Abstract
The ELISpot assay is used in vaccine studies for the quantification of antigen-specific memory B cells (BMEM), and can be performed using cryopreserved samples. The effects of cryopreservation on BMEM detection and the consistency of cultured ELISpot assays when performed by different operators or laboratories are unknown. In this study, blood was taken from healthy volunteers, and a cultured ELISpot assay was used to count BMEM specific for 2 routine vaccine antigens (diphtheria and tetanus toxoid). Results were assessed for intra- and inter-operator variation, and the effects of cryopreservation. Cryopreserved samples were shipped to a second laboratory in order to assess inter-laboratory variation. BMEM frequencies were very strongly correlated when comparing fresh and frozen samples processed by the same operator, and were also very strongly correlated when comparing 2 operators in the same laboratory. Results were slightly less consistent when samples were processed in different laboratories but correlation between the 2 measurements was still very strong. Although cell viability was reduced in some cryopreserved samples due to higher temperatures during transportation, BMEM could still be quantified. These results demonstrate the reproducibility of the ELISpot assay across operators and laboratories, and support the use of cryopreserved samples in future BMEM studies.
Introduction
The measurement of antigen-specific B cell numbers using Enzyme-Linked Immunosorbent Spot (ELISpot) assays has provided new insights into the biology of the immune response following immunization and has been used in many clinical vaccine trials.Citation1-Citation6 This method relies on the isolation of peripheral blood mononuclear cells (PBMCs) from blood specimens collected at study visits before and after a stimulus to the immune system such as an immunization. Although frequently used, the variability of the B cell ELISpot assay between different laboratories or operators or between fresh and frozen PBMC samples has not been formally studied. These parameters are important in order to combine data produced in different laboratories in multi-center studies or at different time points.
Storage and cryopreservation of human lymphocytes
Cryopreservation allows lymphocytes to be stored long-term before being used in immunological assays. It involves cooling the sample before transferring it into liquid nitrogen for long-term storage. In this way, samples from multiple time points may be stored until they can be analyzed simultaneously, reducing variation arising from different assay runs. Frozen samples may also be transported to a single laboratory for analysis, reducing potential variation introduced by different operators and laboratory conditions. It is important that freezing, storage, and thawing does not have an excessive negative impact on assay performance. One parameter which can be affected by cryopreservation is cell viability. A median viable cell recovery of 50% following the thawing of frozen PBMCs was reported in one study,Citation7 although there was no significant subsequent progressive reduction in the percentage of viable cells recovered after prolonged storage over the 12-y study period. A more recent study in which all samples were processed by a single experienced technician in the same laboratory, reported over 80% recovery of viable cells.Citation8
The effects of cryopreservation on detection of memory B cells have not previously been studied. The effect of freezing of PBMCs on B cell responses has been investigated by Kyu et al. who studied the antibody-secreting cell (ASC) response to 3 influenza antigens (H1, H3 and H7).Citation9 No difference in H1 and H3-specific ASC frequencies was found when comparing fresh and frozen samples, and detection of H7-specific ASCs was minimal but similar in both groups. The effect of cryopreservation on T cell responses has been more extensively studied, but results have been mixed. Although cryopreservation of PBMCs can alter cell surface markers,Citation10-Citation12 cytokine production,Citation11,Citation13 and cell proliferation,Citation14 such discrepancies can be minimized when an optimized protocol is used.Citation12,Citation15-Citation17 Preservation of cell function has been shown to be improved if PBMCs are frozen within 12 h of blood collectionCitation17 and temperature fluctuations are minimized during storage.Citation18
Variability of the ELISpot assay
The B cell ELISpot assay was initially designed for the detection of spontaneously antibody-secreting cells,Citation19 but has been further developed to enable quantification of memory B cells (BMEM) by the inclusion of a stimulation step in which BMEM differentiate into detectable antibody-secreting cells.Citation20 Although the basic principles remain unchanged, assay protocols are not standardized between laboratories and are continually undergoing optimization.Citation21 Similarly protocols for ELISpot assays detecting T cell cytokine secretion vary,Citation22 and studies of their reproducibility have shown substantial inter-laboratory variation.Citation23-Citation25 However, reproducible T cell ELISpot results can be achieved if assay and data analysis procedures are standardized across centers,Citation26 suggesting that the compilation of results from different laboratories may be appropriate in the context of multi-center clinical trials.
