Abstract
This phase II, randomized, double-blind study evaluated the immunogenicity of RTS,S vaccines containing Adjuvant System AS01 or AS02 as compared with non-adjuvanted RTS,S in healthy, malaria-naïve adults (NCT00443131). Thirty-six subjects were randomized (1:1:1) to receive RTS,S/AS01, RTS,S/AS02, or RTS,S/saline at months 0, 1, and 2. Antibody responses to Plasmodium falciparum circumsporozoite (CS) and hepatitis B surface (HBs) antigens were assessed and cell-mediated immune responses evaluated by flow cytometry using intracellular cytokine staining on peripheral blood mononuclear cells. Anti-CS antibody avidity was also characterized. Safety and reactogenicity after each vaccine dose were monitored. One month after the third vaccine dose, RTS,S/AS01 (160.3 EU/mL [95%CI: 114.1–225.4]) and RTS,S/AS02 (77.4 EU/mL (95%CI: 47.3–126.7)) recipients had significantly higher anti-CS antibody geometric mean titers (GMTs) than recipients of RTS,S/saline (12.2 EU/mL (95%CI: 4.8–30.7); P < 0.0001 and P = 0.0011, respectively). The anti-CS antibody GMT was significantly higher with RTS,S/AS01 than with RTS,S/AS02 (P = 0.0135). Anti-CS antibody avidity was in the same range in all groups. CS- and HBs-specific CD4+ T cell responses were greater for both RTS,S/AS groups than for the RTS,S/saline group. Reactogenicity was in general higher for RTS,S/AS compared with RTS,S/saline. Most grade 3 solicited adverse events (AEs) were of short duration and grade 3 solicited general AEs were infrequent in the 3 groups. No serious adverse events were reported. In conclusion, in comparison with non-adjuvanted RTS,S, both RTS,S/AS vaccines exhibited better CS-specific immune responses. The anti-CS antibody response was significantly higher with RTS,S/AS01 than with RTS,S/AS02. The adjuvanted vaccines had acceptable safety profiles.
Introduction
The RTS,S/AS candidate malaria vaccine is under clinical development for possible use in the Expanded Program on Immunization for infants and children in sub-Saharan Africa as an addition to existing preventive and treatment measures, such as insecticide-treated bed nets, indoor residual spraying, and intermittent preventive treatment with sulfadoxine-pyrimethamine.Citation1,Citation2 The antigen component of the candidate malaria vaccine, RTS,S, consists of repeat sequences of the Plasmodium falciparum circumsporozoite (CS) protein fused to the hepatitis B surface antigen (HBs).Citation3 Two adjuvant systems have been evaluated with the RTS,S antigen: AS02, which consists of an oil-in-water emulsion with monophosphoryl lipid A (MPL) and Quillaja saponaria Molina, fraction 21 (QS21, Antigenics Inc., a wholly owned subsidiary of Agenus Inc., Lexington, MA, USA), as immunostimulants, and AS01, a related liposome-based adjuvant system that also contains MPL and QS21.Citation3,Citation4
Anti-CS antibody titers and, to a lesser extent, CS-specific CD4+ T cells elicited by RTS,S have been identified as immunological markers associated with protection.Citation5-Citation7 CS-specific CD4+ T cells induced by RTS,S produce a mixture of cytokines, such as interleukin (IL)-2, tumor necrosis factor (TNF)-α, and interferon (IFN)-γ.Citation7-Citation11 In phase 2 clinical trials of adults and children, the RTS,S/AS01 formulation had an improved immunogenicity profile, in terms of humoral and cell-mediated immune (CMI) responses, and an equally favorable safety profile as compared with RTS,S/AS02.Citation11-Citation14 The RTS,S/AS01 formulation was consequently selected for phase 3 development. First results from the ongoing phase 3 trial in Africa show the vaccine candidate provides significant protection against clinical and severe malaria in young children and infants.Citation15,Citation16
The Committee for Medicinal Products for Human Use (CHMP) of the European Medicines Agency (EMA) recommended to establish in small studies the effect of vaccine adjuvants on immune responses to the antigens with which they are combined.Citation17 The present study was therefore designed to evaluate the humoral and CMI responses elicited by RTS,S/AS01 and RTS,S/AS02 as compared with non-adjuvanted RTS,S antigen. The study also evaluated antibody avidity against the CS repeat antigen.
This trial was conducted in healthy, malaria-naïve adults in order to control for factors associated with immune responses following malaria exposure. As subjects with pre-existing anti-HBs immunity may have improved immune responses against both HBs and CS when compared with HBs-naïve subjects,Citation14 for uniformity, only adults seroprotected for HBs at baseline were enrolled in the trial.
Results
Study population
A total of 56 malaria-naïve volunteers were screened of which 36 were randomized (1:1:1) to the vaccination groups (); all participants completed the study. Two were excluded from the according-to-protocol (ATP) cohort for immunogenicity because of incomplete vaccination. The demographic profile of participants was balanced across groups (). All participants were white (Caucasian/European heritage).
Figure 1. CONSORT diagram of study flow in phase II randomized, double-blind study of humoral and cell-mediated immune responses against 3 doses of RTS,S malaria vaccine formulated with AS01 (RTS,S/AS01) or AS02 (RTS,S/AS02) compared with 3 doses of RTS,S reconstituted with saline (RTS,S/saline).
