Abstract
Intravital microscopy is a valuable tool in studying the liver, a complex organ with a unique sinusoidal microcirculation and both metabolic and immune functions. The liver is also subject to a large variety of diseases including viral, bacterial and parasitic infections. We developed novel recording techniques to visualize dynamic events in the hepatic microvasculature without the need of fluorescent markers. In combination with cellular and molecular probes, reporter mice and Plasmodium as a hepatotropic model microorganism, we demonstrate the power of these techniques in monitoring the development of the malaria parasite and the response of the hepatic microenvironment to infection.
Introduction
The liver is unique in that it receives not only oxygenated arterial, but also nutrient-rich portal venous blood from the gastrointestinal tract. Upon entry into the liver lobule, the two blood sources merge and flow along the sinusoids toward the central venule. Because portal venous blood carries food antigens and microbial products from the intestines, the liver is exposed to a plethora of innocuous dietary and commensal antigens, to which it typically responds with tolerance, as well as potential pathogens or toxins against which it normally mounts an immune response.Citation1,Citation2 Kupffer cells, resident professional phagocytes of the liver, are a key component of the unique hepatic innate immune system and crucially involved in the dichotomy between induction of tolerance or immunity.Citation3 Based on these unique features, combined with easy accessibility and the continuously improving imaging technology, the liver has been a major focus of intravital microscopy (IVM) for many years. While early studies relied on video microscopy and transillumination, which limited analysis to the edge of the liver, the subsequent use of wide-field epifluorescence microscopy greatly extended the area of examination.Citation4-Citation7 Technical advances in instrument design over the last years have to led to confocal, spinning disc and 2P microscopes with vastly improved IVM capabilities.Citation8-Citation14
Designed as a barrier between the digestive system and the rest of the body, the liver is subject to a multitude of microbial infections. For example, Plasmodium undergoes a round of multiplication in hepatocytes and, by choosing this route of entry into the host, may be able to exploit the tolerogenic properties of the liver.Citation15,Citation16 IVM has been instrumental in clarifying the dynamics of Plasmodium sporozoite entry into the liver and the release of thousands of merozoites into the blood,Citation13,Citation17,Citation18 which eventually leads to the typical malaria-associated symptoms and pathology. Despite decades of research, however, the elusive clinically silent Plasmodium liver stage (LS) is still a major focus of anti-malarial intervention and vaccine development,Citation19-Citation22 highlighting our incomplete understanding of the basic immunobiology of Plasmodium within the hepatic microenvironment.
Here we present novel recording techniques that visualize dynamic events in the hepatic microvasculature without the need of fluorescent markers. Using Plasmodium yoelii as a hepatotropic model microorganism, we demonstrate, in real-time and under normal blood flow conditions, the development of the malaria parasite in the liver of live animals with an intact immune system and preserved innervation.
Results
To obtain a more detailed view of the hepatic architecture, in particular the sinusoidal microvasculature, we developed a novel approach based on simultaneous recording of reflected and autofluorescent signal for use in non-fluorescent mice. This technique, which we call intravital reflection recording (IRR), visualizes the sinusoidal walls and blood cells in the absence of a fluorescent tracer (). When IRR is recorded in inverse color mode, blood cells appear as dark objects traveling through the microvasculature in bright (non-fluorescent) plasma (). Combined with reporter mice, for example ECFP () or DsRed () mice that express fluorescence predominantly in hepatocytes, IRR dramatically improves visualization of the overall liver architecture by generating an image of the sinusoidal microvasculature complimentary to the fluorescent parenchymal signal. Direct (Vid. S1) and inverse IRR (Vid. S2) are ideally suited to monitor the blood flow or immune cells patrolling the sinusoidal microvasculature. Considering the multitude of fluorescent markers and reporter mice available, the ability to use any laser line for IRR in a complementary color presents a major advantage of this technique.
