Abstract
DNA vaccines are being developed as a potentially safe and effective immunization platform. However, translation of DNA vaccines into a clinical setting has produced results that have fallen short of those generated in a preclinical setting. Various strategies are being developed to address this lack of potency, including improvements in delivery methods. Electroporation (EP) creates transient increases in cell membrane permeability, thus enhancing DNA uptake and leading to a more robust immune response. Here, we report on the safety and tolerability of delivering sterile saline via intramuscular (IM) or intradermal (ID) injection followed by in vivo electroporation using the CELLECTRA® adaptive constant current device in healthy adults from two open-label studies. Pain, as assessed by VAS, was highest immediately after EP but diminishes by about 50% within 5 min. Mean VAS scores appear to correlate with the amount of energy delivered and depth of needle insertion, especially for intramuscular EP. Mean scores did not exceed 7 out of 10 or 3 out of 10 for IM and ID EP, respectively. The majority of adverse events included mild to moderate injection site reactions that resolved within one day. No deaths or serious adverse events were reported during the course of either study. Overall, injection followed by EP with the CELLECTRA® device was well-tolerated and no significant safety concerns were identified. These studies support the further development of electroporation as a vaccine delivery method to enhance immunogenicity, particularly for diseases in which traditional vaccination approaches are ineffective.
Introduction
With the advent of DNA immunization, many challenges associated with more traditional vaccination approaches are being addressed. The advantages of this non-live vaccine platform extend not only into improvements in safety but also into managing logistical concerns, including manufacturing and maintenance.Citation1 Despite its initial promise, DNA vaccination has shown inferior performance in meeting immunogenicity standards compared with conventional vaccines when applied to large animals and humans. Although many factors may contribute to lack of potency, one clearly identifiable issue is cellular delivery. The development of novel tools to support efficient delivery of DNA plasmids could help maximize the potential of this vaccine platform.
Electroporation (EP) involves the application of brief electric pulses to tissue in order to permeabilize cell membranes in a transient and reversible manner. The temporary formation of pores facilitates successful transport of macromolecules, like DNA, that occurs on the order of microseconds vs. minutes in the case of passive diffusion alone.Citation2 This technique has demonstrated versatility in use, as shown by its functionality in combination with a range of molecules, tissue types, disease indications, and across species.Citation3 It has been used extensively over more than 25 y in in vitro settings, but has more recently gained widespread use as an in vivo transfection tool.Citation4 Vaccine efficacy may be enhanced 10- to 100-fold when EP is employed, as reflected by increases in immune responses.Citation1,Citation5 Indeed, cellular uptake and expression of DNA material at the injection site, as well as antigen-specific antibody titers, are greatly improved in mice in the presence of EP compared to DNA alone.Citation6
EP-mediated immune response enhancement may mechanistically be due to local inflammatory processes caused by the procedure itself. Electrical stimulation induces the secretion of inflammatory chemokines and cytokines and recruitment of monocytes, lymphocytes, and antigen-presenting cells to the site of EP. This has been shown in a porcine model, whereby an influx of macrophages and neutrophils and mild eosinophilia was observed in muscle tissue post-EP with bovine herpes virus I glycoprotein D DNA immunization, and this corresponded with high neutralizing antibody titers in serum to the virus.Citation7 As a result of these processes, both humoral and cell-mediated immunity is augmented compared with DNA injection alone, thus potentially enhancing vaccine efficacy.
The CELLECTRA® electroporation device developed by Inovio Pharmaceuticals, Inc. is currently being assessed in clinical studies for investigational purposes, for both prophylactic and therapeutic indications. This device delivers square-wave electric pulses by applying an adaptive electric field based on constant current, rather than constant voltage.Citation8 These electrical parameters measure and adjust for changes in tissue resistance in real-time that may otherwise result in tissue damage and reduced DNA uptake.Citation9 To date, the device has been evaluated in several animal models, including mice, pigs, and rhesus macaques, and has demonstrated favorable immunogenicity.Citation8,Citation10
The majority of studies in the past have focused on intramuscular (IM) EP delivery, but more recently, intradermal (ID) EP administration has gained momentum. The skin is not only an easily accessible tissue, but also allows for a shallower needle depth and reduced energy. These characteristics are associated with less invasiveness and conceivably more tolerable delivery. Additionally, skin is highly immunocompetent and populated with resident antigen-presenting cells.Citation11,Citation12 For instance, mice vaccinated with DNA followed by skin EP exhibit increased antigen-specific CD4+ and CD8+ T cell responses, including elevated IFNγ levels.Citation13
Despite these advantages, a primary drawback of EP is pain and discomfort at the application site compared with conventional injections.Citation14 Here, we present the tolerability of the CELLECTRA® electroporation device from two separate open-label, single dose studies evaluating IM and ID delivery. The purpose of these studies was to assess pain and safety of in vivo electroporation after intramuscular and intradermal injection in healthy adults.