The aim of this study was to compare the results of quantification of antigen-specific BMEM using ELISpot when performed by different operators and in different laboratories using a standardized protocol and likewise to compare results using both fresh and frozen samples.
Materials and Methods
PBMC isolation and ELISpot
The cultured ELISpot assay to detect antigen-specific BMEM was performed as previously described.Citation27,Citation28 PBMCs were first isolated from whole blood using lymphoprep™ (Axis-Shield Diagnostics) density gradient centrifugation. After a wash step, PBMCs were cultured at a concentration of 2 × 105 cells/well in a mixture of SAC (S. aureus Cowan strain, 1:5000, Calbiochem), CpG oligodeoxynucleotide 2006 (1.8 µg/mL, Source Bioscience) and pokeweed mitogen (PWM, 83 ng/mL, Sigma) for 6 d at 37 °C, 5% CO2, and 95% humidity. Cells were then harvested, washed, and seeded at 2 × 105 viable cells per well onto a 96-well plate with PVDF-membranes (Millipore) pre-coated with diphtheria or tetanus toxoid. Plates also included phosphate buffered saline (PBS) wells and polyvalent goat anti-human Ig (10 μg/mL) wells as negative and positive controls respectively. Cell viability was assessed using the trypan blue dye exclusion test.Citation29 In this test, only cells with an intact cell membrane exclude the dye, therefore viable cells remain unstained. Following overnight incubation, plates were washed before incubating the wells with a goat anti-human IgG conjugated to alkaline phosphatase (Calbiochem) to detect bound antibody. The antibody was visualized using the alkaline phosphatase substrate kit (Bio-Rad) and the reaction stopped with distilled water. Plates were then dried overnight in a drying-oven before being read using an automated ELISpot reader (AID ELR03, AID Diagnostika).
Cryopreservation of PBMCs
Following PBMC separation, cells were re-suspended (5 × 106 cells/mL) in a cell freezing mix (Recovery™, Invitrogen, UK). One mL aliquots were placed into a freezing container ‘Mr Frosty’ (Nalgene®, VWR International, UK, pre-chilled at −20 °C), and held overnight at −80 °C before being transferred into liquid nitrogen for long-term storage. For thawing, vials were immersed in a 37 °C water bath and the PBMC solution transferred into 10 mL of pre-warmed medium (‘complete medium’), containing RPMI-1640 (Hepes modification), 10% newborn bovine serum, 1% Penicillin-Streptomycin, 1% L-Glutamine (all from Sigma-Aldrich), 1% MEM Non-Essential Amino Acids, 1% MEM Sodium Pyruvate 100 mM, and 0.1% 2-Mercaptoethanol 1000× (all from Invitrogen). Following a wash step, the cells were processed further as described above.
Study design
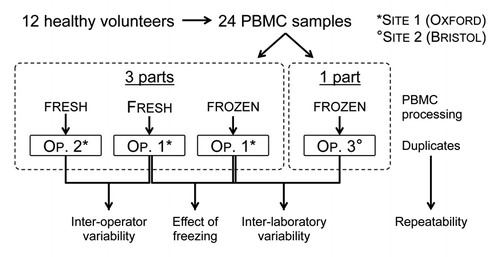
A prospective study was conducted across 2 sites. Twelve healthy volunteers each donated 40 mL of venous blood each divided into 2 aliquots anticoagulated with 350 μL preservative-free heparin, and PBMCs were isolated within 2 h at site 1. Samples were divided into 4 parts: 2 parts were processed immediately for culture and ELISpot, and 2 parts were cryopreserved for 6 mo. One of the cryopreserved samples was shipped to site 2, and the other was processed fully at site 1. This study design () allowed comparison between operators, between fresh and cryopreserved samples and between laboratories. Duplicate samples processed by each operator were assessed for intra-operator variability. During the 2 d transit period to site 2, cryopreserved samples were kept on dry ice, and were transferred to storage at −80 °C on arrival where they were kept for 1 wk before processing. Following thawing, cell viability was assessed before performing the ELISpot assay. ELISpot assays were performed using plates pre-coated with diphtheria and tetanus toxoids at a concentration of 10 and 5 μg/mL, respectively, both included among routinely administered vaccines.