Figure 1. IRR visualizes blood flow in the non-fluorescent sinusoidal microvasculature. (A) IRR was obtained with a 633 nm HeNe laser at 25% power output and collected at 630–850 nm. In this 3D projection, sinusoids appear as red tubes due to the sinusoidal wall and dense packing of blood cells reflecting the excitation light. (B) ECFP mouse liver fluorescence was excited with a 405 nm Diode laser to visualize the hepatocyte plates. (C) Overlay shows the intricate network of liver parenchyma and highly anastomozed sinusoids. (D) Inverse IRR highlights the sinusoidal microvasculature, while DsRed mouse fluorescence, obtained with 543 nm HeNe laser, visualizes the hepatocyte plates (E). (F) Overlay allows for a 3D representation of the non-fluorescent structural details of the sinusoids (red) and the DsRed fluorescence of the liver tissue (green pseudocolor). Scale bars 20 μm.
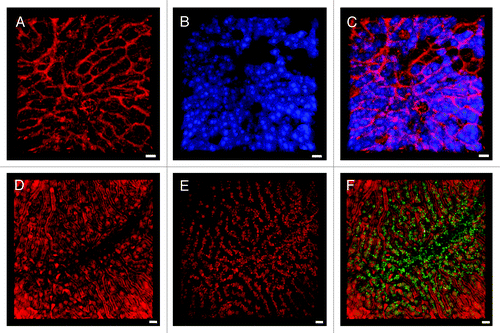
Another novel approach for IVM of the hepatic microvasculature includes new membrane stains. CellMask Orange, for example, provides a detailed outline of the sinusoidal wall due to the high cell membrane density along the Disse space () and visualizes the sinusoidal blood flow (Vid. S3). In the avascular subcapsular region of the liver, CellMask Orange primarily stains hepatocyte membranes (). Conventional MitoTracker dyes represent excellent parenchymal markers due to the high concentration of mitochondria in hepatocytes (). In combination with MitoTracker, CellMask () provides a detailed view of the sinusoidal microvasculature and the radial hepatic plates, thus visualizing the overall architecture of the liver lobule (). Even though representing a more traditional approach, endothelial labeling with CD31-specific antibodies () offers enhanced information on the microvasculature if used in conjunction with IRR.
Figure 2. Cell membrane and mitochondrial staining visualizes the sinusoidal liver architecture. (A–C) In the liver, the cell membrane stain CellMask Orange (shown in green) highlights the sinusoidal microvasculature due to the high density of cell membranes along the space of Disse. (A) 3D reconstruction of a z-stack. (B) A z-slice from the avascular subcapsular region of the liver demonstrates that CellMask Orange predominantly outlines hepatocytes. (C) Another z-slice, acquired 40 μm from the liver surface, visualizes that CellMask Orange outlines the microvasculature as well as individual blood cells within the sinusoidal lumen. (D–F) Liver from a mouse injected with MitoTracker Deep Red (D) and CellMask Orange (E). The overlay (F) visualizes the sinusoidal microvasculature outlined with CellMask Orange, while the hepatocyte cytoplasm is filled with red fluorescent mitochondria. Distortion of the RBCs in the sinusoidal lumen indicates preserved blood flow. Scale bar 20 μm.
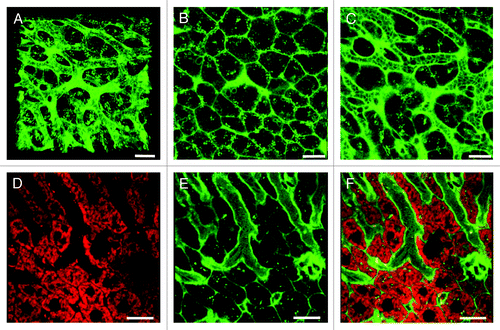
Figure 3. Novel complementary approaches for visualization of the sinusoidal microvasculature. (A) In this 3D reconstruction of a lysM-EGFP mouse liver z-stack, the sinusoidal microvasculature is labeled with anti-CD31 Alexa Fluor 647 (red). Myelomonocytic cells in this reporter mouse fluoresce green and nuclei are blue (Hoechst). (B) Z-slice of a Tie2-GFP mouse liver with fluorescent endothelia (green). Hepatocytes are red (MitoTracker), CD8+ T cells were labeled with anti-mouse CD8a-PE (green outline) and nuclei are blue (Hoechst). See Vid. S8 for complete z-stack. (C) 3D projection of a liver z-stack showing anti-CD8a-PE labeled CD8+ T cells (red circles) patrolling the sinusoidal endothelium (green). Nuclei are blue (Hoechst).(D) Z-slice showing red hepatocyte plates (MitoTracker) lined with green fluorescent sinusoidal endothelia. Nuclei are blue (Hoechst). Scale bars 20 μm.