Results
Study population
Screening procedures to determine eligibility were performed within 30 d prior to study entry. All eligible subjects provided written informed consent, were enrolled, and completed the study. A total of 15 subjects were screened for the IM study, of which 10 were eligible. The mean age was 32 y (range 21–39 y) with 90% male and 90% white. All subjects had normal calculated BMI, with a mean of 24 kg/m2. For the ID study, 10 subjects were screened and all were found eligible. The mean age was 33.9 y (range 25–40 y) with 60% male and 60% white. One subject had a calculated BMI below the normal range (17.5 kg/m2) and two subjects had a calculated BMI above the normal range (42.6 kg/m2 and 30.1 kg/m2). The mean BMI for the ID cohort was 26.1 kg/m2. Subjects in both studies were administered a single dose of sterile saline without any DNA followed by the EP procedure using the CELLECTRA® electroporation device (). As detailed in , one set of 3 electric pulses were delivered for the IM study and two sets of 2 pulses were delivered in the ID study.
Figure 1. CELLECTRA® adaptive constant current electroporation device. (A) User-prompted operating console (B) Intramuscular hand-held applicator and 5-needle array (C) Intradermal hand-held applicator and 3-needle array.
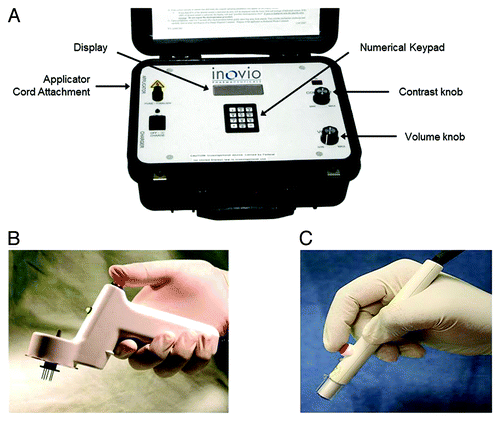
Table 1. Electrical parameters of EP
Pain assessment following EP treatment
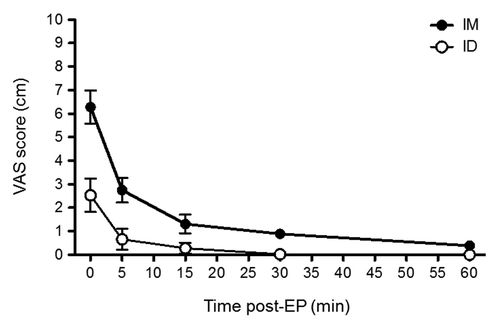
At various time-points post-EP delivery, subjects were asked to gauge their level of pain by completing the visual analog scale (VAS), a 10 cm horizontal line anchored by word descriptors at each end (No Pain = 0; Worst Pain = 10) as well as by using a stopwatch to measure time to meaningful pain relief. By VAS, all subjects reported some level of pain at the injection site. VAS scores were consistently highest immediately after EP, with a mean score of 6.28 cm for IM administration and 2.5 cm for ID administration (). Peak scores declined rapidly, such that the highest pain level reported was 0.8 cm at 60 min post-EP for IM and 0.2 cm at 30 min post-EP for ID (no pain was reported at 60 min post-EP for ID). Furthermore, the median time until meaningful pain relief following EP, as measured by a stopwatch, was 30 sec for IM and 11 sec for ID (data not shown). Overall, pain levels were greater when EP was delivered IM vs. ID (). The majority of subjects in the IM study reported pain at less than 2 cm within 15 min after EP, and those in the ID study reported pain at less than 1 cm within 5 min after EP.
Table 2. Mean pain intensity score as measured by visual analog scale (VAS) over time
Figure 2. Mean VAS score post-EP delivery with CELLECTRA® device over time. Subject VAS score as reported on the day of EP. IM, intramuscular study, n = 10; ID, intradermal study, n = 10.