Blood was taken from laboratory personnel at site 1 in agreement with Oxford University Occupational Health Service Policy document 1/03 for taking blood samples from colleagues or students for research (https://www.admin.ox.ac.uk/uohs/policies-guidance/blood/). Written informed consent was obtained from all study participants and samples were subsequently anonymized so that all operators were blinded to the study participant the samples came from during processing of the samples, and also when plates were read and counted.
Data analysis
All ELISpot plates were read and counted automatically using pre-defined settings at study site 1 and spot numbers were manually corrected for the presence of artifacts identified as spots by the machine. If artifacts had been included in the automatic spot count, this number was subtracted from the total spot count from that well. Antigen-specific spot counts were calculated as the mean of 4 wells minus the mean spot count from PBS control wells. Samples with a low spot count in IgG control wells were excluded. Spot counts were multiplied by 5 to obtain BMEM frequencies per million cultured PBMCs. All analyses were performed with these final frequencies, i.e., spots per million cultured PBMCs.
Correlation between samples was assessed using the Pearson correlation coefficient and correlation was graded as low (r-values between 0.2–0.39), moderate (0.4–0.59), strong (0.6–0.79), and very strong (≥0.8). Agreement between BMEM numbers obtained from duplicates of the same participants’ samples was assessed by generating Bland-Altman plots for each antigen and each operator. Overall, we defined assay reliability as acceptable for correlation coefficients ≥0.8. Statistical analysis and plots were performed using RCitation30 and the ggplot2 package for R.Citation31
Results
Numbers of viable PBMC obtained after thawing of frozen samples were significantly lower in samples which were kept on dry ice for transport to the other laboratory, both before and after culture ( and ). However, when adjusted for numbers of viable cells that were put into culture, post-culture viable cell counts were not different between the 2 groups (data not shown).
Figure 2. Number of viable PBMCs (A) following thawing of the samples and (B) after 6 d in culture. Significant differences in the PBMC counts were found both before and after culturing the cells between the frozen samples processed by operator 1 at site 1 and the frozen samples that were processed by operator 3, which were shipped to site 2. P values are calculated by two-tailed paired t test and horizontal bars indicate medians. Op, operator.
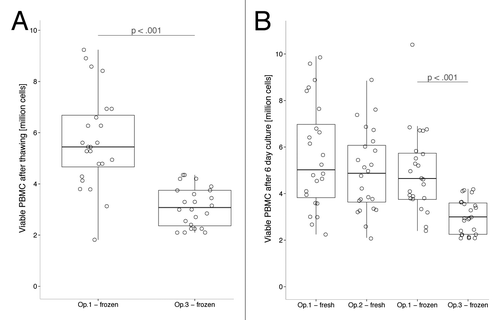
Table 1. Summary of PBMC counts following thawing and after the 6 d culture as determined by the different operators
A subset of automatically counted plates was corrected for artifacts independently by 2 operators who were blinded to the samples. A very strong correlation (r ≥ 0.97, P < 0.0001) between the 2 spot counts was found when numbers of spots for each sample were compared. Subsequently, analysis of all plates was performed using spot counts from operator 1.
When comparing diphtheria and tetanus toxoid-specific BMEM between 2 independent operators within the same laboratory using fresh PBMC samples (operator 1 fresh vs. operator 2 fresh), correlation was very strong ( and ).
Figure 3. Scatterplot matrix of diphtheria toxoid-specific ASC/106 cultured PBMC using (A) fresh samples operator 1 vs. operator 2, (B) fresh operator 2 (Oxford) vs. frozen operator 3 (Bristol), (C) fresh vs. frozen samples operator 1, and (D) frozen samples operator 1 (Oxford) vs. operator 3 (Bristol).