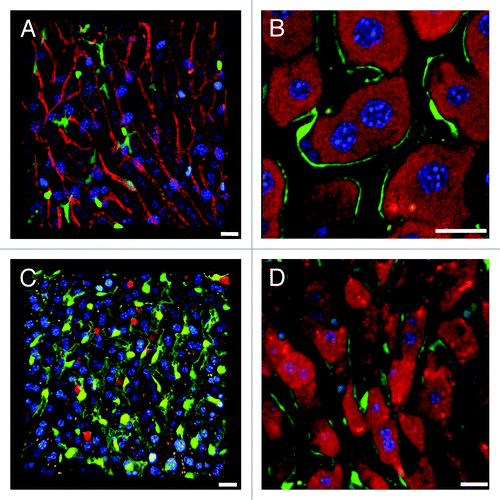
A large variety of reporter mice are available to study interactions between liver antigen presenting cells (APC) and immune cells. As sinusoidal endothelia can function as APC,Citation3,Citation23 Tie2-GFP reporter miceCitation24 are useful tools for monitoring CD8+ T cell adhesion to the sinusoidal microvasculature (). When separate PMTs are used to collect signal from of anti-CD8a-phycoerythrin (PE) labeled CD8+ T cells and GFP expressing endothelia (; Vid. S4), the blood flow remains invisible without IRR (Vid. S5). When PE and GFP signal is acquired with one detector and IRR with another (Vid. S6), CD8+ T cells patrolling the sinusoids can be monitored together with the blood flow (compare Vids. S5 and S6). Tie2-GFP mice may also be used in conjunction with MitoTracker to visualize the Disse space () or with anti-CD8a-PE to monitor CD8+ T cells (Vids. S7 and S8). Further, lysM-EGFP reporter mice with fluorescent myelomonocytic cellsCitation25 are valuable to study innate immune cells or interactions between antigen-presenting Kupffer cells and CD8+ T cells. Anti-CD8a-PE surface labeling allows differentiation of CD8+ T cells from solid green GFP+ myelomonocytic cells, despite considerable overlap of the two emission spectra (), but the blood flow remains invisible without IRR (Vid. S9). Capturing surface and cytosolic signal from different cell types in one PMT provides the advantage that other detectors remain available for IRR (Vid. S10), inverse IRR (Vid. S11), or additional fluorochromes such as nuclear stains (Vid. S12). CD11c-EYFP miceCitation26 injected with mitochondrial markers allow monitoring dendritic cell motility within the liver parenchyma () and together with IRR, observation of blood flow (Vid. S13). Finally, crossbreeding reporter mice that express complimentary fluorochromes in different cell types represents another useful scheme for IVM. For example, DsRed x lysM-EGFP hybrid mice enable study of the behavior of myelomonocytic cells in the uniformly red fluorescent liver parenchyma ().
Figure 4. Complementary imaging techniques for reporter mice with fluorescent immune cells. (A) Single z-slice of a lysM-EGFP mouse liver with fluorescent myelomonocytic cells (solid green cytoplasm, in this case a Kupffer cell). MitoTracker was used to visualize hepatocytes (red), CD8+ T cells were labeled with anti-mouse CD8a-PE (green outline) and nuclei are blue (Hoechst). (B) 3D reconstruction of a z-stack from a CD11c-EYFP mouse liver with fluorescent dendritic cells (yellow). Parenchymal cells are red (MitoTracker), nuclei are blue (Hoechst). Scale bar 50 μm. (C) A DsRed x lysM-EGFP hybrid mouse has red fluorescent hepatocytes and green fluorescent Kupffer and myelomonocytic cells. All other scale bars 20 µm.
