 2010; 28:623 - 67; http://dx.doi.org/10.1146/annurev-immunol-030409-101330; PMID: 20192812
- Whitman WB, Coleman DC, Wiebe WJ. Prokaryotes: the unseen majority. Proc Natl Acad Sci U S A 1998; 95:6578 - 83; http://dx.doi.org/10.1073/pnas.95.12.6578; PMID: 9618454
- Ley RE, Peterson DA, Gordon JI. Ecological and evolutionary forces shaping microbial diversity in the human intestine. Cell 2006; 124:837 - 48; http://dx.doi.org/10.1016/j.cell.2006.02.017; PMID: 16497592
- Kalliomäki M, Kirjavainen P, Eerola E, Kero P, Salminen S, Isolauri E. Distinct patterns of neonatal gut microflora in infants in whom atopy was and was not developing. J Allergy Clin Immunol 2001; 107:129 - 34; http://dx.doi.org/10.1067/mai.2001.111237; PMID: 11150002
- Kummeling I, Stelma FF, Dagnelie PC, Snijders BE, Penders J, Huber M, et al. Early life exposure to antibiotics and the subsequent development of eczema, wheeze, and allergic sensitization in the first 2 years of life: the KOALA Birth Cohort Study. Pediatrics 2007; 119:e225 - 31; http://dx.doi.org/10.1542/peds.2006-0896; PMID: 17200248
- Marra F, Marra CA, Richardson K, Lynd LD, Kozyrskyj A, Patrick DM, et al. Antibiotic use in children is associated with increased risk of asthma. Pediatrics 2009; 123:1003 - 10; http://dx.doi.org/10.1542/peds.2008-1146; PMID: 19255032
- Lambrecht BN, Hammad H. The role of dendritic and epithelial cells as master regulators of allergic airway inflammation. Lancet 2010; 376:835 - 43; http://dx.doi.org/10.1016/S0140-6736(10)61226-3; PMID: 20816550
- Paul WE, Zhu J. How are T(H)2-type immune responses initiated and amplified?. Nat Rev Immunol 2010; 10:225 - 35; http://dx.doi.org/10.1038/nri2735; PMID: 20336151
- Herbst T, Sichelstiel A, Schär C, Yadava K, Bürki K, Cahenzli J, et al. Dysregulation of allergic airway inflammation in the absence of microbial colonization. Am J Respir Crit Care Med 2011; 184:198 - 205; http://dx.doi.org/10.1164/rccm.201010-1574OC; PMID: 21471101
- Morin S, Bernard H, Przybylski-Nicaise L, Corthier G, Rabot S, Wal JM, et al. Allergenic and immunogenic potential of cow’s milk β-lactoglobulin and caseins evidenced without adjuvant in germ-free mice. Mol Nutr Food Res 2011; 55:1700 - 7; http://dx.doi.org/10.1002/mnfr.201100024; PMID: 22045656
- Rodriguez B, Prioult G, Hacini-Rachinel F, Moine D, Bruttin A, Ngom-Bru C, et al. Infant gut microbiota is protective against cow’s milk allergy in mice despite immature ileal T-cell response. FEMS Microbiol Ecol 2012; 79:192 - 202; http://dx.doi.org/10.1111/j.1574-6941.2011.01207.x; PMID: 22029421
- Noverr MC, Falkowski NR, McDonald RA, McKenzie AN, Huffnagle GB. Development of allergic airway disease in mice following antibiotic therapy and fungal microbiota increase: role of host genetics, antigen, and interleukin-13. Infect Immun 2005; 73:30 - 8; http://dx.doi.org/10.1128/IAI.73.1.30-38.2005; PMID: 15618138
- Noverr MC, Noggle RM, Toews GB, Huffnagle GB. Role of antibiotics and fungal microbiota in driving pulmonary allergic responses. Infect Immun 2004; 72:4996 - 5003; http://dx.doi.org/10.1128/IAI.72.9.4996-5003.2004; PMID: 15321991
- Bashir ME, Louie S, Shi HN, Nagler-Anderson C. Toll-like receptor 4 signaling by intestinal microbes influences susceptibility to food allergy. J Immunol 2004; 172:6978 - 87; PMID: 15153518