On Day 2 after the EP procedure, subjects rated pain level or discomfort at the injection site by responding to a categorical prompt. For the IM study, eight out of ten subjects responded with “none,” while two subjects rated it as “a little.” All subjects rated their pain or discomfort as “none” in the ID study.
Injection site reactions following EP treatment
On the day of the EP procedure, as well as on Days 2 and 7 post-EP, injection site reactions were assessed. Injection site pain, tenderness, erythema, and swelling were captured and presented in . In the IM study, a total of eight subjects reported experiencing an injection site reaction. One subject reported mild (grade 1) pain that resolved within one day without intervention. Three subjects reported mild and four reported moderate (grade 2) tenderness, which resolved within 3 d for all.
Table 3. Number of subjects who reported injection site reactions*
In the ID study, nine subjects experienced injection site reactions. Of those, six subjects reported injection site pain; four mild in grade and two moderate, all of which lasted one day. Eight subjects reported erythema; seven were mild and one was moderate. All reactions resolved within one day, except for one subject who reported mild erythema for 9 d, without treatment. One subject reported mild swelling at the injection site, which resolved without treatment. No grade 3 or 4 events were reported with either route of EP administration.
Adverse events (AEs) experienced following EP treatment
No deaths or serious adverse events were reported during the course of either study. All reported adverse events were mild or moderate, as summarized in . In the IM study, five total subjects experienced AEs following injection and EP delivery. These included injection site burning (one subject), anesthesia (one subject), warmth (one subject), and hematoma (one subject); elevated blood CPK level (one subject); and nervous system disorders (two subjects). The subject with a significantly elevated CPK level (2-fold higher than upper limit of normal range) on Day 2 post-EP was asymptomatic and levels returned to within normal range by Day 7. Five additional subjects displayed mild increases in CPK levels, but none were clinically significant. All AEs reported were most likely related to treatment, but typically resolved within one day without sequelae.
Table 4. Summary of adverse events by system organ class and preferred term with route of delivery and relationship to investigational product*
In the ID study, four total subjects reported AEs throughout the study. Systemic adverse events included gastrointestinal disorders (one subject); myalgia (one subject); headache (one subject); involuntary muscle contraction (one subject); and respiratory disorders (two subjects). The subject experiencing mild headache reported this condition on two separate occasions prior to EP delivery, the first of which required medication. This subject also reported a mild cough (prior to EP) and moderate wheezing (5 d after EP), both of which were ongoing at study discharge. All AEs with the exception of involuntary muscle contraction were determined to be unrelated to the study. CPK levels for all subjects did not change significantly after EP.
Discussion
Collectively, the IM and ID studies examined the safety and tolerability of electroporation using the CELLECTRA® device without any DNA in healthy individuals. Overall, injection followed by EP with the CELLECTRA® device appeared to be well tolerated and no significant safety signals were detected. Subject-reported pain level was gauged by a stopwatch and VAS scores. This type of scale is an accepted tool for assessment of pain and discomfort that is able to generate reproducible results across different subject populations.Citation15,Citation16 The VAS score was highest immediately after EP for both IM and ID administration but declined to about 50% within 5 min for the majority of subjects. In the IM group, most subjects experienced some, albeit extremely low, level of pain at 60 min post-EP. Pain levels in the ID group appeared to dissipate at a faster rate, with the majority of subjects reporting no pain as early as 15 min post-EP. By Day 2 post-EP, only two subjects (both in the IM study) reported any pain. Several published dermal studies have reported comparable pain profiles using other EP delivery devices.Citation17,Citation18 The difference in time to pain resolution may in part be due to needle depth of the device during administration. During IM injections, the 5-needle array penetrates intramuscularly to a depth of approximately 18 mm, while needle depth maximally penetrates 3 mm into the dermal tissue layers for ID electroporation. The reduced pain level associated with ID EP may make this route of administration more acceptable to patients.