Imaging malaria parasites in the liver
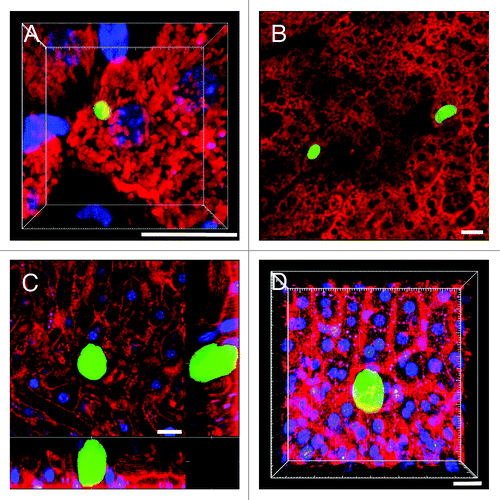
Using the P. yoelii infected BALB/c mouse, currently the model of choice due to its superior LS development rate and negligible induction of unspecific inflammation,Citation27 we demonstrate the ability of IRR to visualize dynamic events in the microvasculature surrounding the parasites. Fluorescence emission by GFP expressing P. yoelii XNL (PyXNL-GFP) allows detection of both immature (; Vid. S14) and mature LS (). Direct () and inverse () IRR dramatically enhances visualization of the sinusoidal architecture. Supported by traditional organelle markers that visualize the non-fluorescent liver parenchyma, these techniques readily demonstrate vascular perfusion around the LS (Vid. S15).
Figure 5. Using IRR to visualize the sinusoidal blood flow surrounding Plasmodium LS in non-fluorescent mice. (A) 3D volume view of a mouse liver harboring a 22 h PyXNL-GFP LS (green). Hepatocytes are labeled with MitoTracker (red), nuclei are blue (Hoechst). (B) MitoTracker (red) labels the hepatocyte plates surrounding two 42 h PyXNL-GFP LS (green). (C) IRR highlights the sinusoidal microvasculature (red) surrounding a 42 h PyXNL-GFP LS (green). Optical xy-xz-yz slice view, obtained with 50% 594 nm and 633 nm HeNe laser output power and PMT set to 590–850 nm. Nuclei are blue (Hoechst). (D) 3D volume view shows a 42 h PyXNL-GFP LS (green) embedded in non-fluorescent liver tissue. The dense sinusoidal network (red) was visualized using 50% 594 nm and 633 nm HeNe laser output power and setting the PMT to 590–850 nm. Nuclei are blue (Hoechst). Scale bars 20 μm.
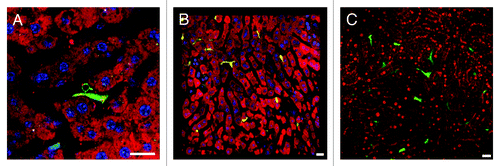
In situations where reporter mice and parasites share the same fluorescent protein, for example PyXNL-GFP infected Tie2-GFP mice, Plasmodium LS can nevertheless be distinguished from endothelia based on morphology (). Parasite host combinations with complementary fluorochromes, for instance PyXNL-DsRed infected lysM-EGFP mice, are preferable as they allow analysis of close encounters between parasite and host immune cells (Fig. S1). Combined with IRR, this approach significantly improves visualization of immune cell adhesion to the endothelium (Vid. S16). Similarly, dendritic cell interactions with LS are readily traceable in PyXNL-DsRed infected CD11c-EYFP mice ().