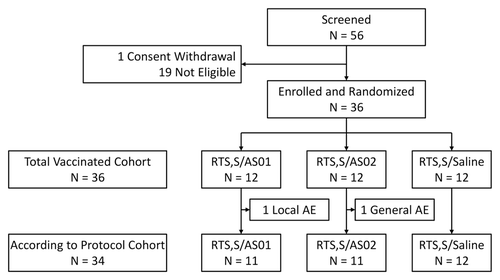
Table 1. Demographic characteristics (ATP cohort for immunogenicity)
Immunogenicity
Humoral responses
Antibodies to CS were determined by evaluating immunoglobulin G (IgG) responses to the CS-repeat region using a standard enzyme-linked immunosorbent assay (ELISA). The antibody response was evaluated in the ATP cohort for immunogenicity. Before vaccination, none of the subjects had detectable anti-CS antibody responses (). One month after each dose, all vaccine recipients in each group were seropositive for anti-CS antibodies (≥0.5 EU/mL), apart from one participant in the RTS,S/saline group who was seronegative after the third vaccine dose.
Table 2. Anti-CS and anti-HBs antibody GMTs by vaccine group 1 mo after each vaccine dose (ATP cohort for immunogenicity)
One month after the third vaccine dose, anti-CS antibody geometric mean titers (GMTs) were significantly higher in the RTS,S/AS01 and RTS,S/AS02 groups than in the RTS,S/saline group (P < 0.0001, RTS,S/AS01 vs. RTS,S/saline; P = 0.0011, RTS,S/AS02 vs. RTS,S/saline) (). Anti-CS GMTs were 13-fold and 6-fold higher for recipients of RTS,S/AS01 and RTS,S/AS02, respectively, than for recipients of RTS,S/saline. In the adjuvanted RTS,S groups, GMTs increased with subsequent doses () and significantly higher responses (P = 0.0135) were observed with RTS,S/AS01 than with RTS,S/AS02 ().
Table 3. Anti-CS antibody geometric mean titer (GMT) ratios (first group over second group) at 1 mo after the third vaccine dose (ATP cohort for immunogenicity)
Anti-CS antibody avidity (as determined by ELISA using the chaotropic agent, ammonium thiocyanate, and expressed as the avidity index) was in the same range for the 3 groups at each time point ().
Figure 2. Box plots of anti-CS antibody avidity index (percentage of antibodies that remained bound to antigen after ammonium thiocyanate treatment) in each group 1 mo after each vaccine dose (ATP cohort for immunogenicity). Box indicates median and Q1 (median minus 25%) and Q3 (median plus 25%) values, whiskers indicate minimum and maximum values. M, month.
All participants had seroprotective anti-HBs antibody titers (≥10 mIU/mL) before vaccination, and anti-HBs antibody GMTs increased after the first dose of RTS,S (range: 356888–536123 mIU/mL), but did not increase further with subsequent doses ().
Cell-mediated immunity
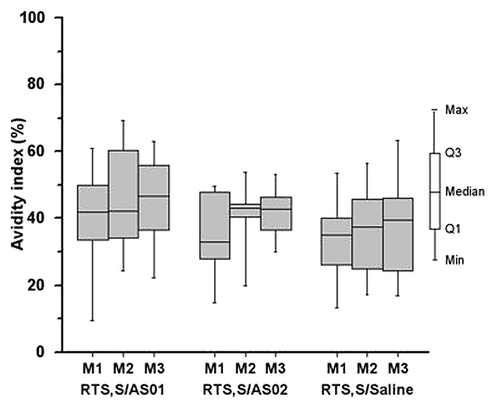
CMI responses to the CS and HBs antigens were assessed by flow cytometry using intracellular cytokine staining (ICS) analyses. Following vaccination, CS-specific CD4+ T cell responses, defined as CD4+ cells expressing at least 2 of the immune markers CD40L, IL-2, TNF-α, and/or IFN-γ, were detected in all vaccine groups with a trend for higher responses in the adjuvanted RTS,S groups over the RTS,S/saline group ().
Figure 3. Box plots for cytokine-positive T cell frequencies, defined as the percentage of CD4+ cells expressing at least 2 immune markers (CD40L, IL-2, TNF-α, and/or IFN-γ) per 106 CD4+ T cells, on stimulation with circumsporozoite (CS) and hepatitis B surface (HBs) antigens (ATP cohort for immunogenicity). Peripheral blood mononuclear cells were harvested, surface-labeled for CD4 and CD8 and then stained for intracellular detection of immune markers (see Methods). Cells were analyzed by flow cytometry. Box indicates median and Q1 (median minus 25%) and Q3 (median plus 25%) values, whiskers indicate minimum and maximum values. Pre, pre-vaccination; M, month. (A) CS-specific CD4+ T cell responses. (B) HBs-specific CD4+ T cell responses.
As expected from the primed status of the participants in terms of anti-HBs antibody titers, CD4+ T cell frequencies are much higher following stimulation with HBs than with CS (). HBs-specific CD4+ T cell responses were detected in all groups after vaccination, with a trend for higher median values in the adjuvanted RTS,S groups. Although some CD8+ T cell proliferation was observed following CS stimulation of peripheral blood mononuclear cells (PBMCs) harvested at screening, no vaccine-induced CS- or HBs-specific CD8+ T cell responses were detected in any group (data not shown).