The majority of adverse events in the IM study were transient localized reactions in response to the EP procedure. Most of the adverse events reported in the ID study were systemic in nature and so the duration was longer compared with those in the IM study. However, the onset for all but one of these adverse events was prior to initiation of electroporation and thus deemed unrelated to study procedures. The only AE that initiated post-EP in the ID study was an involuntary muscle contraction, reported in one subject, which resolved the same day. The EP procedure has been shown to carry some potential of transient muscle damage in animal models, evident as increased numbers of fibers with central nucleoli and damaged myofibrillar bundles.Citation19,Citation20 Since muscle damage is a concern, serum CPK levels were assessed both at baseline (prior to EP) and after the procedure. One subject out of both study cohorts had a clinically significant but asymptomatic CPK elevation, which resolved within 5 d.
Although the current studies evaluated EP without delivering any biologic product, it has been shown that EP following DNA plasmid injection significantly enhances DNA uptake and retention.Citation21 Interestingly, EP must be applied immediately after DNA injection to exert its effect on gene expression. In a porcine model, EP administered prior to plasmid injection did not significantly affect the expression of reporter genes compared with plasmid injection alone.Citation7 Inflammatory cell infiltration associated with the EP process may partially account for the dramatic increase in uptake. It is possible that antigen presentation is more efficient under stressful conditions in electrically stimulated cells and may create an adjuvant effect.Citation22 Along these lines, EP has been shown to induce considerable lymphocytic infiltration, in addition to CPK elevation and skeletal muscle damage evident as lesions in mice.Citation20 Because muscle tissue does not generally harbor many resident antigen-presenting cells, it is likely that recruitment to the injection site contributes to the efficacy of EP. The skin, however, contains resident Langerhans and other dermal dendritic cells well-suited for antigen presentation, making this tissue advantageous for EP studies.Citation23,Citation24
In another murine study, lymphocyte response was detectable post DNA injection with EP and undetectable after DNA injection alone at the same dose.Citation25 Similarly, the CELLECTRA® device imparted a greater and longer-lasting immune response with a lower dose of human immunodeficiency virus plasmid compared with DNA injection alone in rhesus macaques.Citation26 These data suggest that EP may impart a dose-sparing effect, and may potentially contribute to limiting toxicity associated with higher doses. More recently, rhesus macaques injected with simian immunodeficiency virus DNA in combination with EP using the CELLECTRA® device showed marked increases in memory T cells and reduced viral loads upon viral challenge.Citation27 Taken together, EP following DNA injection elicits more robust humoral and cellular immune responses, which in effect contributes to the establishment of an effector memory pool for long-term immunity.Citation28-Citation30
In conclusion, IM and ID delivery of EP was well-tolerated in healthy adults. Local pain was present but subsided quickly, and most related adverse events were mild injection site reactions. As a corollary to these data, two Phase I clinical trials have been conducted using the CELLECTRA® device to deliver a therapeutic human papillomavirus vaccine.Citation31,Citation32 The tolerability of the device has been demonstrated in these Phase I studies and has led to the initiation of a Phase II clinical trial, which is currently ongoing. In summary, EP has emerged as a promising method of biologic delivery for the purposes of increasing DNA vaccine potency.
Materials and Methods
Study design and subjects
These open-label studies were conducted at two centers, both in the United States. Separate centers were used to evaluate each route of electroporation (EP) administration, either intramuscular (IM) or intradermal (ID). Study protocols were approved by the Institutional Review Board and adhered to the guidelines of Good Clinical Practice and the Declaration of Helsinki. Written informed consent was obtained prior to study enrollment. Male and female subjects deemed eligible were between 18–45 y of age, inclusive, and healthy as assessed by medical history, physical examination, creatine phosphokinase (CPK) evaluation, and 12-lead ECGs. Female subjects must not have been nursing or pregnant and have had a negative urine pregnancy test 48 h prior to electroporation. For the IM study, a minimum weight of 110 lbs and a BMI less than 30 kg/m2 (normal range considered 18–30 kg/m2) was required. No weight or BMI criteria were imposed for the ID study. Ten subjects each were enrolled in the IM and ID studies and all subjects completed the study.