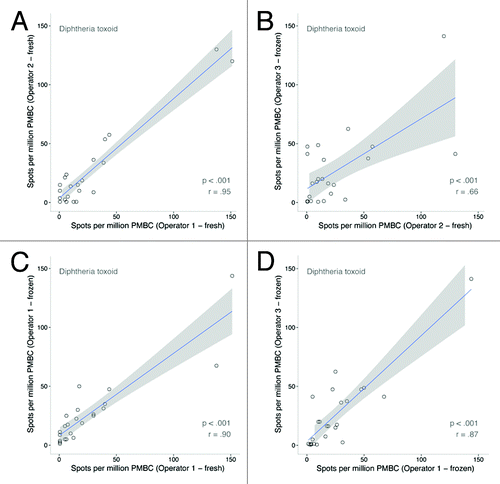
Figure 4. Scatterplot matrix of tetanus toxoid-specific ASC/106 cultured PBMC using (A) fresh samples operator 1 vs. operator 2, (B) fresh operator 2 [Oxford] vs. frozen operator 3 (Bristol), (C) fresh vs. frozen samples operator 1, and (D) frozen samples operator 1 (Oxford) vs. operator 3 (Bristol).
![Figure 4. Scatterplot matrix of tetanus toxoid-specific ASC/106 cultured PBMC using (A) fresh samples operator 1 vs. operator 2, (B) fresh operator 2 [Oxford] vs. frozen operator 3 (Bristol), (C) fresh vs. frozen samples operator 1, and (D) frozen samples operator 1 (Oxford) vs. operator 3 (Bristol).](/cms/asset/f432491b-57e2-450e-a9fb-46f41824f677/khvi_a_10929318_f0004.gif)
Comparison of fresh vs. frozen samples also showed very strong correlation for both diphtheria and tetanus toxoid-specific BMEM frequencies when processed by the same operator ( and ). Inter-laboratory correlation, i.e., samples being processed by 2 different operators in 2 different laboratories (operator 1 frozen vs. operator 3 frozen), was slightly lower but still significant when comparing diphtheria and tetanus toxoid-specific BMEM frequencies ( and ). Not unexpectedly, the lowest correlation was measured for both diphtheria and tetanus toxoid-specific BMEM when samples were processed by different operators in different laboratories with one operator working with fresh and the other operator working with frozen samples ( and ).
The correlation coefficients for each of the comparisons discussed is summarized in Figure S1, which shows that each additional change during processing was associated with a small reduction in the strength of correlation.
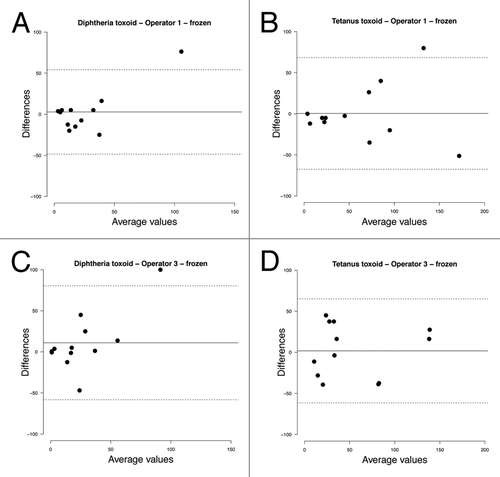
The agreements between the duplicate samples processed by each operator (intra-operator agreement) are shown in and , for fresh and frozen samples respectively. For fresh and frozen samples, systematic bias was equally low (0–11 spots/million cultured PBMCs) in both groups of samples compared with the mean of duplicate samples (continuous lines in and ). In general, the 95% limits of agreement were wider in frozen compared with fresh samples and for tetanus-specific compared with diphtheria-specific ASC frequencies (dashed lines in and ). For higher average values, variability increased as suggested by larger scatter around the bias line, which was especially true for tetanus-specific ASC frequencies.
Figure 5. Bland-Altman plots of intra-operator variability on fresh samples showing the difference between replicates processed by the same operator against the mean of 2 measurements. Bias (mean difference, continuous line) and 95% limits of agreement (dashed lines) are shown. (A) Operator 1, diphtheria toxoid; (B) Operator 1, tetanus toxoid; (C) Operator 2, diphtheria toxoid; (D) Operator 2, tetanus toxoid.
