Abstract
Interleukin-22 (IL-22) is mainly produced by activated Th1 cells, Th17 cells and NK cells and promotes anti-microbial defense, pro-inflammatory and tissue remodeling responses. However, its potential use as a vaccine adjuvant has not been tested. In this study, we tested if a DNA construct expressing IL-22 (pVAX-IL-22) could be used as a molecular adjuvant to enhance host immune responses induced by HBV DNA vaccination (pcD-S2). After immunizing mice with pcD-S2 combined with pVAX-IL-22, we didn’t find enhancement of HBsAg-specific antibody responses in comparison to mice immunized with pcD-S2 alone. However, there was an enhancement of the level of IL-17 expression in antigen specific CD8+ cytotoxic T lymphocytes (Tc17). By using CD8 T-cell knockout (KO) and IL-17 KO mice, Tc17 cells were found to be a dominant population driving cytotoxicity. Importantly, there was a correlation between pVAX-IL-22 enhancement of T lymphocytes and a reduction of HBsAg-positive hepatocytes in HBsAg transgenic mice. These results demonstrate that IL-22 might be used as an effective adjuvant to enhance cellular immune responses during HBsAg DNA vaccination since it can induce Tc17 cells to break tolerance in HBsAg transgenic mice.
Keywords: :
Introduction
Chronic viral hepatitis B, is an infectious inflammatory disease of liver caused by hepatitis B virus (HBV),Citation1-Citation3 and it remains a large public health problem in the world. HBV is a species of the genus Orthohepadnavirus, part of the Hepadnaviridae family of viruses that cause chronic liver disease and are transmitted via body fluids.Citation4-Citation6 Infection can lead to cirrhosis and hepatocellular carcinoma. About 350 million people worldwide are HBV chronic carriers. It is estimated that 1 million people die each year from HBV infection and it’s complications.Citation7,Citation8
Although several recombinant protein based vaccines have been developed and shown to prevent hepatitis B virus infection in people successfully, certain percentage of people does not respond this kind of vaccine well. Furthermore, the current recombinant vaccines have not been effective to clear viral infected cells in hosts. This is mostly due to that this type of vaccines are the weaker inducers for a powerful cellular immunity, especially CD8+ T-cell-mediated immunity that is required to clear the virus infection. Recently, reports have described IFN-γ-producing Tc1 cells and IL-17-producing Tc17 cells involved in efficient clearance of HBV and influenza A virus infections.Citation9 Tc1 cells mainly clean up virus by perforin-mediated cytolytic activity, while the Tc17 cells rely on the Fas-FasL pathway.Citation10,Citation11
DNA vaccination has emerged as an attractive approach for immunotherapeutic vaccine development.Citation12 HBV DNA vaccination effectively induces CD8+ T-cell activation in mice, but the effect has been reported to be weak in human trials when no adjuvant is used.Citation13,Citation14 The addition of adjuvants may facilitate therapeutic HBV DNA vaccine development.
Interleukin-22, also known as IL-TIF (IL-10-related T-cell-derived inducible factor), belongs to a family of cytokines structurally related to IL-10 that includes IL-19 and IL-26. Initially, IL-22 was identified by DumoutieCitation15,Citation16 as the product of a gene specifically induced by IL-9 in mouse T cells. Unlike IL-10, IL-22 signals through a receptor complex consisting of IL-22RA1 and IL-10RB subunits, the latter being shared with the IL-10R. IL-22 plays an important role in inflammation, including chronic inflammatory and infectious diseases.Citation17-Citation19 IL-22 induces antimicrobial proteins such as S100 family molecules (S100A7, S100A8, and S100A9), α-defensins, lipocalin-2, and CXCL5 chemokine in keratinocytes and mucosal surfaces.Citation20-Citation23 IL-22-injected mice showed acute reactive protein expression in hepatocytes. Accumulated evidence also shows that IL-22 can be associated with autoimmune diseases and pulmonary inflammation. AujlaCitation24 found that IL-22 increased pneumonia by inducing lipocalin-2. Conversely, intestinal IL-22 produced by innate lymphoid cells acted as a critical regulator of tissue sensitivity to graft-vs.-host disease (GVHD) and a protector during inflammatory damage.Citation25,Citation26 Studies also showed that IL-22 appeared to be an important mediator of inflammatory response and played as a protective role in chronically HBV infected liver.Citation27-Citation29 Taken all together, the existing evidence makes it unclear whether IL-22 can be a candidate adjuvant to enhance HBV DNA vaccine cellular responses.
In our study, we examined whether IL-22 could act a molecular adjuvant with HBV DNA vaccine. When we used pVAX-IL-22 plasmid together with HBV DNA we elicited IL-17 producing- CD8+ T cells and a strong CTL response. Further study using HBsAg transgenic mice indicated a correlation between the level of IL-22-enhanced CTL and reduction of HBsAg-positive hepatocytes. Thus, IL-22 may be exploited as a potent adjuvant for DNA vaccines through inducing a strong Tc17 response.
Results
Cloning of murine IL-22 and expression in BHK cells
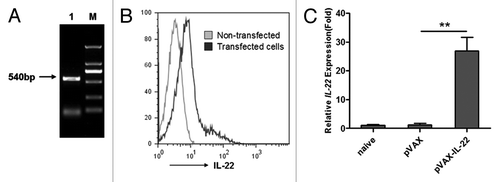
To generate the mouse IL-22 expression plasmid pVAX-IL-22, 5 pairs of mouse IL-22 primers were designed and the full-length IL-22 (540bp) nucleotide sequence was amplified by overlap PCR, verified by sequencing, and then sub-cloned into eukaryotic expression vector pVAX (). To test the expression of protein IL-22, we transfected pVAX-IL-22 plasmid into BHK cells. Forty-eight hours after the transfection, cells were used in intracellular staining analysis with anti-IL-22-PE. The expression of IL-22 was observed as shown in . Expression of IL-22 in mouse muscle tissue was found by quantitative RT-PCR on mRNA extracted on day 3 after the initial immunization ().