- Hill DA, Hoffmann C, Abt MC, Du Y, Kobuley D, Kirn TJ, et al. Metagenomic analyses reveal antibiotic-induced temporal and spatial changes in intestinal microbiota with associated alterations in immune cell homeostasis. Mucosal Immunol 2010; 3:148 - 58; http://dx.doi.org/10.1038/mi.2009.132; PMID: 19940845
- Russell SL, Gold MJ, Hartmann M, Willing BP, Thorson L, Wlodarska M, et al. Early life antibiotic-driven changes in microbiota enhance susceptibility to allergic asthma. EMBO Rep 2012; 13:440 - 7; http://dx.doi.org/10.1038/embor.2012.32; PMID: 22422004
- Tulic MK, Hodder M, Forsberg A, McCarthy S, Richman T, D’Vaz N, et al. Differences in innate immune function between allergic and nonallergic children: new insights into immune ontogeny. J Allergy Clin Immunol 2011; 127:470 - 8.e1; http://dx.doi.org/10.1016/j.jaci.2010.09.020; PMID: 21093030
- Olszak T, An D, Zeissig S, Vera MP, Richter J, Franke A, et al. Microbial exposure during early life has persistent effects on natural killer T cell function. Science 2012; 336:489 - 93; http://dx.doi.org/10.1126/science.1219328; PMID: 22442383
- Hammad H, Plantinga M, Deswarte K, Pouliot P, Willart MA, Kool M, et al. Inflammatory dendritic cells--not basophils--are necessary and sufficient for induction of Th2 immunity to inhaled house dust mite allergen. J Exp Med 2010; 207:2097 - 111; http://dx.doi.org/10.1084/jem.20101563; PMID: 20819925
- Sokol CL, Barton GM, Farr AG, Medzhitov R. A mechanism for the initiation of allergen-induced T helper type 2 responses. Nat Immunol 2008; 9:310 - 8; http://dx.doi.org/10.1038/ni1558; PMID: 18300366
- Skokos D, Botros HG, Demeure C, Morin J, Peronet R, Birkenmeier G, et al. Mast cell-derived exosomes induce phenotypic and functional maturation of dendritic cells and elicit specific immune responses in vivo. J Immunol 2003; 170:3037 - 45; PMID: 12626558
- Padigel UM, Hess JA, Lee JJ, Lok JB, Nolan TJ, Schad GA, et al. Eosinophils act as antigen-presenting cells to induce immunity to Strongyloides stercoralis in mice. J Infect Dis 2007; 196:1844 - 51; http://dx.doi.org/10.1086/522968; PMID: 18190266
- Charles N, Hardwick D, Daugas E, Illei GG, Rivera J. Basophils and the T helper 2 environment can promote the development of lupus nephritis. Nat Med 2010; 16:701 - 7; http://dx.doi.org/10.1038/nm.2159; PMID: 20512127
- Gessner A, Mohrs K, Mohrs M. Mast cells, basophils, and eosinophils acquire constitutive IL-4 and IL-13 transcripts during lineage differentiation that are sufficient for rapid cytokine production. J Immunol 2005; 174:1063 - 72; PMID: 15634931
- Mohrs K, Wakil AE, Killeen N, Locksley RM, Mohrs M. A two-step process for cytokine production revealed by IL-4 dual-reporter mice. Immunity 2005; 23:419 - 29; http://dx.doi.org/10.1016/j.immuni.2005.09.006; PMID: 16226507
- Min B, Prout M, Hu-Li J, Zhu J, Jankovic D, Morgan ES, et al. Basophils produce IL-4 and accumulate in tissues after infection with a Th2-inducing parasite. J Exp Med 2004; 200:507 - 17; http://dx.doi.org/10.1084/jem.20040590; PMID: 15314076
- Durkin HG, Bazin H, Waksman BH. Origin and fate of IgE-bearing lymphocytes. I. Peyer’s patches as differentiation site of cells. Simultaneously bearing IgA and IgE. J Exp Med 1981; 154:640 - 8; http://dx.doi.org/10.1084/jem.154.3.640; PMID: 6974216