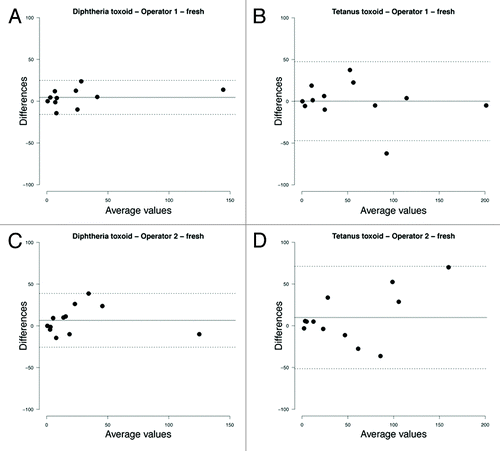
Figure 6. Bland-Altman plots of intra-operator variability on frozen samples showing the difference between replicates processed by the same operator against the mean of 2 measurements. Bias (mean difference, continuous line) and 95% limits of agreement (dashed lines) are shown. (A) Operator 1, diphtheria toxoid; (B) Operator 1, tetanus toxoid; (C) Operator 3, diphtheria toxoid; (D) Operator 3, tetanus toxoid.
Discussion
In this study, an ELISpot assay measuring numbers of BMEM was assessed for intra and inter-operator variability as well as inter-laboratory performance and the effect of freezing of PBMCs. We found that intra-operator agreement of sample replicates was best for fresh samples with somewhat greater variability between replicates for higher average spot counts. The limits of agreement were slightly wider for frozen samples but comparable between laboratories ( and ).
BMEM frequencies were very strongly correlated when 2 different operators within the same laboratory processed samples. This is an encouraging finding, as there are a number of stages in the ELISpot assay where variability may be introduced, particularly when different operators are processing samples. The method used to count BMEM when analyzing plates may also introduce variability,Citation32 therefore standardization of equipment and parameters for plate analysis are important. In this study, counting of spots in a semi-automated manner using pre-defined plate reading settings followed by manual correction for artifacts, resulted in a very strong correlation when assessed by 2 different operators.
BMEM counts from fresh and frozen samples were also very strongly correlated, demonstrating that cryopreservation does not have a negative impact on the detection of BMEM in the cultured ELISpot, consistent with studies demonstrating reliable ASC and T cell ELISpot results when using cryopreserved samples.Citation9,Citation15-Citation17 Although, as expected, fluctuating temperatures experienced by the cells in transit resulted in a lower yield of viable cells, antigen-specific BMEM frequencies could still be reliably measured. Compared with T cells, which have been shown to display reduced antigen-specific responses after experiencing fluctuating storage temperatures,Citation18 BMEM may be more resilient under these conditions. Although previous studies have shown that low cell viability following cryopreservation can compromise lymphocyte proliferation in response to mitogens including PWM,Citation33 the present study shows that after allowing for lower viable cell numbers following storage and shipment, freezing of PBMC did not affect the numbers of viable PBMCs that were harvested after a 6-d culture compared with fresh samples. This suggests that in this case, the remaining viable antigen-specific B cells responded to the polyclonal stimulators (PWM, CpG, and SAC) in the culture medium to the same degree as the fresh cells, and that these cells were not more susceptible to lower storage temperatures compared with other B cell subsets. Nevertheless, lower cell viability after shipping should be taken into account when planning the transport of samples between centers. Care should be taken to minimize the time cells are exposed to higher storage temperatures in order to maintain cell viability as much as possible.
As demonstrated in Figure S1, variability increases as additional processing variables are introduced e.g., when one sample is fresh and the other is frozen, or when samples are processed in different laboratories. Although correlation was lower when samples were processed in different laboratories, inter-laboratory variability was still relatively low and within the range of the variability that was found when 2 different operators within the same laboratory processed fresh samples, suggested by similar correlation coefficients between the 2 pairs ( and ). Reproducibility of the ELISpot assay between laboratories is important in order to combine data in the context of multi-center studies. We, along with investigators studying the reproducibility of T cell ELISpots,Citation26 have demonstrated that low inter-laboratory variability is achievable when using a standardized protocol.