Reactogenicity and safety
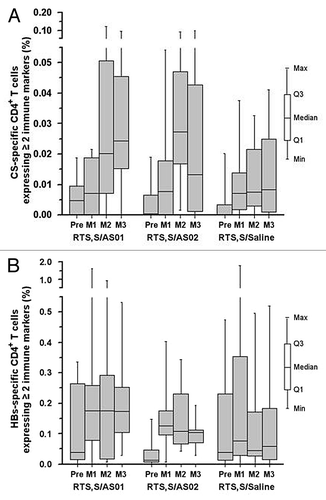
Incidences of all solicited adverse events (AEs), apart from gastrointestinal symptoms, tended to be higher with the adjuvanted antigen than with unadjuvanted RTS,S (). There was no trend suggesting an increase in solicited AE incidence with subsequent vaccine doses (data not shown). Injection site pain was the most frequently reported solicited local AE in all vaccine groups (). All grade 3 local AEs resolved within the 7-d follow up, except for 2 separate episodes of grade 3 redness after the first dose of RTS,S/AS01 that resolved on day 8 (participant received no further vaccine doses) and day 9, respectively. Fatigue and headache were the most frequently reported solicited general AEs (). Grade 3 solicited general AEs were infrequent and resolved within the 7-d follow-up, apart from one report of grade 3 gastrointestinal discomfort following the first dose of RTS,S/AS02, which resolved 14 d after vaccination; the participant received no further vaccine doses.
Figure 4. Frequency of solicited local and general adverse events (overall per dose) occurring within 7 d of vaccination (total vaccinated cohort). Grade 3 defined as preventing normal daily activity, apart from grade 3 fever (>39.0 °C) and grade 3 swelling or redness (diameter >50 mm) (A) Solicited local adverse events (B) Solicited general adverse events.
At least one unsolicited AE was reported in 10 (83.3%) subjects in each of the RTS,S/AS groups and 6 (50.0%) subjects in the RTS,S/saline group. The incidence of unsolicited events reported by more than one subject in a single group is shown in ; few were reported by more than 2 subjects. Unsolicited AEs that were considered to be causally related to vaccination were reported by 4 recipients of RTS,S/AS01 (33.3%), 5 recipients of RTS,S/AS02 (41.7%), and 3 recipients of RTS,S/saline (25.0%). Each vaccine-related unsolicited AE occurred in one subject only for each group, except for injection site pruritus (reported in 2 subjects in the RTS,S/AS01 group), arthralgia (reported in 2 subjects in the RTS,S/AS01 group), and myalgia (reported in 3 subjects in the RTS,S/AS01 group). One related unsolicited AE had grade 3 intensity: myalgia, which followed the first dose of RTS,S/AS01 and resolved within 2 d.
Table 4. Frequency of unsolicited symptoms (reported in more than one subject in a single group) during the 30-d post-vaccination period (total vaccinated cohort)
No serious AEs were reported during the study. No clinically relevant changes in clinical laboratory parameters were reported as AEs or serious AEs.
Discussion
The present study was designed to evaluate the humoral and cellular immune responses elicited by adjuvanted RTS,S as compared with non-adjuvanted RTS,S in healthy, malaria-naïve adults. As priming with hepatitis B vaccine has been shown to influence immune responses against both CS and HBs,Citation14 the immunological determinants contained in RTS,S, we enrolled subjects with detectable anti-HBs responses (≥10 mIU/mL) in an attempt to ensure baseline comparability. Adjuvantation was shown to strongly enhance immune responses, with RTS,S/AS01 and RTS,S/AS02 eliciting anti-CS antibody GMT responses that were 13- and 6-fold higher, respectively, than the response to non-adjuvanted RTS,S. CS- and HBs-specific CD4+ T cell responses were also stronger with the adjuvanted RTS,S formulations as compared with RTS,S/saline, with a trend toward higher CMI responses in the RTS,S/AS01 group. Paradoxically one subject in the saline group showed a CS-specific immune response after dose 1 which decreased over time and was undetectable at study end. We have no clear reason for this. However, although highly improbable considering the fact that the subject reported only mild fatigue after vaccination, we can’t completely rule out the possibility that the subject erroneously received an adjuvanted vaccine at month 0.
The results of this trial confirm those from a study of malaria-naïve adults conducted in the USA, which reported significantly greater CS-specific humoral immune responses and a tendency toward higher CD4+ T cell responses with RTS,S/AS01 than with RTS,S/AS02.Citation11 In that study, vaccine efficacy against malaria challenge was 50% with RTS,S/AS01 and 32% with RTS,S/AS02, and significant correlations were found between protection against malaria challenge and both CS-specific antibody responses and CMI responses induced by the RTS,S vaccine.Citation7,Citation11 CD4+ T cells predominantly expressed CD40L, a co-stimulatory ligand required for T cell help that also induces the differentiation of B cells,Citation18,Citation19 and IL-2, a cytokine associated with memory T cells and T cell proliferation and differentiation.Citation20 There was also a strong association between the frequency of IL-2 producing CD4+ T cells and titers of CS-specific antibodies in the same individual, suggesting that IL-2 may contribute to protection by promoting both cellular and humoral responses.Citation7 Methods available at the time of the study, however, did not allow for a phenotypic analysis of the CS CD4+ T cell data.