Figure 6. Visualization of the sinusoidal architecture aids in analysis of the Plasmodium infected liver. (A) Optical xy-xz-yz slice view of a 42 h PyXNL-GFP LS in a Tie2-GFP mouse liver. Despite expression of the same green fluorescent protein, endothelia can readily be distinguished from the large LS (arrows). Kupffer cells were identified by phagocytic uptake of dextran-rhodamine (red). (B) 3D reconstruction showing a 42 h PyXNL-DsRed LS (red) surrounded by dendritic cells (green) in a CD11c-EYFP mouse liver. Nuclei are blue (Hoechst). (C) Optical xy-xz-yz slice view of a non-fluorescent 42 h PyXNL-wt LS in a DsRed mouse liver. The parasite is identifiable by the large number of small blue merozoite nuclei (Hoechst). The sinusoidal architecture was visualized by IRR (green) using 40% output power of a 633 nm HeNe laser and PMT set to 630–750 nm. DsRed fluorescence is most pronounced in hepatocytes. See Figure S2 for individual color channels. (D) A non-fluorescent 42 h PyXNL-wt LS in a lysM-EGFP mouse with green Kupffer and myelomonocytic cells. Hepatocytes are red (MitoTracker). The small punctate merozoite nuclei of the mature LS can readily be distinguished from the much larger hepatocyte nuclei (both stained blue with Hoechst). Scale bars 20 µm.
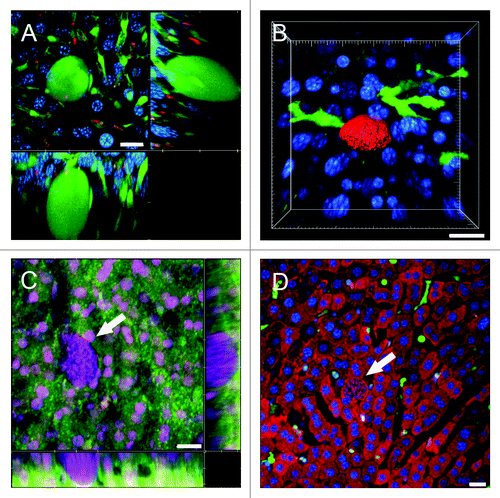
In contrast to fluorescent parasites, wt parasites are inherently difficult to locate in the liver. Because wt parasites frequently produce considerably higher sporozoite loads in the mosquitoes’ salivary glands compared with fluorescent mutants, and consequently less time needs to be spent on purification of the several million sporozoites required for IVM, we deemed it essential to develop a method for identification of wt LS in the liver when fluorescent parasites are scarce or not available.
Distinct punctate DNA staining of the hundreds of merozoite nuclei that eventually fill the entire volume of mature parasites readily identifies the location of non-fluorescent PyXNL-wt LS in the liver parenchyma of DsRed (; Fig. S2) or Tie2-GFP mice (Fig. S3). In lysM-EGFP mice, combined mitochondrial and nuclear staining facilitates determining the position of PyXNL-wt LS relative to recruited myelomonocytic cells (; Fig. S4). IRR dramatically improves visualization of blood flow and the behavior of myelomonocytic cells patrolling the sinusoids surrounding PyXNL-wt LS (Vid. S17). By providing structural details of the liver parenchyma, mitochondrial staining also reveals the space occupied by non-fluorescent LS (Fig. S4). We anticipate that these methods will be useful to visualize, in the absence of fluorescent reporters, P. falciparum parasites in humanized mouse models.Citation28
Discussion
Collection of IRR signal from non-fluorescent structures not only greatly improves visualization of the sinusoidal microvasculature and the flow of blood cells, but also provides advantages over more traditional approaches. IRR generates a completely uniform image of the microvasculature, while vascular endothelia of Tie2-GFP mice, for example, fluoresce in the arterial rather than the venous portion of the microvasculature and thus highlight the periphery rather than the center of the liver lobuleCitation18 (Frevert, unpublished data). Similarly, the widely used endothelial marker PECAM-1 is not universally expressed throughout the entire microvascular tree.Citation29 Also, cross-linking of cell surface molecules by antibody-mediated labeling may elicit unwanted cellular responses, an important consideration for IVM studies.