Figure 1. Cloning of murine IL-22 and expression in vitro and vivo; (A) The DNA sequence of IL-22 was cloned by overlap PCR (Lane M: DL2000 markers, Lane 1: the cloned IL-22); (B) 48 h after transfection with pVAX-IL-22, BHK cells and non-transfected cells were intracellularly stained with anti-IL-22-PE and analyzed by FACSCalibur; (C) RNA was extracted from injected mouse muscle on day 3 after primary immunization. The expression of IL-22 gene was quantified by Q-PCR, normalized with GAPDH (**p < 0.01).
IL-22 as a molecular adjuvant does not enhance anti-HBsAg humoral response
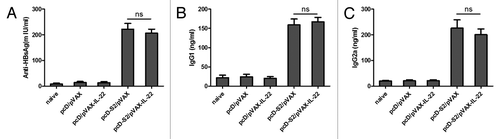
Humoral immunity can be an important component of overall responses to DNA vaccination. To evaluate the adjuvant effect of IL-22 on humoral responses, C57BL/6 mice were immunized three times and serum was collected on day 7 after the third immunization. Serum IgG, IgG1 and IgG2a antibodies against HBsAg were determined by quantitative ELISA. Compared with the group immunized with pcD-S2 plus pVAX empty vector, no significant change was seen in the levels of HBsAg-specific total IgG, IgG1 or IgG2a in the group immunized with pcD-S2 plus pVAX-IL-22 (). Hence, these findings suggested that IL-22 as a molecular adjuvant did not augment humoral immune responses to the DNA vaccine.
Figure 2. IL-22 as a molecular adjuvant failed to enhance anti-HBsAg humoral response. Sera from immunized mice was collected and pooled 7 d after the third immunization and analyzed by ELISA; (A) Total IgG was quantified with a commercial IgG standard; (B) IgG1 and (C) IgG2a isotypes against HBsAg were quantified against dilutions of standard mouse IgG subtypes. Data shown are representative of three independent experiments (ns, p > 0.05).
Effects of IL-22 on cytokines expressions in CD4+ T and CD8+ T cells
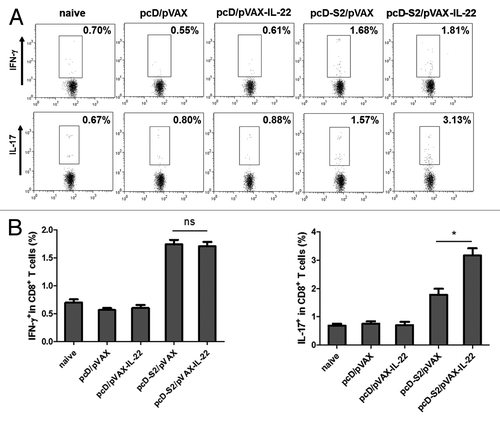
To evaluate T cell responses, total splenic cells were isolated at day 7 after the final vaccination and re-stimulated with I-Ab-restricted peptide (HBsAg aa126–138, RGLYFPAGGSSSG) or H-2Kb-restricted peptide (HBsAg aa208–216, ILSPFLPLL). After intracellular staining, FACS assay was performed with gates set on CD4+ T and CD8+ T cells. As shown in , and , we observed that pcD-S2 plus pVAX-IL-22 induced a higher level of expression of IL-17 in antigen-specific CD8+ T cells. In contrast, the percentages of cells expressing cytokines (IL-4, IFN-γ and IL-17 in CD4+ T cells and IFN-γ in CD8+ T cells) were not changed when compared with the effect of pcD-S2 alone. The results indicated that IL-22 could evoke an IL-17-producing subset of CD8+ effector cells known as Tc17 cells.
Figure 3. Effect of IL-22 as an adjuvant on antigen-specific cytokine production in CD4+ T cells; (A) Expression of IFN-γ, IL-4 and IL-17 in CD4+ T cells detected after 6 h stimulation in vitro with HBs126–138 (RGLYFPAGGSSSG). T cells were isolated from C57BL/6 mice on day 7 after final immunization. Intracellular staining of IFN-γ, IL-4 and IL-17 in CD4+ T cells was determined by FACS; (B) Summary of the data in panel A (*p < 0.05; ns, p > 0.05).
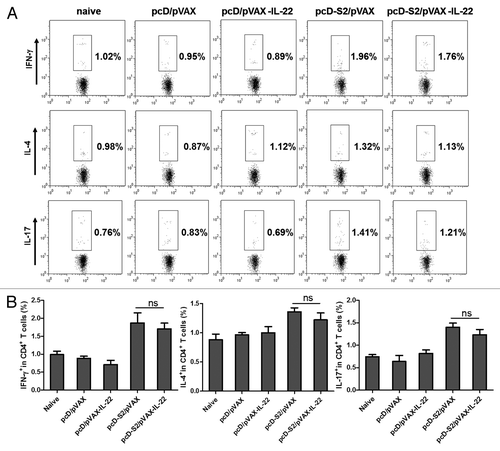
Figure 4. Effects of IL-22 as an adjuvant on antigen-specific cytokine productions in CD8+ T cells; (A) T cells were isolated from C57BL/6 mice on day 7 after final immunization and expression of IFN-γ, and IL-17 in CD8+ T cells was detected after 6h stimulation in vitro with S208–216 (ILSPFLPLL). Intracellular staining of IFN-γ and IL-17 in CD8+ T cells was determined by FACS; (B) Summary of the data in panel A (*p < 0.05; ns, p > 0.05).