Induction of CD4+ T cells directed against P. falciparum CS protein by RTS,S adjuvanted formulations has been shown in clinical field trials in adults and children.Citation6,Citation8,Citation9,Citation21-Citation23 No systematic vaccine-induced CD8+ T cell response was detected in PBMCs in our study, which was consistent with other studies that showed RTS,S/AS induces little or no detectable CD8+ T cell response.Citation6,Citation11,Citation21,Citation24,Citation25
The anti-CS humoral immune responses in this study tended to be lower than those observed following administration of 3 doses of RTS,S/AS01 or RTS,S/AS02 to malaria-experienced children in AfricaCitation13,Citation14 but higher than those in African adults in a high malaria transmission area.Citation12 Overall, in all studies including the present trial, RTS,S/AS formulations produced robust anti-CS antibody responses, with the AS01 adjuvanted vaccine inducing higher responses than the AS02 adjuvanted formulation.Citation12-Citation14 To further assess the quality of the antibody response, the relative avidity of anti-CS antibodies was measured in an ELISA procedure using the chaotropic agent, ammonium thiocyanate. The use of chaotropic agents is based on their ability to dissociate antibody-antigen complexes of low avidity while complexes of high avidity remain intact.Citation26 In the present study, the avidity of the anti-CS antibodies was in the same range for the 3 groups. This suggests that, while adjuvantation can have an impact on magnitude of the anti-CS response, it may have much less influence on the avidity of the elicited antibodies.
Previous HBs-induced immune responses have been shown to enhance the CS-specific antibody response to adjuvanted RTS,S in children, most likely related to the covalently bound CS segment and HBs fusion protein in RTS,S.Citation14 In this population of HBs-primed subjects, anti-HBs antibody titers increased dramatically after the first dose of study vaccine with no further increase upon subsequent doses. Various hypotheses could explain these observations: (1) more T and B cell epitopes are present in HBs than in the CS antigen, making HBs immunodominant over CS and leading to earlier maximum anti-HBs antibody production than for CS; (2) relatively lower doses of CS antigen are administered compared with HBs as there are fewer CS antigens than HBs antigens in RTS,S; (3) competition at the T cell level, resulting in more and earlier T cell responses and B cell help for HBs-specific B cells than for CS; (4) binding of RTS,S by anti-HBs antibodies followed by uptake and presentation of vaccine-derived peptides by HBs-specific B cells, resulting in a rapid increase in HBs-specific antibodies and minimal priming of CS-specific T and B cells; and (5) higher levels of anti-HBs antibodies interfering with HBs boosting by binding and phagocytosis of vaccine particles. Most likely a combination of all or some of these mechanisms leads to the continuing rise of vaccine-induced anti-CS antibodies, while no further increase of anti-HBs responses is observed after the second dose. It was also noted that adjuvanted RTS,S did not induce higher anti-hepatitis B booster responses than non-adjuvanted RTS,S.
Reactogenicity was in general higher in the adjuvanted vaccine groups than in the non-adjuvanted control group but was within acceptable limits and in line with previous experience of RTS,S/AS vaccines.Citation4,Citation27 Most grade 3 solicited symptoms were of short duration and grade 3 solicited general AEs were infrequent in all groups. Further interpretation of the safety results and immunogenicity analyses is limited by the small number of participants in each group. Another limitation of this study was the absence of a group of subjects without seroprotective anti-HBs antibody titers at baseline.
In summary, adjuvanted RTS,S vaccines exhibited superior anti-CS humoral and CMI responses over non-adjuvanted RTS,S, with a tendency toward stronger immune responses induced by RTS,S/AS01 compared with RTS,S/AS02, which was in line with previous studies. The adjuvanted vaccines demonstrated an acceptable safety profile, although reactogenicity was generally higher with the adjuvanted vaccines than with non-adjuvanted RTS,S. These results, together with previously published studies, confirm the immunological basis for adjuvantation of RTS,S.
Methods
Study design and participants
This phase II, randomized, double-blind (observer-blind) study was conducted at the Center for Vaccinology, Ghent University and Ghent University Hospital, Ghent, Belgium, between April and July in 2007 (ClinicalTrials.gov identifier: NCT00443131). Subjects were recruited primarily via advertisements posted at the University Hospital. Healthy malaria-naïve men or women of non-childbearing potential, aged 18 to 45 y at the time of first vaccination, who were seronegative for human immunodeficiency virus (HIV 1 or 2), HBs, and hepatitis C virus antibodies, with seroprotective anti-HBs antibody titers (≥10 mIU/mL) at screening, were eligible for enrolment. All subjects had been immunized with the hepatitis B vaccine. Written informed consent was obtained from all participants before performing any study procedure.
The study was reviewed and approved by the ethics review committee of the University of Ghent. The trial was undertaken according to the International Conference on Harmonization and Good Clinical Practice guidelines, and was monitored by GlaxoSmithKline Vaccines. The primary objective of the study was to demonstrate superiority of anti-CS antibody responses at 1 mo post-dose 3 against RTS,S formulated with AS01 or AS02 compared with RTS,S reconstituted with saline.