Conclusion
In this study we found that the cultured B cell ELISpot is a robust and reproducible assay that can be performed on fresh or frozen samples and in different laboratories. Specific caution is advised when shipment of frozen samples to a different laboratory is considered, although antigen-specific BMEM frequencies can still be reliably measured even with a lower number of viable cells resulting from fluctuating temperatures during shipment and storage.
| Abbreviations: | ||
| BMEM | = | memory B cells |
| ELISpot | = | Enzyme-Linked Immunosorbent Spot |
| PBMCs | = | peripheral blood mononuclear cells |
| PBS | = | phosphate buffered saline |
| ASC | = | antibody-secreting cell |
Additional material
Download Zip (703.6 KB)Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding/Support
This study was funded in part by the UK Department of Health’s NIHR Oxford Biomedical Research Centre with support from the NIHR Thames Valley Comprehensive Local Research Network.
Acknowledgments
The authors are grateful to all the study participants, without whom this study would have not been possible and to the NIHR Oxford Biomedical Research Centre, the NIHR Thames Valley Comprehensive Local Research Network for funding. A.J.P. is a Jenner Institute Investigator and James Martin Senior Fellow. J.T. is the recipient of a European Society of Paediatric Infectious Diseases fellowship award and a James Martin Fellow.
References
- Clutterbuck EA, Lazarus R, Yu L-M, Bowman J, Bateman EAL, Diggle L, Angus B, Peto TE, Beverley PC, Mant D, et al. Pneumococcal conjugate and plain polysaccharide vaccines have divergent effects on antigen-specific B cells. J Infect Dis 2012; 205:1408 - 16; http://dx.doi.org/10.1093/infdis/jis212; PMID: 22457293
- Blanchard Rohner G, Snape MD, Kelly DF, John T, Morant A, Yu LM, Borkowski A, Ceddia F, Borrow R, Siegrist CA, et al. The magnitude of the antibody and memory B cell responses during priming with a protein-polysaccharide conjugate vaccine in human infants is associated with the persistence of antibody and the intensity of booster response. J Immunol 2008; 180:2165 - 73; http://dx.doi.org/10.4049/jimmunol.180.4.2165; PMID: 18250423
- Blanchard-Rohner G, Pulickal AS, Jol-van der Zijde CM, Snape MD, Pollard AJ. Appearance of peripheral blood plasma cells and memory B cells in a primary and secondary immune response in humans. Blood 2009; 114:4998 - 5002; http://dx.doi.org/10.1182/blood-2009-03-211052; PMID: 19843885
- Clarke ET, Williams NA, Findlow J, Borrow R, Heyderman RS, Finn A. Polysaccharide-specific memory B cells generated by conjugate vaccines in humans conform to the CD27+IgG+ isotype-switched memory B Cell phenotype and require contact-dependent signals from bystander T cells activated by bacterial proteins to differentiate into plasma cells. J Immunol 2013; 191:6071 - 83; http://dx.doi.org/10.4049/jimmunol.1203254; PMID: 24227777
- Clarke ET, Williams NA, Dull PM, Findlow J, Borrow R, Finn A, Heyderman RS. Polysaccharide-protein conjugate vaccination induces antibody production but not sustained B-cell memory in the human nasopharyngeal mucosa. Mucosal Immunol 2013; 6:288 - 96; http://dx.doi.org/10.1038/mi.2012.70; PMID: 22806100
- Mitchell R, Kelly DF, Pollard AJ, Trück J. Polysaccharide-specific B cell responses to vaccination in humans. Hum Vaccin Immunother 2014; 10:10; http://dx.doi.org/10.4161/hv.28350; PMID: 24632599
- Kleeberger CA, Lyles RH, Margolick JB, Rinaldo CR, Phair JP, Giorgi JV. Viability and recovery of peripheral blood mononuclear cells cryopreserved for up to 12 years in a multicenter study. Clin Diagn Lab Immunol 1999; 6:14 - 9; PMID: 9874657