Without a fluorescent tracer, the hepatic microvascular plasma appears typically dark when imaged by epifluorescence/video, confocal or 2P microscopy.Citation13,Citation18,Citation30-Citation32 IRR is particularly valuable for organs such as liver, spleen or bone marrow, whose sinusoidal capillaries are lined with fenestrated endothelia. Soluble vascular markers, while widely used for IVM of organs with a continuous endothelium, are less suitable for long-term microcirculatory studies of the liver because the sinusoidal hepatic microvasculature allows tracers such as fluorochrome-conjugated albumin or low molecular weight dextran to rapidly diffuse out of the vascular lumen into the space of Disse where they are internalized by hepatocytes, either for degradation or for secretion into the bile.Citation4,Citation14 In fact, small dyes such as rhodamine 123 have been used to measure the kinetics of hepatocytic uptake.Citation31 Further, Evans blue, a tracer that is commonly used as a fluorescent plasma marker based on its reaction with albumin,Citation33,Citation34 conjugates to other proteins as well and also stains cells thus diminishing its value for liver IVM. Large fluorescent molecules, beads, or liposomes, while ideally suited for clearance studies, are equally inappropriate as tracers for the hepatic microcirculation, because any corpuscular material is rapidly cleared from the blood by Kupffer cells, resident phagocytes that line the liver sinusoids in large numbers.Citation18,Citation35-Citation41 IRR is also well suited for microcirculatory studies of other organs, for example the brain, where fluorescent tracers remain strictly confined to the vascular lumen. Monitoring blood brain barrier impairment and the ensuing uptake of plasma into the parenchyma will still require the use of vascular tracers, however (Movila et al., unpublished data).Citation29
Prerequisite for monitoring microbial intruders in the hepatic microenvironment and the ensuing innate or acquired response is visualization of immune cells and their interaction with the various non-parenchymal antigen-presenting cells of the liver.Citation3,Citation23 Many years of liver IVM in the McCuskey lab have provided detailed knowledge on the biology and function of Kupffer cells and the regulation of the hepatic microcirculation in health and disease.Citation30,Citation36-Citation41 More recently, extensive liver IVM studies in the Kubes lab have revealed that recruitment of neutrophils and platelets in sepsis and sterile inflammation is mediated by CD44/hyaluronan and Mac-1/ICAM-1, respectively.Citation42-Citation47 Liver IVM was used to monitor CD1d-reactive CXCR6-positive NKT cells patrolling the sinusoids based on recognition of endothelial CXCL16,Citation10 to study macrophage and T cell dynamics in the course of mycobacterial granuloma formation,Citation11,Citation12 and to characterize the behavior of CD8+ effector T cells in the livers of Plasmodium infected and immunized mice (Cabrera et al., manuscript in preparation). We envision that future studies aiming at visualizing immune cells traveling in the hepatic microvasculature, adhesion to the endothelium and invasion of the parenchyma will greatly benefit from direct or inverse IRR. Further, IRR will help associate the upregulation of molecules such as ICAM-1, CXCR6, or CD14 during vascular inflammation with specific sections of the microvasculature.Citation10,Citation29,Citation48 Last not least, because inverse IRR depicts (non-fluorescent) blood cells as bright objects on a dark background, this approach may eliminate the need for re-inoculation of ex vivo labeled erythrocytes for velocity measurements.Citation5 Thus, the potential applications of this novel imaging technique are manifold.
Overall, facilitated by the increasing availability of imaging instrumentation with spectral capability and a vast fluorochrome library, the presented approaches are readily adaptable to two-photon microscopy for greater depth of data acquisition or spinning disk confocal microscopy for enhanced speed, for study of the broad spectrum of microbial infections of the liver, analysis of other hepatic functions and IVM of other organs.
Materials and Methods
Ethics statement
This study was conducted in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The protocol was approved by the Institutional Animal Care and Use Committee, NYU School of Medicine (Protocol number 120213-01). All surgery was performed under ketamine/xylazine/acepromazine anesthesia, and all efforts were made to minimize suffering.
Parasites
Wild-type P. yoelii strain 17XNL (PyXNL-wt), P. yoelii 17XNL expressing GFP (PyXNL-GFP)Citation13 and P. yoelii 17XNL expressing DsRed (PyXNL-DsRed)Citation49 were purified from the salivary glands of female Anopheles stephensi mosquitoes. PyXNL-GFP and PyXNL-DsRed parasites emit fluorescence throughout the entire life cycle.