IL-22 adjuvant effect on HBsAg-specific cytotoxic response mainly mediated Tc17 cells
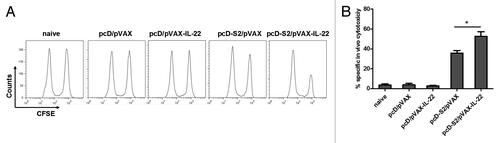
CTL responses play a key role in clearance of infections with viruses and other intracellular pathogens. To test whether IL-22 could enhance an HBsAg-specific CTL response, an in vivo cytotoxic assay was performed on day 7 after the third immunization. As shown in , mice immunized with pcD-S2 plus pVAX-IL-22 had a significantly augmented cytotoxic response against HBsAg compared with mice immunized with pcD-S2 alone.
Figure 5. Effects of IL-22 on antigen-specific cytotoxic T lymphocyte responses to HBV DNA vaccination; (A) CFSE-labeled spleen cells were transferred to immunized mice and cytotoxicity was assayed as described in Methods; (B) The percentage of specific lysis, summarized as the means and SEM of the three independent experiments (*p < 0.05).
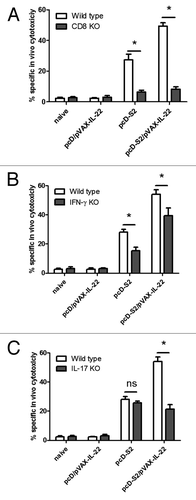
To test the roles for CD8, IFN-γ and IL-17, we immunized CD8 KO mice, IL-17 KO mice and IFN-γ KO mice with pcD-S2 plus pVAX-IL-22. At day 7 after the third immunization, analysis of HBsAg-specific cytotoxic response was performed. As shown in , CTL were completely absent from the CD8 KO mice immunized with or without pVAX-IL-22. In IFN-γ KO mice, the CTL level in pcD-S2 immunized mice was about 50% lower than that of the WT group, whereas the CTL level in the pcD-S2 plus pVAX-IL-22 group was only about 15% reduced compared with the WT counterpart (). Antigen-specific cytotoxicity in IL-17 KO mice was reduced about 60% compared with the wild type counterparts after immunization with pcD-S2 plus pVAX-IL-22. However, this reduction of CTL was not seen in the pcD-S2 immunized IL-17 KO group (). These findings suggest that IL-22 as a molecular adjuvant could augment HBsAg-specific CD8+ T cell-mediated cytolytic responses in mice mainly via activation of a Tc17 cell subset, less so via Tc1.
Figure 6. Impairment of antigen-specific cytotoxic T lymphocyte response in CD8 KO mice and IL-17 KO mice. Mice were immunized with HBV DNA vaccine and pVAX-IL-22 plasmid. CTL assay was performed 7 d after the last immunization as described in Methods. The percentage of specific lysis and SEM is shown for (A) CD8 KO mice and wild type mice; (B) IL-17 KO mice and WT mice; (C) IFN-γ KO mice and WT mice. In each case, the results are representative of three independent experiments (*p < 0.05).
IL-22-induced reduction of HBsAg-positive hepatocytes
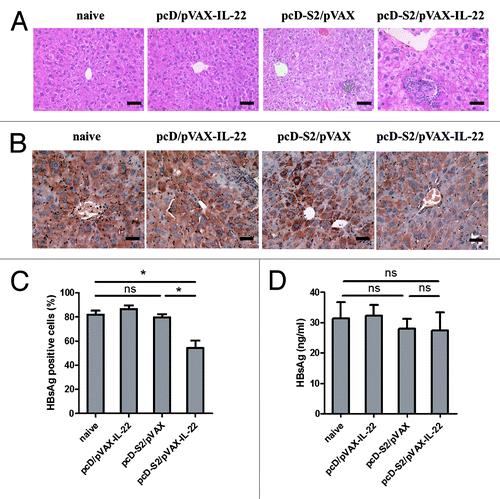
To examine the ability of IL-22 and Tc17 cells to participate in elimination of HBsAg-positive hepatocytes, HBsAg transgenic mice were immunized in the presence or absence of pVAX-IL-22. Liver histology study by H&E stain 14 d after the fourth immunization was performed. IL-22 adjuvant-enhanced T lymphocyte infiltration is evident and showed in . To examine if HBsAg-positive cells were reduced in the mice immunized with pcD-S2 plus pVAX-IL-22, anti-HBsAg antibodies were used to stain the hepatocytes. As depicted in , a reduced level of HBsAg positive hepatocytes was exhibited in pcD-S2 plus pVAX-IL-22 immunized group. However, this reduction in HBsAg was not accompanied in sera (). Thus, these findings suggested that IL-22 as adjuvant increased Tc17 cells which may have contributed to the partial elimination of HBsAg positive hepatocytes.
Figure 7. Histopathology of liver and immunochemical staining in HBsAg-transgenic mice; (A) Livers from each group were isolated on day 14 after the final immunization and fixed, sectioned, and then stained with H&E. Bar = 50 μm; (B) Specific immunostaining of HBsAg on day 14 after final immunization. Bar = 50 μm; (C) The percentage of HBsAg-positive hepatocytes was determined by light microscopy; (D) The concentration of HBsAg in sera on day 14 after final immunization was detected by ELISA. There were four mice in each group (* p < 0.05; ns, p > 0.05).
Discussion
This is the first study to demonstrate an adjuvant effect of IL-22 for DNA vaccination. We found that the use of IL-22 as a molecular adjuvant could significantly augment the levels of IL-17-producing CD8+ T cells and HBsAg-specific CTL elicited by HBV DNA vaccine. In addition, we demonstrated that the IL-22-enhanced antigen-specific killing of HBsAg-positive hepatocytes in HBsAg transgenic mice. Enhanced killing depended upon IL-17-production by CD8+ T cells (Tc17) since the effect was absent from CD8 KO mice and from IL-17 KO mice. This adjuvant effect may be due to a high expression of innate immune cytokine CXCL1 and β-defensins induced in total splenic cells with IL-22 as adjuvant.