- Aziz N, Margolick JB, Detels R, Rinaldo CR, Phair J, Jamieson BD, Butch AW. Value of a quality assessment program in optimizing cryopreservation of peripheral blood mononuclear cells in a multicenter study. Clin Vaccine Immunol 2013; 20:590 - 5; http://dx.doi.org/10.1128/CVI.00693-12; PMID: 23408528
- Kyu SY, Kobie J, Yang H, Zand MS, Topham DJ, Quataert SA, Sanz I, Lee FE. Frequencies of human influenza-specific antibody secreting cells or plasmablasts post vaccination from fresh and frozen peripheral blood mononuclear cells. J Immunol Methods 2009; 340:42 - 7; http://dx.doi.org/10.1016/j.jim.2008.09.025; PMID: 18996127
- Costantini A, Mancini S, Giuliodoro S, Butini L, Regnery CM, Silvestri G, Montroni M. Effects of cryopreservation on lymphocyte immunophenotype and function. J Immunol Methods 2003; 278:145 - 55; http://dx.doi.org/10.1016/S0022-1759(03)00202-3; PMID: 12957403
- Owen RE, Sinclair E, Emu B, Heitman JW, Hirschkorn DF, Epling CL, Tan QX, Custer B, Harris JM, Jacobson MA, et al. Loss of T cell responses following long-term cryopreservation. J Immunol Methods 2007; 326:93 - 115; http://dx.doi.org/10.1016/j.jim.2007.07.012; PMID: 17707394
- Weinberg A, Song L-Y, Wilkening C, Sevin A, Blais B, Louzao R, Stein D, Defechereux P, Durand D, Riedel E, et al, Pediatric ACTG Cryopreservation Working Group. Optimization and limitations of use of cryopreserved peripheral blood mononuclear cells for functional and phenotypic T-cell characterization. Clin Vaccine Immunol 2009; 16:1176 - 86; http://dx.doi.org/10.1128/CVI.00342-08; PMID: 19515870
- Weinberg A, Wohl DA, Brown DG, Pott GB, Zhang L, Ray MG, van der Horst C. Effect of cryopreservation on measurement of cytomegalovirus-specific cellular immune responses in HIV-infected patients. J Acquir Immune Defic Syndr 2000; 25:109 - 14; http://dx.doi.org/10.1097/00126334-200010010-00004; PMID: 11103040
- Weinberg A, Betensky RA, Zhang L, Ray G. Effect of shipment, storage, anticoagulant, and cell separation on lymphocyte proliferation assays for human immunodeficiency virus-infected patients. Clin Diagn Lab Immunol 1998; 5:804 - 7; PMID: 9801338
- Kreher CR, Dittrich MT, Guerkov R, Boehm BO, Tary-Lehmann M. CD4+ and CD8+ cells in cryopreserved human PBMC maintain full functionality in cytokine ELISPOT assays. J Immunol Methods 2003; 278:79 - 93; http://dx.doi.org/10.1016/S0022-1759(03)00226-6; PMID: 12957398
- Maecker HT, Moon J, Bhatia S, Ghanekar SA, Maino VC, Payne JK, Kuus-Reichel K, Chang JC, Summers A, Clay TM, et al. Impact of cryopreservation on tetramer, cytokine flow cytometry, and ELISPOT. BMC Immunol 2005; 6:17; http://dx.doi.org/10.1186/1471-2172-6-17; PMID: 16026627
- Kierstead LS, Dubey S, Meyer B, Tobery TW, Mogg R, Fernandez VR, Long R, Guan L, Gaunt C, Collins K, et al. Enhanced rates and magnitude of immune responses detected against an HIV vaccine: effect of using an optimized process for isolating PBMC. AIDS Res Hum Retroviruses 2007; 23:86 - 92; http://dx.doi.org/10.1089/aid.2006.0129; PMID: 17263637
- Germann A, Oh Y-J, Schmidt T, Schön U, Zimmermann H, von Briesen H. Temperature fluctuations during deep temperature cryopreservation reduce PBMC recovery, viability and T-cell function. Cryobiology 2013; 67:193 - 200; http://dx.doi.org/10.1016/j.cryobiol.2013.06.012; PMID: 23850825
- Czerkinsky CC, Nilsson LA, Nygren H, Ouchterlony O, Tarkowski A. A solid-phase enzyme-linked immunospot (ELISPOT) assay for enumeration of specific antibody-secreting cells. J Immunol Methods 1983; 65:109 - 21; http://dx.doi.org/10.1016/0022-1759(83)90308-3; PMID: 6361139