- McCoy KD, Harris NL, Diener P, Hatak S, Odermatt B, Hangartner L, et al. Natural IgE production in the absence of MHC Class II cognate help. Immunity 2006; 24:329 - 39; http://dx.doi.org/10.1016/j.immuni.2006.01.013; PMID: 16546101
- Kitaura J, Eto K, Kinoshita T, Kawakami Y, Leitges M, Lowell CA, et al. Regulation of highly cytokinergic IgE-induced mast cell adhesion by Src, Syk, Tec, and protein kinase C family kinases. J Immunol 2005; 174:4495 - 504; PMID: 15814670
- Galli SJ, Kalesnikoff J, Grimbaldeston MA, Piliponsky AM, Williams CM, Tsai M. Mast cells as “tunable” effector and immunoregulatory cells: recent advances. Annu Rev Immunol 2005; 23:749 - 86; http://dx.doi.org/10.1146/annurev.immunol.21.120601.141025; PMID: 15771585
- Xiang Z, Möller C, Nilsson G. IgE-receptor activation induces survival and Bfl-1 expression in human mast cells but not basophils. Allergy 2006; 61:1040 - 6; http://dx.doi.org/10.1111/j.1398-9995.2006.01024.x; PMID: 16918505
- Smith K, McCoy KD, Macpherson AJ. Use of axenic animals in studying the adaptation of mammals to their commensal intestinal microbiota. Semin Immunol 2007; 19:59 - 69; http://dx.doi.org/10.1016/j.smim.2006.10.002; PMID: 17118672
- Perrigoue JG, Saenz SA, Siracusa MC, Allenspach EJ, Taylor BC, Giacomin PR, et al. MHC class II-dependent basophil-CD4+ T cell interactions promote T(H)2 cytokine-dependent immunity. Nat Immunol 2009; 10:697 - 705; http://dx.doi.org/10.1038/ni.1740; PMID: 19465906
- Yoshimoto T, Yasuda K, Tanaka H, Nakahira M, Imai Y, Fujimori Y, et al. Basophils contribute to T(H)2-IgE responses in vivo via IL-4 production and presentation of peptide-MHC class II complexes to CD4+ T cells. Nat Immunol 2009; 10:706 - 12; http://dx.doi.org/10.1038/ni.1737; PMID: 19465908
- Sullivan BM, Liang HE, Bando JK, Wu D, Cheng LE, McKerrow JK, et al. Genetic analysis of basophil function in vivo. Nat Immunol 2011; 12:527 - 35; http://dx.doi.org/10.1038/ni.2036; PMID: 21552267
- Ohnmacht C, Schwartz C, Panzer M, Schiedewitz I, Naumann R, Voehringer D. Basophils orchestrate chronic allergic dermatitis and protective immunity against helminths. Immunity 2010; 33:364 - 74; http://dx.doi.org/10.1016/j.immuni.2010.08.011; PMID: 20817571
- Kim S, Prout M, Ramshaw H, Lopez AF, LeGros G, Min B. Cutting edge: basophils are transiently recruited into the draining lymph nodes during helminth infection via IL-3, but infection-induced Th2 immunity can develop without basophil lymph node recruitment or IL-3. J Immunol 2010; 184:1143 - 7; http://dx.doi.org/10.4049/jimmunol.0902447; PMID: 20038645
- Ohnmacht C, Voehringer D. Basophils protect against reinfection with hookworms independently of mast cells and memory Th2 cells. J Immunol 2010; 184:344 - 50; http://dx.doi.org/10.4049/jimmunol.0901841; PMID: 19955520
- Nakanishi K. Basophils are potent antigen-presenting cells that selectively induce Th2 cells. Eur J Immunol 2010; 40:1836 - 42; http://dx.doi.org/10.1002/eji.201040588; PMID: 20518033
- Sokol CL, Chu NQ, Yu S, Nish SA, Laufer TM, Medzhitov R. Basophils function as antigen-presenting cells for an allergen-induced T helper type 2 response. Nat Immunol 2009; 10:713 - 20; http://dx.doi.org/10.1038/ni.1738; PMID: 19465907
- Torrero MN, Hübner MP, Larson D, Karasuyama H, Mitre E. Basophils amplify type 2 immune responses, but do not serve a protective role, during chronic infection of mice with the filarial nematode Litomosoides sigmodontis. J Immunol 2010; 185:7426 - 34; http://dx.doi.org/10.4049/jimmunol.0903864; PMID: 21057084