IL-22, a member of the IL-10 family cytokines, is produced by activated T cells (Th17 cells, Th22 cells) and natural killer (NK) cells.Citation24,Citation30,Citation31 It acts via a heterodimeric receptor complex composed of IL-22R1 and IL-10R2.Citation32-Citation34 To date, IL-22 is known to have biological functions in promoting anti-microbial defense, being anti-apoptotic, protecting liver against damage and facilitating tissue remodeling. Previous studies showed that IL-22 can upregulate keratinocyte genes for anti-microbial peptides including β-defensins (S100A7, S100A8), CXCL5 and MMP3.Citation35-Citation37 IL-22 can enhance innate immune responses, inducing acute phase proteins in ConA-induced hepatitis, in protecting liver from damage,Citation38,Citation39 including from fibrosis and chronic hepatitis B infection,Citation40,Citation41 but little is known about the effect of IL-22 on adaptive immune responses when it is used as a molecular adjuvant.
Cytotoxic CD8+ T cells are considered to be a key factor in host antiviral responses. Two subsets of CD8+T cells are now known to enhance cytotoxic responses, namely IFN-γ-producing CD8+ T cells (Tc1) and IL-17-producing CD8+ T cells (Tc17).Citation10,Citation11,Citation42,Citation43 Zou showed that a chemical adjuvant, praziquantel (PZQ), could be used to elicit Tc17 cells in HBV DNA vaccination and contributed to viral antigen clearance in a HBsAg-transgenic mouse model.Citation11 Tc1 is a classical cytotoxic CD8+ T cell, producing IFN-γ and TNF-α and cytolytic effects by secreting perforin and granzyme B when in contact with target cells.Citation44-Citation46 However, Tc17 cells reportedly enhance anti-fungal responses via neutrophil recruitment, so may not exert their cytotoxic activity directly.Citation10,Citation47 In addition, Tc17 could acquire cytotoxic potential in vivo without IFN-γ being present in a vaccinia virus (VV) model and this was associated with Fas-Fasl interaction.Citation10,Citation48 Here, we observed that IL-22 as a molecular adjuvant could induce a Tc17 cell subpopulation (), which was associated with a high level of cytolytic activities as shown in ; in an HBsAg transgenic mice model the percentage of HBsAg-positive hepatocytes was reduced by 40%. The cytolytic activity was also greatly reduced in the IL-17 KO and CD8+ T KO mice (), confirming that IL-22 as adjuvant could induce Tc17-dependent CTL. Whether the elicited Tc17 could recruit neutrophils or enhance FasL expression remains unknown. Vaccine-induced memory CD8+ T play an important role in vaccine development against virus.Citation49 The long-term memory response of IL-22 in HBV DNA vaccine needed to be investigated in the future.
Humoral immune responses also play a key role in protecting the host from viral invasions. However, we did not observe any beneficial effect on humoral response in the group immunized with pcD-S2 plus pVAX-IL-22 compared with the pcD-S2-immunized group (). This lack of effect of IL-22 on B cells should be further investigated.
We report for the first time that IL-22 as a molecular adjuvant can elicit a Tc17 (IL-17 producing CD8+ T cell) subpopulation, closely associated with an antigen-specific cytotoxic response and with downregulation of expression of HBsAg-positive hepatocytes that is induced in HBsAg transgenic mice by HBsAg DNA vaccination. Thus, IL-22 may be a useful candidate adjuvant for inducing Tc17 responses in the future DNA vaccine development.
Materials and Methods
Reagents and animals
Female C57BL/6 mice at 6–8 weeks of age were purchased from the Animal Institute of Chinese Medical Academy. HBsAg-transgenic mice (C57BL/6J-Tg (Alb1HBV) 44Bri/J), which express S, pre-S and X genes of HBV under the albumin promoterCitation50-Citation52 and IFN-γ KO (B6.129S7-Ifngtm1Ts/J) mice were purchased from the Jackson Laboratory. IL-17 KO mice (C57BL/6 background) were kindly provided by Richard Flavell (Yale University School of Medicine). All animals were housed with pathogen-free food and water under 12 h light-cycle conditions.
The HBsAg-derived peptides aa208–216 (ILSPFLPLL; H-2Kb-restricted) and I-Ab-restricted peptide (HBs aa126–138; RGLYFPAGGSSSG) were synthesized by Science Peptide Co.,Ltd. Fluorescent-labeled anti-mouse mAbs including anti-CD4-FITC (cat.no.11-0041), anti-CD8-APC (cat.no.17-0081), anti-IFN-γ-PE (cat.no.12-7311), anti-IL-17-PE (cat.no.12-7177) and anti-IL-4-PE (cat.no.12-7041) were purchased from eBioscience. The anti-HBsAg antibody for immune-histochemical staining was produced in C57BL/6 mice and serum was collected after immunizing 3 times with recombinant HBsAg and alum as adjuvant as previously described.Citation11
Plasmid construction and preparation
The HBV DNA vaccine containing HBV pre-S2 and S regions was cloned in pcDNA3.1 vector and designated as pcD-S2 and was prepared as described previously.Citation53 pcDNA3.1 vector was designated as pcD. To construct pVAX-IL-22 plasmid, five overlapping PCR pairs of murine IL-22-specific primers (NM_016971.2) were designed. The coding sequence for mouse IL-22 was obtained by the overlap PCR method, and sub-cloned into pVAX vector. Both plasmids were maxi-prepared by the alkaline method and purified with Qiagen Maxi prep kit (Qiagen Inc., cat.no.12165), then diluted in PBS.
Immunization
Mice were randomly divided into five groups (n = 6 each) and were immunized intramuscularly in thigh of right hind on days 0, 14, and 28 according to the design of different experiments, as listed in . For evaluate the adjuvant effect of IL-22, HBV transgenic mice were divided into 4 groups (n = 4 each).
Table 1. Immunization groups
Transfection of the BHK cell lines
The purified plasmid pVAX-IL-22 was transfected into Baby Hamster Kidney (BHK) cells with lipofectamine 2000 according to the manufacturer’s protocol (Invitrogen, cat.no.11668-019). The transfected cells were harvested at 48 h post-transfection and fixed with 4% paraformaldehyde and permeabilized with 0.5% saponin in PBS. Cells were intracellularly stained with anti-IL-22-PE (eBioscience, cat.no.12-7221-82) for 0.5 h on ice, and analyzed with FACSCalibur using Cell Quest ProSoftware (BD Biosciences).