Mice and crossbreeding
Swiss Webster mice (Taconic Farms, Inc.) were used for propagation of the parasite cycles. Because of the limited liver surface area available for IVM analysis, high parasite densities are essential for acquisition of sufficient data sets for statistical analysis. BALB/c mice (Taconic Farms, Inc.), in particular the BALB/cAnNHsd strain (Harlan Laboratories), were chosen based on their superior susceptibility to infection with P. yoelii. To generate maximum numbers of Plasmodium LS in fluorescent reporter mice, the following mouse strains were backcrossed into BALB/c background: DsRed mice that express red fluorescent protein in all cells (B6.Cg-Tg(CAG-DsRed*MST)1Nagy/J, Stock 006051, Jackson Laboratory); ECFP mice that express cyan fluorescent protein in all cells (B6.129(ICR)-Tg(CAG-ECFP)CK6Nagy/J, Stock 004218, Jackson Laboratory); Tie2-GFP mice that express GFP in vascular endothelia (TgN(TIE2GFP)287Sato/J, Stock 003658, Jackson Laboratory)Citation24; lysM-EGFP mice that express EGFP in the myelomonocytic lineage (a kind gift from Dr Thomas Graf)Citation25; and CD11c-YFP mice that express EYFP in dendritic cells [B6.Cg-Tg(Itgax-EYFP)1Mnz/J, Stock 008829, Jackson Laboratory].Citation26
Anesthesia, surgery and intravital microscopy
Mice were infected by intravenous inoculation of at least 1–2 million sporozoites. At various time points after infection, mice were anesthetized with a cocktail of 50 mg/kg Ketamine HCl (Ketaset, Fort Dodge Animal Health), 10 mg/kg Xylazine (Lloyd Laboratories, 4821) and 1.7 mg/kg Acepromazine Maleate (Butler Animal Health Supply, 003845) (KXA mix) as described.Citation13,Citation18 The mouse peritoneal cavity was opened along the rib cage and the liver exposed for IVM. The mouse was then positioned on its left side on a custom-made microscope stage adaptor with a 3 cm opening covered with a 45 × 50 mm coverslip (Fisher Scientific, 12-545H). The left liver lobe was gently teased out onto the coverslip with a cotton-tipped applicator and immobilized with gauze moistened with PBS pH 7.0. The adaptor was then inserted into the stage of an inverted Leica DMIRE2 microscope equipped with a temperature controlled Ludin chamber and the liver was analyzed with a Leica TCS SP2 AOBS confocal system (40× HCX PL APO 1.25–0.75 oil lens) with the following excitation light sources and laser lines: 405 nm (Diode laser), 488 nm (Argon/ Krypton laser) and 543, 594 and 633 nm (HeNe lasers). Periodic reinjection of KXA mix at 60–90 min intervals allowed imaging for at least 4 h.
Direct and inverse IRR
To visualize the architecture of the liver tissue and the sinusoidal blood flow in non-fluorescent mice, a combination of reflected light and autofluorescence was recorded. Liver autofluorescence was collected at a distance of least 5 nm from the excitation line using any one of the HeNe lasers. Reflected light was collected in a narrow wavelength setting of 3 nm on either side of the excitation line. IRR was done by collecting reflection plus autofluorescence in one of the AOBS photomultiplier tubes (PMT) by setting the spectra cutoffs to include a reflection excitation line and its corresponding autofluorescence emission, or by collecting autofluorescence from a shorter HeNe line plus reflection from the next longer laser excitation source. Inverse IRR was done correspondingly except that one of the inverse color Leica look-up tables (LUT) was used.
Cellular markers
Prior to imaging, mice were intravenously or intraperitoneally inoculated with 648 nmol of the nuclear stain Hoechst 33342 (λex = 405 nm, Invitrogen, H3570), 18.4 nmol MitoTracker Deep Red (λex = 633 nm, Invitrogen, M22426), 7.5 μg CellMask Orange plasma membrane stain (λex = 543 nm, Invitrogen, C10045), 125 pmol rhodamine-dextran (MW 2,000 kDa, λex = 543 nm) to visualize Kupffer cell phagocytosis (all from Invitrogen), 2 μg of PE-conjugated anti-mouse CD8a (Clone 53–6.7, λex = 488 nm; eBiosciences, 12-0081), or 4.5 μg of Alexa Fluor 647 conjugated anti-mouse CD31 (Clone MEC13.3, λex = 633nm; BioLegend, 102516) in matching color combinations.