The participants were randomized (1:1:1), by a centralized randomization system on the internet administered by the investigator, to receive vaccination at months 0, 1, and 2 with lyophilized RTS,S (50 µg) reconstituted with 500 µL of either AS01A, AS02B (referred to elsewhere in this paper as AS01 and AS02, respectively), or saline. The RTS,S vaccine has been described previously.Citation3 The vaccines were administered intramuscularly to the deltoid muscle of the non-dominant arm and vaccine recipients were observed for at least 30 min following each vaccination.
All laboratory assays were performed at the Center for Vaccinology, Ghent University and Hospital, or at the laboratories of GlaxoSmithKline Vaccines, using standardized, validated procedures.
Humoral immune response assessments
Assessment of anti-CS and anti-HBs antibody titers was conducted on serum samples taken before dose 1 (at enrolment), 1 mo after dose 1 (month 1), 1 mo after dose 2 (month 2), and 1 mo after dose 3 (month 3). Antibodies against CS were measured by evaluating IgG responses to the CS-repeat region, using a standard ELISA with R32LR as the capture antigen.Citation28 An anti-CS antibody titer of 0.5 EU/mL or greater was considered to be positive. Anti-HBs antibodies were measured using an in-house ELISA; an antibody titer of 10 mIU/mL or greater was considered to be seroprotective.
The avidity of anti-CS antibodies in sera was assessed at months 1, 2, and 3. The relative avidity of IgG antibodies was determined by ELISA with R32LR as coating antigen. The assay was an adaptation of the anti-CS assayCitation28 and based on previous methodology on the dissociation of low avidity antibody-antigen complexes by the chaotropic agent, ammonium thiocyanate (NH4SCN).Citation26 After sample addition, formed antigen-antibody complexes were treated with a 1M ammonium thiocyanate solution and remaining complexes were quantified. The result was compared with the concentration obtained when no treatment was applied and expressed as the avidity index, indicating the percentage of antibodies that remained bound to antigens.
CMI response assessments
Blood samples for CMI response analysis were collected at months 1, 2, and 3. CMI responses to the CS and HBs antigens were assessed using frozen PBMCs, which were isolated by standard Ficoll-Hypaque density gradient centrifugation and cryopreserved in liquid nitrogen within 12 h of blood collection.
CS-specific and HBs-specific CD4+/CD8+ T cells expressing the cytokines CD40L and/or IL-2 and/or TNF-α and/or IFN-γ were detected using ICS and flow cytometry, based on previously described methodology.Citation29,Citation30 Briefly, PBMCs were stimulated in vitro for 2 h with antigen or pools of peptides, which covered the entire sequence of the antigens, in the presence of anti-CD28 and anti-CD49d antibodies. The cells were then incubated overnight with brefeldin A to prevent cytokine excretion. The cells were stained for surface markers (CD4 and CD8), fixed and permeabilized, and stained with fluorochrome-conjugated monoclonal antibodies to detect the immune markers by flow cytometry.
Safety and reactogenicity evaluation
Solicited local (injection site pain, redness, and swelling) and general (fatigue, fever, gastrointestinal symptoms [nausea, vomiting, diarrhea, abdominal pain], and headache) AEs were recorded by participants on diary cards during the 7-d follow-up after each vaccination. Information on unsolicited AEs were collected over 30 d after each vaccination. Serious AEs were reported throughout the study. Duration, causality, and outcome of AEs were recorded. All solicited local reactions were considered causally related to vaccination; the relationship of other AEs was classified as possible or not causally related. AE intensity was scored on a scale from 1 to 3. Grade 3 AEs were defined as preventing normal daily activity, apart from grade 3 solicited fever, which was defined as axillary temperature >39.0 °C, and grade 3 solicited swelling or redness, defined as diameter >50 mm. Complete blood count, renal (creatinine) and hepatic functional tests (alanine aminotransferase and aspartate aminotransferase) were taken at screening and 1 mo after the third vaccine dose.
Statistical analyses
A sample size of 10 evaluable subjects per group had 98% power to demonstrate superiority of RTS,S/AS01 over RTS,S/saline, assuming a log standard deviation not exceeding 0.7 and anti-CS GMTs of 5 EU/mL and 143 EU/mL for RTS,S/saline and RTS,S/AS01, respectively, and 91% power to demonstrate superiority of RTS,S/AS02 over RTS,S/saline, assuming a log standard deviation not exceeding 0.7 and anti-CS GMTs of 5 EU/mL and 82 EU/mL for RTS,S/saline and RTS,S/AS02, respectively.
Immunogenicity analysis was performed on the ATP cohort for immunogenicity, defined as those meeting all eligibility criteria, complying with the procedures defined in the protocol, with no elimination criteria during the study, and for whom data concerning immunogenicity endpoint measures were available. Anti-CS and anti-HBs antibody GMTs were calculated with 95% CIs. The percentages of subjects with seropositive levels of anti-CS antibodies (≥0.5 EU/mL) and seroprotective levels of anti-HBs antibodies (≥10 mIU/mL) were determined. Superiority of RTS,S/AS01 or RTS,S/AS02 over RTS,S/saline in terms of anti-CS antibody GMTs 1 mo after the third vaccine dose was evaluated using a 2-sided T-test on the log10 transformed anti-CS titers (analysis of variance [ANOVA] model, pooled variance). The superiority condition was met if the P value was <0.025.