Detection of anti-HBsAg-specific antibody
The detection of anti-HBsAg-specific antibodies in the serum was by quantitative ELISA. The sera from immunized mice were tested individually on day 7 after the third immunization. The 96 wells were coated with 2 μg/ml of rHBsAg at 4 °C overnight at 100 μl/well. Wells were subsequently blocked with 5% of no-fat milk in PBST for 1 h at 37 °C, and recoated with serial dilutions of mouse serum. After 5 time washes with PBST, 1:1000 diluted goat anti-mouse IgG, IgG1, or IgG2a conjugated with HPR was added to the wells, and the plates were incubated at 37 °C for 1 h. After five times of wash, 100 μl TMB buffer (Sigma, cat.no.T2885, 10 mg TMB powder dissolved in 200 μl H2O and 800 μl ethanol) was added to each well. The reaction was stopped by the addition of 2 M of H2SO4. OD values at 450/620 nm were analyzed with a plate reader (Magellan). The OD ratios at a standard serum dilution were calculated as the means of three independent assays.
In vivo CTL assay
In vivo CTL was previously described with some modifications.Citation53 Briefly, splenocytes from naive C57BL/6 mice were pulsed with 10−6 M HBsAg peptide and labeled with a high concentration of CFSE (20 μM, CFSEhigh cells) to provide target cells. A portion of the same splenocyte suspension was labeled with a low concentration of CFSE (1 μM, CFSElow cells) during incubation without peptide to provide a non-target control. The target and control cells were mixed in a 1:1 ratio, and injected into immunized mice at 2 × 10Citation7 total cells per mouse via the tail vein on day 7 after the third immunization. Eight hours later, the spleens were isolated and analyzed for differential CFSE fluorescence intensities using FACSCalibur. Specific lysis was calculated using the following formula: ratio = percentage CFSE low/percentage CFSE high. Percentage specific lysis = [1-(ratio unprimed/ratio primed) × 100].
Flow cytometric analysis
Splenic cells were isolated on day 7 after the third immunization. Splenocytes were adjusted to 2 × 10Citation6 cells/ml, and stimulated with HBsAg peptide (S208–215, HBs126–138, 10 μg/ml) for 6 h at 37 °C and 5% CO2 in the presence of brefeldin A (BD PharMingen, cat.no.555029, 5 μg/ml). The cells were blocked with Fc-Block (BD PharMingen, cat.no.553140) in PBS for 30 min at 4 °C before being fixed with 4% paraformaldehyde and permeabilized with saponin, then stained for IFN-γ, IL-17, IL-4, and surface CD4 and CD8 for 1.5 h at 4 °C. The cells were washed then analyzed with FACSCalibur using CellQuest Pro Software.
Real-time PCR analysis
Total RNA was isolated with a Total RNA Extraction Kit (Omega, cat.no.R6834-01) and used for cDNA synthesis using the Toyobo ReverTra Ace (TOYOBO, cat.no.TRT-101) and oligo(dT)18 primers. q-PCR was done using an ABI 7900 Real Time PCR system with SYBR Green Realtime PCR Master Mix-plus-Kit (TOYOBO, cat.no.QPK-212T). Gene expression was normalized with the housekeeping gene GAPDH. IL-22: forward; 5′-TTGAGGTGTC CAACTTCCAG CA-3′: reverse; 5′-AGCCGGACGT CTGTGTTGTT A-3′; GAPDH: forward; 5′-GCACAGTCAA GGCCGAGAAT-3′: reverse; 5′-GCCTTCTCCA TGGTGGTGAA-3′.
Histopathology
Fourteen days after the final immunization, liver samples were collected from each mouse group, fixed in 4% paraformaldehyde and embedded in paraffin blocks. Paraffin-embedded samples were cut into 5μm thick sections and antigen retrieval was accomplished by boiling the slides in 0.01 M citrate buffer (pH 6.0) followed by staining with H&E or immunostaining with anti-HBsAg. Sections were incubated with anti-HBs overnight at 4 °C in a humidified chamber after blocking endogenous peroxidase with 0.3% H2O2. Slides were incubated with goat-anti-mouse secondary antibody for 30min. After 3 time washes with PBS, staining was performed with DAB (3,3′-diaminobenzidine). Ten visual fields in × 400 magnification of each section were analyzed under a light microscope to determine histological changes.
Statistical analysis
Results are presented as means plus standard error of the mean (SEM). Nonparametric test was used for statistical assay (p < 0.05 was considered statistically significant).