Electroporation using CELLECTRA® device
The CELLECTRA® 2000 adaptive constant current electroporation device delivers short, controlled electric pulses through a sterile, disposable array consisting of 5 needles (for IM applications) or 3 needles (for ID applications) (). When inserted into tissue, the needle array surrounds the site of injection. For the IM study, 1 ml of sterile 0.9% saline solution was injected intramuscularly into the deltoid muscle of one arm followed 4 sec later by three electroporation pulses, each 52 msec in duration and spaced in 1 sec intervals. The IM device delivered a range of 80–130 V to maintain a constant current of 0.5 Amp for each pulse. For the ID study, 0.15 ml of sterile 0.9% saline solution was injected intradermally into the back of the upper arm. The ID EP session delivered four total pulses, each 52 msec in duration. The ID device delivered a range of 58–194 V to maintain a constant current of 0.2 Amp. The first two pulses were delivered in 0.2 sec intervals followed by a 3 sec delay, and the last two pulses were again spaced in 0.2 sec intervals. For each route of administration, the complete EP procedure, including injection, was no longer than 1.5 min. Data pertaining to EP was automatically collected by the device and downloaded for further data analysis.
Pain assessment
Two methods were employed to capture pain response after injection and EP delivery on the day of administration. On the day of injection and EP, subjects were instructed to measure the length of time pain was experienced by using a stopwatch. Study staff started a stopwatch at the start of electroporation. The subject was then given the stopwatch to be held in the hand of the non-injected arm. Each subject was instructed to stop the stopwatch when they experienced meaningful relief from pain caused by the procedure. Additionally, subjects completed the Visual Analog Scale (VAS) to rate the level of pain experienced immediately and at 5 min, 15 min, 30 min, and 60 min post-EP. Subjects marked pain level on a 10 cm horizontal line, anchored by word descriptors (“No Pain” and “Worst Pain”) at opposite ends. The VAS score, reported in cm, was determined by measuring from “No Pain” to the marked location. On Day 2 post-EP, subjects rated pain or discomfort level using an ordinal word descriptor scale, comprised of “none,” “a little,” “some,” and “a lot” categories.
Safety assessment
Injection site reactions, including pain, tenderness, erythema, and swelling were assessed on the day of EP, Day 2 post-EP, and Day 7 post-EP. Systemic adverse events were monitored continuously over the course of the study beginning from the date of consent. CPK levels were assessed on Day 2 post-EP. All events were graded in accordance with the 2007 FDA toxicity grading scale for healthy adult and adolescent volunteers enrolled in preventive vaccine clinical trials (grade 1 = mild, grade 2 = moderate, grade 3 = severe, grade 4 = life-threatening).
| Abbreviations: | ||
| EP | = | electroporation |
| IM | = | intramuscular |
| ID | = | intradermal |
| CPK | = | Creatine Phosphokinase |
| VAS | = | visual analog scale |
| AE | = | adverse event |
Conflict of Interest/Financial Disclosure
All authors, with the exception of SED and PT, are under employ of Inovio Pharmaceuticals, Inc. No financial interests were reported for SED and PT.
Acknowledgments
We thank all study participants for their cooperation and study completion. We also extend much appreciation to C. Jo White (formerly Inovio Pharmaceuticals, Blue Bell, PA), Lindsey Steel (formerly VGX Pharmaceuticals, The Woodlands, TX) and Ruxandra Draghia-Akli (formerly VGX Pharmaceuticals, The Woodlands, TX) for their contribution to the IM study; and Phuong Diep-Lam (formerly Inovio Pharmaceuticals, Blue Bell, PA), Larissa Zifchak (University of Pennsylvania, Philadelphia, PA), and Joel Maslow (VA Medical Center, Philadelphia, PA) for their contribution to the ID study. The IM study was conducted at Premier Research Group in Austin, TX, USA. The ID study was conducted at the University of Pennsylvania, Division of Infectious Disease in Philadelphia, PA, USA.