The avidity of anti-CS antibodies was expressed as the avidity index, indicating the percentage of antibodies that remained bound to antigens after ammonium thiocyanate treatment. CMI responses were determined as the frequency of CS- and HBs-specific CD4+ and CD8+ T cells expressing at least 2 immune markers (CD40L, IL-2, TNF-α, and/or IFN-γ), presented as the percentage of T cells expressing at least 2 cytokines per million cells.
The safety analysis was conducted on the total vaccinated cohort. Percentages of solicited or unsolicited AEs were calculated with exact 95% CIs. Clinically relevant abnormal laboratory values were determined according to predefined normal ranges.
| Abbreviations: | ||
| AE | = | adverse event |
| ANOVA | = | analysis of variance |
| ATP | = | according-to-protocol |
| CHMP | = | Committee for Medicinal Products for Human Use |
| CI | = | confidence interval |
| CMI | = | cell-mediated immune |
| CS | = | circumsporozoite |
| ELISA | = | enzyme-linked immunosorbent assay |
| EMA | = | European Medicines Agency |
| GMT | = | geometric mean titer |
| HBs | = | hepatitis B surface antigen |
| HIV | = | human immunodeficiency virus |
| ICS | = | intracellular cytokine staining |
| IFN | = | interferon |
| Ig | = | immunoglobulin |
| IL | = | interleukin |
| MPL | = | monophosphoryl lipid A |
| PBMC | = | peripheral blood mononuclear cell |
| SD | = | standard deviation |
| TNF | = | tumor necrosis factor |
Disclosure of Potential Conflicts of Interest
The study was supported by GlaxoSmithKline Biologicals SA. O.O-A., M.L.. E.J., P.M., W.R.B., and J.C. are employees of the GlaxoSmithKline group of companies and own GlaxoSmithKline stock and/or stock options. G.L.-R., I.L.-R., and F.C. received funding from GlaxoSmithKline via their institute to cover study costs. G.L.-R. received payments from GlaxoSmithKline for lectures on HPV vaccines and vaccines in general, and for consultancy on influenza vaccines and adjuvants, from Novartis Vaccine and Diagnostics and Immune Targeting Systems (UK) for consultancy on influenza vaccines, and from Baxter Vaccines for lectures on influenza vaccines. I.L.-R. received fees from GlaxoSmithKline and Sanofi Pasteur for lectures on vaccine-related topics and received registration and travel expenses from GlaxoSmithKline to attend vaccine-related conferences.
Acknowledgments
The authors would like to thank all trial participants, and are indebted to the clinicians, nurses and laboratory technicians at CEVAC, Ghent University and Ghent University Hospital, for their contributions. The authors also thank, from GlaxoSmithKline Vaccines, Priya Pavithran for writing the study protocol, Thomas Moens for writing the clinical study report, Didier Lapierre for review of the clinical study report, Marie Ange Demoitie for clinical read outs, Grégory Catteau for statistical analysis, and Amanda Leach for critical review of this manuscript, Jarno Jansen (Keyrus Biopharma, on behalf of GlaxoSmithKline Vaccines) for publication management, and Joanne Knowles (independent medical writer, on behalf of GlaxoSmithKline Vaccines) for initial drafting of the manuscript and incorporation of comments received from the authors.
Contributions
G.L.-R., I.L.-R., and F.C. were investigators in this study and were responsible for the recruitment of subjects, collection and assembly of data, and provided critical input in the protocol, interpretation of results and writing of the manuscript. G.L.R., I.L.R., W.R.B., and O.O.-A. were involved in all steps of the study from study design to analysis and interpretation of results. F.C., P.M., E.J. ,and J.C. were responsible for the testing and interpretation of humoral and cellular immune response assessments. M.L. was responsible for the design, execution and interpretation of statistical analyses. G.L.R., I.L.R., and O.O.-A. supervised the design of the study, analysis and interpretation of results. All authors have critically reviewed the manuscript drafts and approved the final article.
Financial Disclosure
This trial was supported by GlaxoSmithKline Biologicals SA, Rixensart, Belgium. GlaxoSmithKline Biologicals SA was involved in all stages of the study conduct and analysis and took responsibility for all costs associated with the development and publishing of the present manuscript.