Acknowledgments
This work was supported in part by Chinese National Natural Science Foundation (30930068) and the National High-Tech 863 Project of China (2010AA022907) to BW. We would like to thank Dr Douglas Lowrie for his critical review of the manuscript and Dr Jane QL Yu, Dr Shuang Geng, and Mr Zhonghuai He for their assistance in this work.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- Arora D, Arora B, Khetarpal A. Seroprevalence of HIV, HBV, HCV and syphilis in blood donors in Southern Haryana. Indian J Pathol Microbiol 2010; 53:308 - 9; http://dx.doi.org/10.4103/0377-4929.64295; PMID: 20551540
- Guo Y, Ren D, He X, Wang F, Jiang L, Song S, He Y, Sun S. A novel mouse model for immunogenic evaluation of human HBV vaccines. Vaccine 2009; 27:5692 - 9; http://dx.doi.org/10.1016/j.vaccine.2009.06.088; PMID: 19615480
- Takahashi K, Brotman B, Usuda S, Mishiro S, Prince AM. Full-genome sequence analyses of hepatitis B virus (HBV) strains recovered from chimpanzees infected in the wild: implications for an origin of HBV. Virology 2000; 267:58 - 64; http://dx.doi.org/10.1006/viro.1999.0102; PMID: 10648183
- Chen H, Chuai X, Deng Y, Wen B, Wang W, Xiong S, Ruan L, Tan W. Optimisation of prime-boost immunization in mice using novel protein-based and recombinant vaccinia (Tiantan)-based HBV vaccine. PLoS One 2012; 7:e43730; http://dx.doi.org/10.1371/journal.pone.0043730; PMID: 22970140
- Dandri M, Lütgehetmann M, Petersen J. Experimental models and therapeutic approaches for HBV. Semin Immunopathol 2013; 35:7 - 21; http://dx.doi.org/10.1007/s00281-012-0335-7; PMID: 22898798
- Seed CR, Jones NT, Pickworth AM, Graham WR. Two cases of asymptomatic HBV “vaccine breakthrough” infection detected in blood donors screened for HBV DNA. Med J Aust 2012; 196:651 - 2; http://dx.doi.org/10.5694/mja11.11589; PMID: 22676882
- Evans AA, London WT, Gish RG, Cohen C, Block TM. Chronic HBV infection outside treatment guidelines: is treatment needed?. Antivir Ther 2013; 18:229 - 35; http://dx.doi.org/10.3851/IMP2325; PMID: 22914436
- Changotra H, Sehajpal PK. Quantitative detection of serum HBV DNA levels employing a new S gene based cPCR assay. Arch Virol 2005; 150:481 - 91; http://dx.doi.org/10.1007/s00705-004-0432-6; PMID: 15747164
- Zou Q, Wu B, He X, Zhang Y, Kang Y, Jin J, Xu H, Liu H, Wang B. Increasing a robust antigen-specific cytotoxic T lymphocyte response by FMDV DNA vaccination with IL-9 expressing construct. J Biomed Biotechnol 2010; 2010:562356; PMID: 20445754
- Yeh N, Glosson NL, Wang N, Guindon L, McKinley C, Hamada H, Li Q, Dutton RW, Shrikant P, Zhou B, et al. Tc17 cells are capable of mediating immunity to vaccinia virus by acquisition of a cytotoxic phenotype. J Immunol 2010; 185:2089 - 98; http://dx.doi.org/10.4049/jimmunol.1000818; PMID: 20624947
- Zou Q, Yao X, Feng J, Yin Z, Flavell R, Hu Y, Zheng G, Jin J, Kang Y, Wu B, et al. Praziquantel facilitates IFN-γ-producing CD8+ T cells (Tc1) and IL-17-producing CD8+ T cells (Tc17) responses to DNA vaccination in mice. PLoS One 2011; 6:e25525; http://dx.doi.org/10.1371/journal.pone.0025525; PMID: 21998665
- Geng S, Zhong Y, Wang S, Liu H, Zou Q, Xie X, Li C, Yu Q, He Z, Wang B. Amiloride enhances antigen specific CTL by faciliting HBV DNA vaccine entry into cells. PLoS One 2012; 7:e33015; http://dx.doi.org/10.1371/journal.pone.0033015; PMID: 22438887
- Yang FQ, Yu YY, Wang GQ, Chen J, Li JH, Li YQ, Rao GR, Mo GY, Luo XR, Chen GM. A pilot randomized controlled trial of dual-plasmid HBV DNA vaccine mediated by in vivo electroporation in chronic hepatitis B patients under lamivudine chemotherapy. J Viral Hepat 2012; 19:581 - 93; http://dx.doi.org/10.1111/j.1365-2893.2012.01589.x; PMID: 22762143
- Ferraro B, Morrow MP, Hutnick NA, Shin TH, Lucke CE, Weiner DB. Clinical applications of DNA vaccines: current progress. Clin Infect Dis 2011; 53:296 - 302; http://dx.doi.org/10.1093/cid/cir334; PMID: 21765081
- Lemaire MM, Vanhaudenarde A, Nizet Y, Dumoutier L, Renauld JC. Induction of autoantibodies against mouse soluble proteins after immunization with living cells presenting the autoantigen at the cell surface in fusion with a human type 2 transmembrane protein. J Immunol Methods 2011; 367:56 - 62; http://dx.doi.org/10.1016/j.jim.2011.02.001; PMID: 21334341
- Dumoutier L, Van Roost E, Ameye G, Michaux L, Renauld JC. IL-TIF/IL-22: genomic organization and mapping of the human and mouse genes. Genes Immun 2000; 1:488 - 94; http://dx.doi.org/10.1038/sj.gene.6363716; PMID: 11197690
- Ciccia F, Guggino G, Rizzo A, Ferrante A, Raimondo S, Giardina A, Dieli F, Campisi G, Alessandro R, Triolo G. Potential involvement of IL-22 and IL-22-producing cells in the inflamed salivary glands of patients with Sjogren’s syndrome. Ann Rheum Dis 2012; 71:295 - 301; http://dx.doi.org/10.1136/ard.2011.154013; PMID: 21979002