References
- Sardesai NY, Weiner DB. Electroporation delivery of DNA vaccines: prospects for success. Curr Opin Immunol 2011; 23:421 - 9; http://dx.doi.org/10.1016/j.coi.2011.03.008; PMID: 21530212
- Mir LM. Therapeutic perspectives of in vivo cell electropermeabilization. Bioelectrochemistry 2001; 53:1 - 10; http://dx.doi.org/10.1016/S0302-4598(00)00112-4; PMID: 11206915
- Bodles-Brakhop AM, Heller R, Draghia-Akli R. Electroporation for the delivery of DNA-based vaccines and immunotherapeutics: current clinical developments. Mol Ther 2009; 17:585 - 92; http://dx.doi.org/10.1038/mt.2009.5; PMID: 19223870
- Cemazar M, Sersa G. Electrotransfer of therapeutic molecules into tissues. Curr Opin Mol Ther 2007; 9:554 - 62; PMID: 18041666
- van Drunen Littel-van den Hurk S, Hannaman D. Electroporation for DNA immunization: clinical application. Expert Rev Vaccines 2010; 9:503 - 17; http://dx.doi.org/10.1586/erv.10.42; PMID: 20450325
- Dupuis M, Denis-Mize K, Woo C, Goldbeck C, Selby MJ, Chen M, et al. Distribution of DNA vaccines determines their immunogenicity after intramuscular injection in mice. J Immunol 2000; 165:2850 - 8; PMID: 10946318
- Babiuk S, Baca-Estrada ME, Foldvari M, Middleton DM, Rabussay D, Widera G, et al. Increased gene expression and inflammatory cell infiltration caused by electroporation are both important for improving the efficacy of DNA vaccines. J Biotechnol 2004; 110:1 - 10; http://dx.doi.org/10.1016/j.jbiotec.2004.01.015; PMID: 15099900
- Khan AS, Pope MA, Draghia-Akli R. Highly efficient constant-current electroporation increases in vivo plasmid expression. DNA Cell Biol 2005; 24:810 - 8; http://dx.doi.org/10.1089/dna.2005.24.810; PMID: 16332178
- Draghia-Akli R, Smith LC. Electrokinetic Enhancement of Plasmid Delivery In Vivo. In: Templeton NS, Lasic DD (eds). Gene Therapy–Therapeutic Mechanisms and Strategies. New York: Marcel Dekker, Inc., 2003: 245-263.
- Hirao LA, Wu L, Khan AS, Satishchandran A, Draghia-Akli R, Weiner DB. Intradermal/subcutaneous immunization by electroporation improves plasmid vaccine delivery and potency in pigs and rhesus macaques. Vaccine 2008; 26:440 - 8; http://dx.doi.org/10.1016/j.vaccine.2007.10.041; PMID: 18082294
- Tobin DJ. Biochemistry of human skin--our brain on the outside. Chem Soc Rev 2006; 35:52 - 67; http://dx.doi.org/10.1039/b505793k; PMID: 16365642
- Martinon F, Kaldma K, Sikut R, Culina S, Romain G, Tuomela M, et al. Persistent immune responses induced by a human immunodeficiency virus DNA vaccine delivered in association with electroporation in the skin of nonhuman primates. Hum Gene Ther 2009; 20:1291 - 307; http://dx.doi.org/10.1089/hum.2009.044; PMID: 19627235
- Bråve A, Nyström S, Roos AK, Applequist SE. Plasmid DNA vaccination using skin electroporation promotes poly-functional CD4 T-cell responses. Immunol Cell Biol 2011; 89:492 - 6; http://dx.doi.org/10.1038/icb.2010.109; PMID: 20838412
- Saade F, Petrovsky N. Technologies for enhanced efficacy of DNA vaccines. Expert Rev Vaccines 2012; 11:189 - 209; http://dx.doi.org/10.1586/erv.11.188; PMID: 22309668
- Paul-Dauphin A, Guillemin F, Virion JM, Briançon S. Bias and precision in visual analogue scales: a randomized controlled trial. Am J Epidemiol 1999; 150:1117 - 27; http://dx.doi.org/10.1093/oxfordjournals.aje.a009937; PMID: 10568628
- Grant S, Aitchison T, Henderson E, Christie J, Zare S, McMurray J, et al. A comparison of the reproducibility and the sensitivity to change of visual analogue scales, Borg scales, and Likert scales in normal subjects during submaximal exercise. Chest 1999; 116:1208 - 17; http://dx.doi.org/10.1378/chest.116.5.1208; PMID: 10559077