- Siracusa MC, Saenz SA, Hill DA, Kim BS, Headley MB, Doering TA, et al. TSLP promotes interleukin-3-independent basophil haematopoiesis and type 2 inflammation. Nature 2011; 477:229 - 33; http://dx.doi.org/10.1038/nature10329; PMID: 21841801
- Fink LN, Metzdorff SB, Zeuthen LH, Nellemann C, Kristensen MB, Licht TR, et al. Establishment of tolerance to commensal bacteria requires a complex microbiota and is accompanied by decreased intestinal chemokine expression. Am J Physiol Gastrointest Liver Physiol 2012; 302:G55 - 65; http://dx.doi.org/10.1152/ajpgi.00428.2010; PMID: 21960522
- Redhu NS, Saleh A, Lee HC, Halayko AJ, Ziegler SF, Gounni AS. IgE induces transcriptional regulation of thymic stromal lymphopoietin in human airway smooth muscle cells. J Allergy Clin Immunol 2011; 128:892 - 6.e2; http://dx.doi.org/10.1016/j.jaci.2011.06.045; PMID: 21835441
- Maurer D, Fiebiger S, Ebner C, Reininger B, Fischer GF, Wichlas S, et al. Peripheral blood dendritic cells express Fc epsilon RI as a complex composed of Fc epsilon RI alpha- and Fc epsilon RI gamma-chains and can use this receptor for IgE-mediated allergen presentation. J Immunol 1996; 157:607 - 16; PMID: 8752908
- Tunon-De-Lara JM, Redington AE, Bradding P, Church MK, Hartley JA, Semper AE, et al. Dendritic cells in normal and asthmatic airways: expression of the alpha subunit of the high affinity immunoglobulin E receptor (Fc epsilon RI -alpha). Clin Exp Allergy 1996; 26:648 - 55; http://dx.doi.org/10.1111/j.1365-2222.1996.tb00591.x; PMID: 8809422
- Ekman AK, Erjefält JS, Jansson L, Cardell LO. Allergen-induced accumulation of CD68-,CD123+ dendritic cells in the nasal mucosa. Int Arch Allergy Immunol 2011; 155:234 - 42; http://dx.doi.org/10.1159/000320480; PMID: 21282962
- Afifi SS, Helal AM. CD11c+ and CD123+ dendritic cell subsets in peripheral blood of lung cancer patients. Egypt J Immunol 2009; 16:9 - 15; PMID: 22059349
- Rajakulasingam K, Durham SR, O’Brien F, Humbert M, Barata LT, Reece L, et al. Enhanced expression of high-affinity IgE receptor (Fc epsilon RI) alpha chain in human allergen-induced rhinitis with co-localization to mast cells, macrophages, eosinophils, and dendritic cells. J Allergy Clin Immunol 1997; 100:78 - 86; http://dx.doi.org/10.1016/S0091-6749(97)70198-2; PMID: 9257791
- Bouskra D, Brézillon C, Bérard M, Werts C, Varona R, Boneca IG, et al. Lymphoid tissue genesis induced by commensals through NOD1 regulates intestinal homeostasis. Nature 2008; 456:507 - 10; http://dx.doi.org/10.1038/nature07450; PMID: 18987631
- Umesaki Y, Setoyama H, Matsumoto S, Okada Y. Expansion of alpha beta T-cell receptor-bearing intestinal intraepithelial lymphocytes after microbial colonization in germ-free mice and its independence from thymus. Immunology 1993; 79:32 - 7; PMID: 8509140
- Macpherson AJ. IgA adaptation to the presence of commensal bacteria in the intestine. Curr Top Microbiol Immunol 2006; 308:117 - 36; http://dx.doi.org/10.1007/3-540-30657-9_5; PMID: 16922088
- Atarashi K, Tanoue T, Shima T, Imaoka A, Kuwahara T, Momose Y, et al. Induction of colonic regulatory T cells by indigenous Clostridium species. Science 2011; 331:337 - 41; http://dx.doi.org/10.1126/science.1198469; PMID: 21205640