- Nograles KE, Zaba LC, Shemer A, Fuentes-Duculan J, Cardinale I, Kikuchi T, Ramon M, Bergman R, Krueger JG, Guttman-Yassky E. IL-22-producing “T22” T cells account for upregulated IL-22 in atopic dermatitis despite reduced IL-17-producing TH17 T cells. J Allergy Clin Immunol 2009; 123:1244 - , e2; http://dx.doi.org/10.1016/j.jaci.2009.03.041; PMID: 19439349
- Zenewicz LA, Flavell RA. IL-22 and inflammation: leukin’ through a glass onion. Eur J Immunol 2008; 38:3265 - 8; http://dx.doi.org/10.1002/eji.200838655; PMID: 19016525
- Eyerich S, Wagener J, Wenzel V, Scarponi C, Pennino D, Albanesi C, Schaller M, Behrendt H, Ring J, Schmidt-Weber CB, et al. IL-22 and TNF-α represent a key cytokine combination for epidermal integrity during infection with Candida albicans. Eur J Immunol 2011; 41:1894 - 901; http://dx.doi.org/10.1002/eji.201041197; PMID: 21469124
- Wang P, Bai F, Zenewicz LA, Dai J, Gate D, Cheng G, Yang L, Qian F, Yuan X, Montgomery RR, et al. IL-22 signaling contributes to West Nile encephalitis pathogenesis. PLoS One 2012; 7:e44153; http://dx.doi.org/10.1371/journal.pone.0044153; PMID: 22952908
- Guilloteau K, Paris I, Pedretti N, Boniface K, Juchaux F, Huguier V, Guillet G, Bernard FX, Lecron JC, Morel F. Skin Inflammation Induced by the Synergistic Action of IL-17A, IL-22, Oncostatin M, IL-1alpha, and TNF-alpha Recapitulates Some Features of Psoriasis. J Immunol 2010; http://dx.doi.org/10.4049/jimmunol.0902464; PMID: 20335534
- Boniface K, Bernard FX, Garcia M, Gurney AL, Lecron JC, Morel F. IL-22 inhibits epidermal differentiation and induces proinflammatory gene expression and migration of human keratinocytes. J Immunol 2005; 174:3695 - 702; PMID: 15749908
- Aujla SJ, Chan YR, Zheng M, Fei M, Askew DJ, Pociask DA, Reinhart TA, McAllister F, Edeal J, Gaus K, et al. IL-22 mediates mucosal host defense against Gram-negative bacterial pneumonia. Nat Med 2008; 14:275 - 81; http://dx.doi.org/10.1038/nm1710; PMID: 18264110
- Hanash AM, Dudakov JA, Hua G, O’Connor MH, Young LF, Singer NV, West ML, Jenq RR, Holland AM, Kappel LW, et al. Interleukin-22 protects intestinal stem cells from immune-mediated tissue damage and regulates sensitivity to graft versus host disease. Immunity 2012; 37:339 - 50; http://dx.doi.org/10.1016/j.immuni.2012.05.028; PMID: 22921121
- Carlson MJ, West ML, Coghill JM, Panoskaltsis-Mortari A, Blazar BR, Serody JS. In vitro-differentiated TH17 cells mediate lethal acute graft-versus-host disease with severe cutaneous and pulmonary pathologic manifestations. Blood 2009; 113:1365 - 74; http://dx.doi.org/10.1182/blood-2008-06-162420; PMID: 18957685
- Zhang Y, Cobleigh MA, Lian JQ, Huang CX, Booth CJ, Bai XF, Robek MD. A proinflammatory role for interleukin-22 in the immune response to hepatitis B virus. Gastroenterology 2011; 141:1897 - 906; http://dx.doi.org/10.1053/j.gastro.2011.06.051; PMID: 21708106
- Feng D, Kong X, Weng H, Park O, Wang H, Dooley S, Gershwin ME, Gao B. Interleukin-22 promotes proliferation of liver stem/progenitor cells in mice and patients with chronic hepatitis B virus infection. Gastroenterology 2012; 143:188 - 98, e7; http://dx.doi.org/10.1053/j.gastro.2012.03.044; PMID: 22484119
- Cobleigh MA, Robek MD. Protective and pathological properties of IL-22 in liver disease: implications for viral hepatitis. Am J Pathol 2013; 182:21 - 8; http://dx.doi.org/10.1016/j.ajpath.2012.08.043; PMID: 23159948
- Wolk K, Sabat R. Interleukin-22: a novel T- and NK-cell derived cytokine that regulates the biology of tissue cells. Cytokine Growth Factor Rev 2006; 17:367 - 80; http://dx.doi.org/10.1016/j.cytogfr.2006.09.001; PMID: 17030002
- Cella M, Fuchs A, Vermi W, Facchetti F, Otero K, Lennerz JK, Doherty JM, Mills JC, Colonna M. A human natural killer cell subset provides an innate source of IL-22 for mucosal immunity. Nature 2009; 457:722 - 5; http://dx.doi.org/10.1038/nature07537; PMID: 18978771
- Sonnenberg GF, Fouser LA, Artis D. Functional biology of the IL-22-IL-22R pathway in regulating immunity and inflammation at barrier surfaces. Adv Immunol 2010; 107:1 - 29; http://dx.doi.org/10.1016/B978-0-12-381300-8.00001-0; PMID: 21034969
- Jones BC, Logsdon NJ, Walter MR. Structure of IL-22 bound to its high-affinity IL-22R1 chain. Structure 2008; 16:1333 - 44; http://dx.doi.org/10.1016/j.str.2008.06.005; PMID: 18599299
- Kotenko SV, Izotova LS, Mirochnitchenko OV, Esterova E, Dickensheets H, Donnelly RP, Pestka S. Identification of the functional interleukin-22 (IL-22) receptor complex: the IL-10R2 chain (IL-10Rbeta ) is a common chain of both the IL-10 and IL-22 (IL-10-related T cell-derived inducible factor, IL-TIF) receptor complexes. J Biol Chem 2001; 276:2725 - 32; http://dx.doi.org/10.1074/jbc.M007837200; PMID: 11035029
- Schulz SM, Köhler G, Schütze N, Knauer J, Straubinger RK, Chackerian AA, Witte E, Wolk K, Sabat R, Iwakura Y, et al. Protective immunity to systemic infection with attenuated Salmonella enterica serovar enteritidis in the absence of IL-12 is associated with IL-23-dependent IL-22, but not IL-17. J Immunol 2008; 181:7891 - 901; PMID: 19017979