- Crotty S, Aubert RD, Glidewell J, Ahmed R. Tracking human antigen-specific memory B cells: a sensitive and generalized ELISPOT system. J Immunol Methods 2004; 286:111 - 22; http://dx.doi.org/10.1016/j.jim.2003.12.015; PMID: 15087226
- Jahnmatz M, Kesa G, Netterlid E, Buisman A-M, Thorstensson R, Ahlborg N. Optimization of a human IgG B-cell ELISpot assay for the analysis of vaccine-induced B-cell responses. J Immunol Methods 2013; 391:50 - 9; http://dx.doi.org/10.1016/j.jim.2013.02.009; PMID: 23454005
- Janetzki S, Britten CM. The impact of harmonization on ELISPOT assay performance. Methods Mol Biol 2012; 792:25 - 36; http://dx.doi.org/10.1007/978-1-61779-325-7_2; PMID: 21956498
- Cox JH, Ferrari G, Kalams SA, Lopaczynski W, Oden N, D’souza MP, Elispot Collaborative Study Group. Results of an ELISPOT proficiency panel conducted in 11 laboratories participating in international human immunodeficiency virus type 1 vaccine trials. AIDS Res Hum Retroviruses 2005; 21:68 - 81; http://dx.doi.org/10.1089/aid.2005.21.68; PMID: 15665646
- Janetzki S, Panageas KS, Ben-Porat L, Boyer J, Britten CM, Clay TM, Kalos M, Maecker HT, Romero P, Yuan J, et al, Elispot Proficiency Panel of the CVC Immune Assay Working Group. Results and harmonization guidelines from two large-scale international Elispot proficiency panels conducted by the Cancer Vaccine Consortium (CVC/SVI). Cancer Immunol Immunother 2008; 57:303 - 15; http://dx.doi.org/10.1007/s00262-007-0380-6; PMID: 17721781
- Janetzki S, Britten CM, Kalos M, Levitsky HI, Maecker HT, Melief CJM, Old LJ, Romero P, Hoos A, Davis MM. “MIATA”-minimal information about T cell assays. Immunity 2009; 31:527 - 8; http://dx.doi.org/10.1016/j.immuni.2009.09.007; PMID: 19833080
- Zhang W, Caspell R, Karulin AY, Ahmad M, Haicheur N, Abdelsalam A, Johannesen K, Vignard V, Dudzik P, Georgakopoulou K, et al. ELISPOT assays provide reproducible results among different laboratories for T-cell immune monitoring--even in hands of ELISPOT-inexperienced investigators. J Immunotoxicol 2009; 6:227 - 34; http://dx.doi.org/10.3109/15476910903317546; PMID: 19908941
- Clutterbuck EA, Oh S, Hamaluba M, Westcar S, Beverley PCL, Pollard AJ. Serotype-specific and age-dependent generation of pneumococcal polysaccharide-specific memory B-cell and antibody responses to immunization with a pneumococcal conjugate vaccine. Clin Vaccine Immunol 2008; 15:182 - 93; http://dx.doi.org/10.1128/CVI.00336-07; PMID: 18032593
- Trück J, Lazarus R, Clutterbuck EA, Bowman J, Kibwana E, Bateman EAL, Pollard AJ. The zwitterionic type I Streptococcus pneumoniae polysaccharide does not induce memory B cell formation in humans. Immunobiology 2013; 218:368 - 72; http://dx.doi.org/10.1016/j.imbio.2012.05.008; PMID: 22704520
- Strober W. Trypan blue exclusion test of cell viability. Curr Protoc Immunol. Appendix 2001; 3:Appendix 3B
- R Core Team. R Foundation for Statistical Computing. R Foundation for Statistical Computing 2013
- Wickham H. ggplot2: elegant graphics for data analysis. New York: Springer; 2009.
- Janetzki S, Schaed S, Blachere NEB, Ben-Porat L, Houghton AN, Panageas KS. Evaluation of Elispot assays: influence of method and operator on variability of results. J Immunol Methods 2004; 291:175 - 83; http://dx.doi.org/10.1016/j.jim.2004.06.008; PMID: 15345315
- Weinberg A, Zhang L, Brown D, Erice A, Polsky B, Hirsch MS, Owens S, Lamb K. Viability and functional activity of cryopreserved mononuclear cells. Clin Diagn Lab Immunol 2000; 7:714 - 6; PMID: 10882680