- Ivanov II, Atarashi K, Manel N, Brodie EL, Shima T, Karaoz U, et al. Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell 2009; 139:485 - 98; http://dx.doi.org/10.1016/j.cell.2009.09.033; PMID: 19836068
- Liu N, Ohnishi N, Ni L, Akira S, Bacon KB. CpG directly induces T-bet expression and inhibits IgG1 and IgE switching in B cells. Nat Immunol 2003; 4:687 - 93; http://dx.doi.org/10.1038/ni941; PMID: 12766768
- Macpherson AJ, Uhr T. Induction of protective IgA by intestinal dendritic cells carrying commensal bacteria. Science 2004; 303:1662 - 5; http://dx.doi.org/10.1126/science.1091334; PMID: 15016999
- Saenz SA, Noti M, Artis D. Innate immune cell populations function as initiators and effectors in Th2 cytokine responses. Trends Immunol 2010; 31:407 - 13; http://dx.doi.org/10.1016/j.it.2010.09.001; PMID: 20951092
- Saenz SA, Siracusa MC, Perrigoue JG, Spencer SP, Urban JF Jr., Tocker JE, et al. IL25 elicits a multipotent progenitor cell population that promotes T(H)2 cytokine responses. Nature 2010; 464:1362 - 6; http://dx.doi.org/10.1038/nature08901; PMID: 20200520
- Atarashi K, Nishimura J, Shima T, Umesaki Y, Yamamoto M, Onoue M, et al. ATP drives lamina propria T(H)17 cell differentiation. Nature 2008; 455:808 - 12; http://dx.doi.org/10.1038/nature07240; PMID: 18716618
- Zaph C, Du Y, Saenz SA, Nair MG, Perrigoue JG, Taylor BC, et al. Commensal-dependent expression of IL-25 regulates the IL-23-IL-17 axis in the intestine. J Exp Med 2008; 205:2191 - 8; http://dx.doi.org/10.1084/jem.20080720; PMID: 18762568
- Engelhardt KR, McGhee S, Winkler S, Sassi A, Woellner C, Lopez-Herrera G, et al. Large deletions and point mutations involving the dedicator of cytokinesis 8 (DOCK8) in the autosomal-recessive form of hyper-IgE syndrome. J Allergy Clin Immunol 2009; 124:1289 - 302.e4; http://dx.doi.org/10.1016/j.jaci.2009.10.038; PMID: 20004785
- Zhang Q, Davis JC, Lamborn IT, Freeman AF, Jing H, Favreau AJ, et al. Combined immunodeficiency associated with DOCK8 mutations. N Engl J Med 2009; 361:2046 - 55; http://dx.doi.org/10.1056/NEJMoa0905506; PMID: 19776401
- Grimbacher B, Holland SM, Puck JM. Hyper-IgE syndromes. Immunol Rev 2005; 203:244 - 50; http://dx.doi.org/10.1111/j.0105-2896.2005.00228.x; PMID: 15661034
- Herbst T, Esser J, Prati M, Kulagin M, Stettler R, Zaiss MM, et al. Antibodies and IL-3 support helminth-induced basophil expansion. Proc Natl Acad Sci U S A 2012; 109:14954 - 9; http://dx.doi.org/10.1073/pnas.1117584109; PMID: 22930820
- Holgate S, Buhl R, Bousquet J, Smith N, Panahloo Z, Jimenez P. The use of omalizumab in the treatment of severe allergic asthma: A clinical experience update. Respir Med 2009; 103:1098 - 113; http://dx.doi.org/10.1016/j.rmed.2009.03.008; PMID: 19362459
- Pace E, Ferraro M, Bruno A, Chiappara G, Bousquet J, Gjomarkaj M. Clinical benefits of 7 years of treatment with omalizumab in severe uncontrolled asthmatics. J Asthma 2011; 48:387 - 92; http://dx.doi.org/10.3109/02770903.2011.561512; PMID: 21391878
- Saini SS, MacGlashan DW Jr.. Assessing basophil functional measures during monoclonal anti-IgE therapy. J Immunol Methods 2012; 383:60 - 4; http://dx.doi.org/10.1016/j.jim.2012.05.016; PMID: 22664098
- Hill DA, Siracusa MC, Abt MC, Kim BS, Kobuley D, Kubo M, et al. Commensal bacteria-derived signals regulate basophil hematopoiesis and allergic inflammation. Nat Med 2012; 18:538 - 46; http://dx.doi.org/10.1038/nm.2657; PMID: 22447074