- Wolk K, Kunz S, Witte E, Friedrich M, Asadullah K, Sabat R. IL-22 increases the innate immunity of tissues. Immunity 2004; 21:241 - 54; http://dx.doi.org/10.1016/j.immuni.2004.07.007; PMID: 15308104
- Wolk K, Witte E, Wallace E, Döcke WD, Kunz S, Asadullah K, Volk HD, Sterry W, Sabat R. IL-22 regulates the expression of genes responsible for antimicrobial defense, cellular differentiation, and mobility in keratinocytes: a potential role in psoriasis. Eur J Immunol 2006; 36:1309 - 23; http://dx.doi.org/10.1002/eji.200535503; PMID: 16619290
- Wahl C, Wegenka UM, Leithäuser F, Schirmbeck R, Reimann J. IL-22-dependent attenuation of T cell-dependent (ConA) hepatitis in herpes virus entry mediator deficiency. J Immunol 2009; 182:4521 - 8; http://dx.doi.org/10.4049/jimmunol.0802810; PMID: 19342625
- Radaeva S, Sun R, Pan HN, Hong F, Gao B. Interleukin 22 (IL-22) plays a protective role in T cell-mediated murine hepatitis: IL-22 is a survival factor for hepatocytes via STAT3 activation. Hepatology 2004; 39:1332 - 42; http://dx.doi.org/10.1002/hep.20184; PMID: 15122762
- Xiang X, Gui H, King NJ, Cole L, Wang H, Xie Q, Bao S. IL-22 and non-ELR-CXC chemokine expression in chronic hepatitis B virus-infected liver. Immunol Cell Biol 2012; 90:611 - 9; http://dx.doi.org/10.1038/icb.2011.79; PMID: 21946664
- Xu M, Morishima N, Mizoguchi I, Chiba Y, Fujita K, Kuroda M, Iwakura Y, Cua DJ, Yasutomo K, Mizuguchi J, et al. Regulation of the development of acute hepatitis by IL-23 through IL-22 and IL-17 production. Eur J Immunol 2011; 41:2828 - 39; http://dx.doi.org/10.1002/eji.201141291; PMID: 21953641
- Tajima M, Wakita D, Satoh T, Kitamura H, Nishimura T. IL-17/IFN-γ double producing CD8+ T (Tc17/IFN-γ) cells: a novel cytotoxic T-cell subset converted from Tc17 cells by IL-12. Int Immunol 2011; 23:751 - 9; http://dx.doi.org/10.1093/intimm/dxr086; PMID: 22039016
- Zhu Y, Ju S, Chen E, Dai S, Li C, Morel P, Liu L, Zhang X, Lu B. T-bet and eomesodermin are required for T cell-mediated antitumor immune responses. J Immunol 2010; 185:3174 - 83; http://dx.doi.org/10.4049/jimmunol.1000749; PMID: 20713880
- Mazur MA, Davis CC, Szabolcs P. Ex vivo expansion and Th1/Tc1 maturation of umbilical cord blood T cells by CD3/CD28 costimulation. Biol Blood Marrow Transplant 2008; 14:1190 - 6; http://dx.doi.org/10.1016/j.bbmt.2008.07.016; PMID: 18804050
- Kleen TO, Asaad R, Landry SJ, Boehm BO, Tary-Lehmann M. Tc1 effector diversity shows dissociated expression of granzyme B and interferon-gamma in HIV infection. AIDS 2004; 18:383 - 92; http://dx.doi.org/10.1097/00002030-200402200-00003; PMID: 15090789
- Wizel B, Nunes M, Tarleton RL. Identification of Trypanosoma cruzi trans-sialidase family members as targets of protective CD8+ TC1 responses. J Immunol 1997; 159:6120 - 30; PMID: 9550413
- Nanjappa SG, Heninger E, Wüthrich M, Gasper DJ, Klein BS. Tc17 cells mediate vaccine immunity against lethal fungal pneumonia in immune deficient hosts lacking CD4+ T cells. PLoS Pathog 2012; 8:e1002771; http://dx.doi.org/10.1371/journal.ppat.1002771; PMID: 22829762
- Hamada H, Garcia-Hernandez MdeL, Reome JB, Misra SK, Strutt TM, McKinstry KK, Cooper AM, Swain SL, Dutton RW. Tc17, a unique subset of CD8 T cells that can protect against lethal influenza challenge. J Immunol 2009; 182:3469 - 81; http://dx.doi.org/10.4049/jimmunol.0801814; PMID: 19265125
- van Duikeren S, Fransen MF, Redeker A, Wieles B, Platenburg G, Krebber WJ, Ossendorp F, Melief CJ, Arens R. Vaccine-induced effector-memory CD8+ T cell responses predict therapeutic efficacy against tumors. J Immunol 2012; 189:3397 - 403; http://dx.doi.org/10.4049/jimmunol.1201540; PMID: 22914049
- Chisari FV, Klopchin K, Moriyama T, Pasquinelli C, Dunsford HA, Sell S, Pinkert CA, Brinster RL, Palmiter RD. Molecular pathogenesis of hepatocellular carcinoma in hepatitis B virus transgenic mice. Cell 1989; 59:1145 - 56; http://dx.doi.org/10.1016/0092-8674(89)90770-8; PMID: 2598264
- Wang J, Zhao W, Cheng L, Guo M, Li D, Li X, Tan Y, Ma S, Li S, Yang Y, et al. CD137-mediated pathogenesis from chronic hepatitis to hepatocellular carcinoma in hepatitis B virus-transgenic mice. J Immunol 2010; 185:7654 - 62; http://dx.doi.org/10.4049/jimmunol.1000927; PMID: 21059892
- Chisari FV, Pinkert CA, Milich DR, Filippi P, McLachlan A, Palmiter RD, Brinster RL. A transgenic mouse model of the chronic hepatitis B surface antigen carrier state. Science 1985; 230:1157 - 60; http://dx.doi.org/10.1126/science.3865369; PMID: 3865369
- Du X, Zheng G, Jin H, Kang Y, Wang J, Xiao C, Zhang S, Zhao L, Chen A, Wang B. The adjuvant effects of co-stimulatory molecules on cellular and memory responses to HBsAg DNA vaccination. J Gene Med 2007; 9:136 - 46; http://dx.doi.org/10.1002/jgm.1004; PMID: 17310492