References
- Cohen J, Nussenzweig V, Nussenzweig R, Vekemans J, Leach A. From the circumsporozoite protein to the RTS, S/AS candidate vaccine. Hum Vaccin 2010; 6:90 - 6; http://dx.doi.org/10.4161/hv.6.1.9677; PMID: 19806009
- WHO Malaria Policy Advisory Committee and Secretariat. Malaria Policy Advisory Committee to the WHO: conclusions and recommendations of September 2012 meeting. Malar J 2012; 11:424; http://dx.doi.org/10.1186/1475-2875-11-424; PMID: 23253143
- Regules JA, Cummings JF, Ockenhouse CF. The RTS,S vaccine candidate for malaria. Expert Rev Vaccines 2011; 10:589 - 99; http://dx.doi.org/10.1586/erv.11.57; PMID: 21604980
- Garçon N, Van Mechelen M. Recent clinical experience with vaccines using MPL- and QS-21-containing adjuvant systems. Expert Rev Vaccines 2011; 10:471 - 86; http://dx.doi.org/10.1586/erv.11.29; PMID: 21506645
- White MT, Bejon P, Olotu A, Griffin JT, Riley EM, Kester KE, Ockenhouse CF, Ghani AC. The relationship between RTS,S vaccine-induced antibodies, CD4⁺ T cell responses and protection against Plasmodium falciparum infection. PLoS One 2013; 8:e61395; http://dx.doi.org/10.1371/journal.pone.0061395; PMID: 23613845
- Ndungu FM, Mwacharo J, Kimani D, Kai O, Moris P, Jongert E, Vekemans J, Olotu A, Bejon P. A statistical interaction between circumsporozoite protein-specific T cell and antibody responses and risk of clinical malaria episodes following vaccination with RTS,S/AS01E. PLoS One 2012; 7:e52870; http://dx.doi.org/10.1371/journal.pone.0052870; PMID: 23300801
- Lumsden JM, Schwenk RJ, Rein LE, Moris P, Janssens M, Ofori-Anyinam O, Cohen J, Kester KE, Heppner DG, Krzych U. Protective immunity induced with the RTS,S/AS vaccine is associated with IL-2 and TNF-α producing effector and central memory CD4 T cells. PLoS One 2011; 6:e20775; http://dx.doi.org/10.1371/journal.pone.0020775; PMID: 21779319
- Ansong D, Asante KP, Vekemans J, Owusu SK, Owusu R, Brobby NA, Dosoo D, Osei-Akoto A, Osei-Kwakye K, Asafo-Adjei E, et al. T cell responses to the RTS,S/AS01(E) and RTS,S/AS02(D) malaria candidate vaccines administered according to different schedules to Ghanaian children. PLoS One 2011; 6:e18891; http://dx.doi.org/10.1371/journal.pone.0018891; PMID: 21556142
- Agnandji ST, Fendel R, Mestré M, Janssens M, Vekemans J, Held J, Gnansounou F, Haertle S, von Glasenapp I, Oyakhirome S, et al. Induction of Plasmodium falciparum-specific CD4+ T cells and memory B cells in Gabonese children vaccinated with RTS,S/AS01(E) and RTS,S/AS02(D). PLoS One 2011; 6:e18559; http://dx.doi.org/10.1371/journal.pone.0018559; PMID: 21494604
- Olotu A, Moris P, Mwacharo J, Vekemans J, Kimani D, Janssens M, Kai O, Jongert E, Lievens M, Leach A, et al. Circumsporozoite-specific T cell responses in children vaccinated with RTS,S/AS01E and protection against P falciparum clinical malaria. PLoS One 2011; 6:e25786; http://dx.doi.org/10.1371/journal.pone.0025786; PMID: 21998698
- Kester KE, Cummings JF, Ofori-Anyinam O, Ockenhouse CF, Krzych U, Moris P, Schwenk R, Nielsen RA, Debebe Z, Pinelis E, et al, RTS,S Vaccine Evaluation Group. Randomized, double-blind, phase 2a trial of falciparum malaria vaccines RTS,S/AS01B and RTS,S/AS02A in malaria-naive adults: safety, efficacy, and immunologic associates of protection. J Infect Dis 2009; 200:337 - 46; http://dx.doi.org/10.1086/600120; PMID: 19569965
- Polhemus ME, Remich SA, Ogutu BR, Waitumbi JN, Otieno L, Apollo S, Cummings JF, Kester KE, Ockenhouse CF, Stewart A, et al. Evaluation of RTS,S/AS02A and RTS,S/AS01B in adults in a high malaria transmission area. PLoS One 2009; 4:e6465; http://dx.doi.org/10.1371/journal.pone.0006465; PMID: 19649245
- Owusu-Agyei S, Ansong D, Asante K, Kwarteng Owusu S, Owusu R, Wireko Brobby NA, Dosoo D, Osei Akoto A, Osei-Kwakye K, Adjei EA, et al. Randomized controlled trial of RTS,S/AS02D and RTS,S/AS01E malaria candidate vaccines given according to different schedules in Ghanaian children. PLoS One 2009; 4:e7302; http://dx.doi.org/10.1371/journal.pone.0007302; PMID: 19806184
- Lell B, Agnandji S, von Glasenapp I, Haertle S, Oyakhiromen S, Issifou S, Vekemans J, Leach A, Lievens M, Dubois MC, et al. A randomized trial assessing the safety and immunogenicity of AS01 and AS02 adjuvanted RTS,S malaria vaccine candidates in children in Gabon. PLoS One 2009; 4:e7611; http://dx.doi.org/10.1371/journal.pone.0007611; PMID: 19859560
- RTS,S Clinical Trials Partnership, Agnandji ST, Lell B, Soulanoudjingar SS, Fernandes JF, Abossolo BP, Conzelmann C, Methogo BG, Doucka Y, Flamen A, Mordmüller B, et al. First results of phase 3 trial of RTS,S/AS01 malaria vaccine in African children. N Engl J Med 2011; 365:1863 - 75; http://dx.doi.org/10.1056/NEJMoa1102287; PMID: 22007715
- RTS,S Clinical Trials Partnership, Agnandji ST, Lell B, Fernandes JF, Abossolo BP, Methogo BG, Kabwende AL, Adegnika AA, Mordmüller B, Issifou S, Kremsner PG, et al. A phase 3 trial of RTS,S/AS01 malaria vaccine in African infants. N Engl J Med 2012; 367:2284 - 95; http://dx.doi.org/10.1056/NEJMoa1208394; PMID: 23136909
- Committee for Medicinal Products for Human Use (CHMP). Guideline on adjuvants in vaccines for human use. The European Medicines Agency; 2005. Report No.: EMEA/CHMP/VEG/134716/2004.