- Wallace M, Evans B, Woods S, Mogg R, Zhang L, Finnefrock AC, et al. Tolerability of two sequential electroporation treatments using MedPulser DNA delivery system (DDS) in healthy adults. Mol Ther 2009; 17:922 - 8; http://dx.doi.org/10.1038/mt.2009.27; PMID: 19277016
- El-Kamary SS, Billington M, Deitz S, Colby E, Rhinehart H, Wu Y, et al. Safety and tolerability of the Easy Vax™ clinical epidermal electroporation system in healthy adults. Mol Ther 2012; 20:214 - 20; http://dx.doi.org/10.1038/mt.2011.235; PMID: 22068424
- Rizzuto G, Cappelletti M, Maione D, Savino R, Lazzaro D, Costa P, et al. Efficient and regulated erythropoietin production by naked DNA injection and muscle electroporation. Proc Natl Acad Sci U S A 1999; 96:6417 - 22; http://dx.doi.org/10.1073/pnas.96.11.6417; PMID: 10339602
- Hartikka J, Sukhu L, Buchner C, Hazard D, Bozoukova V, Margalith M, et al. Electroporation-facilitated delivery of plasmid DNA in skeletal muscle: plasmid dependence of muscle damage and effect of poloxamer 188. Mol Ther 2001; 4:407 - 15; http://dx.doi.org/10.1006/mthe.2001.0483; PMID: 11708877
- Ulmer JB, Wahren B, Liu MA. Gene-based vaccines: recent technical and clinical advances. Trends Mol Med 2006; 12:216 - 22; http://dx.doi.org/10.1016/j.molmed.2006.03.007; PMID: 16621717
- Murtaugh MP, Foss DL. Inflammatory cytokines and antigen presenting cell activation. Vet Immunol Immunopathol 2002; 87:109 - 21; http://dx.doi.org/10.1016/S0165-2427(02)00042-9; PMID: 12072225
- Peachman KK, Rao M, Alving CR. Immunization with DNA through the skin. Methods 2003; 31:232 - 42; http://dx.doi.org/10.1016/S1046-2023(03)00137-3; PMID: 14511956
- Roos AK, Moreno S, Leder C, Pavlenko M, King A, Pisa P. Enhancement of cellular immune response to a prostate cancer DNA vaccine by intradermal electroporation. Mol Ther 2006; 13:320 - 7; http://dx.doi.org/10.1016/j.ymthe.2005.08.005; PMID: 16185933
- Liu J, Kjeken R, Mathiesen I, Barouch DH. Recruitment of antigen-presenting cells to the site of inoculation and augmentation of human immunodeficiency virus type 1 DNA vaccine immunogenicity by in vivo electroporation. J Virol 2008; 82:5643 - 9; http://dx.doi.org/10.1128/JVI.02564-07; PMID: 18353952
- Rosati M, Valentin A, Jalah R, Patel V, von Gegerfelt A, Bergamaschi C, et al. Increased immune responses in rhesus macaques by DNA vaccination combined with electroporation. Vaccine 2008; 26:5223 - 9; http://dx.doi.org/10.1016/j.vaccine.2008.03.090; PMID: 18468743
- Rosati M, Bergamaschi C, Valentin A, Kulkarni V, Jalah R, Alicea C, et al. DNA vaccination in rhesus macaques induces potent immune responses and decreases acute and chronic viremia after SIVmac251 challenge. Proc Natl Acad Sci U S A 2009; 106:15831 - 6; http://dx.doi.org/10.1073/pnas.0902628106; PMID: 19717425
- Grønevik E, Mathiesen I, Lømo T. Early events of electroporation-mediated intramuscular DNA vaccination potentiate Th1-directed immune responses. J Gene Med 2005; 7:1246 - 54; http://dx.doi.org/10.1002/jgm.760; PMID: 15822067
- Selby M, Goldbeck C, Pertile T, Walsh R, Ulmer J. Enhancement of DNA vaccine potency by electroporation in vivo. J Biotechnol 2000; 83:147 - 52; http://dx.doi.org/10.1016/S0168-1656(00)00308-4; PMID: 11000470
- Tollefsen S, Tjelle T, Schneider J, Harboe M, Wiker H, Hewinson G, et al. Improved cellular and humoral immune responses against Mycobacterium tuberculosis antigens after intramuscular DNA immunisation combined with muscle electroporation. Vaccine 2002; 20:3370 - 8; http://dx.doi.org/10.1016/S0264-410X(02)00289-X; PMID: 12213407
- Phase I of Human Papillomavirus (HPV) DNA Plasmid. (VGX-3100) + Electroporation for CIN 2 or 3. http://www.clinicaltrials.gov/ct2/show/NCT00685412.
- Fourth Dose of Human Papillomavirus (HPV) DNA Plasmid. (VGX-3100) + EP in Adult Females Previously Vaccinated With Three Doses of VGX-3100. http://www.clinicaltrials.gov/ct2/show/NCT01188850.