- Ma DY, Clark EA. The role of CD40 and CD154/CD40L in dendritic cells. Semin Immunol 2009; 21:265 - 72; http://dx.doi.org/10.1016/j.smim.2009.05.010; PMID: 19524453
- Cerutti A, Puga I, Cols M. Innate control of B cell responses. Trends Immunol 2011; 32:202 - 11; http://dx.doi.org/10.1016/j.it.2011.02.004; PMID: 21419699
- Liao W, Lin JX, Leonard WJ. IL-2 family cytokines: new insights into the complex roles of IL-2 as a broad regulator of T helper cell differentiation. Curr Opin Immunol 2011; 23:598 - 604; http://dx.doi.org/10.1016/j.coi.2011.08.003; PMID: 21889323
- Reece WH, Pinder M, Gothard PK, Milligan P, Bojang K, Doherty T, Plebanski M, Akinwunmi P, Everaere S, Watkins KR, et al. A CD4(+) T-cell immune response to a conserved epitope in the circumsporozoite protein correlates with protection from natural Plasmodium falciparum infection and disease. Nat Med 2004; 10:406 - 10; http://dx.doi.org/10.1038/nm1009; PMID: 15034567
- Pinder M, Reece WH, Plebanski M, Akinwunmi P, Flanagan KL, Lee EA, Doherty T, Milligan P, Jaye A, Tornieporth N, et al. Cellular immunity induced by the recombinant Plasmodium falciparum malaria vaccine, RTS,S/AS02, in semi-immune adults in The Gambia. Clin Exp Immunol 2004; 135:286 - 93; http://dx.doi.org/10.1111/j.1365-2249.2004.02371.x; PMID: 14738458
- Barbosa A, Naniche D, Aponte JJ, Manaca MN, Mandomando I, Aide P, Sacarlal J, Renom M, Lafuente S, Ballou WR, et al. Plasmodium falciparum-specific cellular immune responses after immunization with the RTS,S/AS02D candidate malaria vaccine in infants living in an area of high endemicity in Mozambique. Infect Immun 2009; 77:4502 - 9; http://dx.doi.org/10.1128/IAI.00442-09; PMID: 19651872
- Stoute JA, Kester KE, Krzych U, Wellde BT, Hall T, White K, Glenn G, Ockenhouse CF, Garcon N, Schwenk R, et al. Long-term efficacy and immune responses following immunization with the RTS,S malaria vaccine. J Infect Dis 1998; 178:1139 - 44; http://dx.doi.org/10.1086/515657; PMID: 9806046
- Lalvani A, Moris P, Voss G, Pathan AA, Kester KE, Brookes R, Lee E, Koutsoukos M, Plebanski M, Delchambre M, et al. Potent induction of focused Th1-type cellular and humoral immune responses by RTS,S/SBAS2, a recombinant Plasmodium falciparum malaria vaccine. J Infect Dis 1999; 180:1656 - 64; http://dx.doi.org/10.1086/315074; PMID: 10515829
- Macdonald RA, Hosking CS, Jones CL. The measurement of relative antibody affinity by ELISA using thiocyanate elution. J Immunol Methods 1988; 106:191 - 4; http://dx.doi.org/10.1016/0022-1759(88)90196-2; PMID: 3339255
- Garçon N, Leroux-Roels G, Cheng W-F. Vaccine adjuvants. In: Garçon N, Stern PL, Cunningham AL, Stanberry LR, editors. Understanding Modern Vaccines: Perspectives in Vaccinology. Elsevier B.V.; 2011. 89-113.
- Clement F, Dewar V, Van Braeckel E, Desombere I, Dewerchin M, Swysen C, Demoitié MA, Jongert E, Cohen J, Leroux-Roels G, et al. Validation of an enzyme-linked immunosorbent assay for the quantification of human IgG directed against the repeat region of the circumsporozoite protein of the parasite Plasmodium falciparum. Malar J 2012; 11:384; http://dx.doi.org/10.1186/1475-2875-11-384; PMID: 23173602
- Leroux-Roels I, Leroux-Roels G, Ofori-Anyinam O, Moris P, De Kock E, Clement F, Dubois MC, Koutsoukos M, Demoitié MA, Cohen J, et al. Evaluation of the safety and immunogenicity of two antigen concentrations of the Mtb72F/AS02(A) candidate tuberculosis vaccine in purified protein derivative-negative adults. Clin Vaccine Immunol 2010; 17:1763 - 71; http://dx.doi.org/10.1128/CVI.00133-10; PMID: 20861328
- Moris P, van der Most R, Leroux-Roels I, Clement F, Dramé M, Hanon E, Leroux-Roels GG, Van Mechelen M. H5N1 influenza vaccine formulated with AS03 A induces strong cross-reactive and polyfunctional CD4 T-cell responses. J Clin Immunol 2011; 31:443 - 54; http://dx.doi.org/10.1007/s10875-010-9490-6; PMID: 21174144