Image processing
After acquisition with Leica LCS software, 2D, 3D or 4D data sets were reconstructed and processed in Imaris 7.4.2 (Bitplane). Composite figures were generated in Adobe Photoshop CS4 (Adobe Systems Inc.).
| Abbreviations: | ||
| APC | = | antigen-presenting cell |
| CFP | = | cyan fluorescent protein |
| LS | = | liver stage |
| GFP | = | green fluorescent protein |
| IRR | = | intravital reflection recording |
| IVM | = | intravital microscopy |
| LUT | = | lookup table |
| PE | = | phycoerythrin |
| PMT | = | photomultiplier tube |
| PyXNL | = | Plasmodium yoelii strain XNL |
| YFP | = | yellow fluorescent protein |
| 2P | = | two-photon |
Additional material
Download Zip (58.6 MB)Acknowledgments
The authors wish to thank Drs Elizabeth Nardin, P’ng Loke, Jerome Vanderberg, and Stefan Kappe for helpful comments on the manuscript. The work was supported by grants RO1 AI070894 and S10 RR019288 from the National Institutes of Health (U.F.).
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Supplemental Materials
Supplemental materials may be found here: www.landesbioscience.com/journals/intravital/article/23423
References
- Racanelli V, Rehermann B. The liver as an immunological organ. Hepatology 2006; 43:Suppl 1 S54 - 62; http://dx.doi.org/10.1002/hep.21060; PMID: 16447271
- Crispe IN. The liver as a lymphoid organ. Annu Rev Immunol 2009; 27:147 - 63; http://dx.doi.org/10.1146/annurev.immunol.021908.132629; PMID: 19302037
- Knolle PA, Gerken G. Local control of the immune response in the liver. Immunol Rev 2000; 174:21 - 34; http://dx.doi.org/10.1034/j.1600-0528.2002.017408.x; PMID: 10807504
- Uhlmann S, Uhlmann D, Spiegel HU. Evaluation of hepatic microcirculation by in vivo microscopy. J Invest Surg 1999; 12:179 - 93; http://dx.doi.org/10.1080/089419399272458; PMID: 10501077
- Clemens MG, Zhang JX. Regulation of sinusoidal perfusion: in vivo methodology and control by endothelins. Semin Liver Dis 1999; 19:383 - 96; http://dx.doi.org/10.1055/s-2007-1007127; PMID: 10643624
- Pries AR, Gaehtgens P. Digital video image-shearing device for continuous microvessel diameter measurement. Microvasc Res 1987; 34:260 - 7; http://dx.doi.org/10.1016/0026-2862(87)90060-4; PMID: 3670118
- Ley K, Pries AR, Gaehtgens P. A versatile intravital microscope design. Int J Microcirc Clin Exp 1987; 6:161 - 7; PMID: 3596913
- Gräf R, Rietdorf J, Zimmermann T. Live cell spinning disk microscopy. Adv Biochem Eng Biotechnol 2005; 95:57 - 75; PMID: 16080265
- Velázquez P, Cameron TO, Kinjo Y, Nagarajan N, Kronenberg M, Dustin ML. Cutting edge: activation by innate cytokines or microbial antigens can cause arrest of natural killer T cell patrolling of liver sinusoids. J Immunol 2008; 180:2024 - 8; PMID: 18250405
- Geissmann F, Cameron TO, Sidobre S, Manlongat N, Kronenberg M, Briskin MJ, et al. Intravascular immune surveillance by CXCR6+ NKT cells patrolling liver sinusoids. PLoS Biol 2005; 3:e113; http://dx.doi.org/10.1371/journal.pbio.0030113; PMID: 15799695
- Egen JG, Rothfuchs AG, Feng CG, Horwitz MA, Sher A, Germain RN. Intravital imaging reveals limited antigen presentation and T cell effector function in mycobacterial granulomas. Immunity 2011; 34:807 - 19; http://dx.doi.org/10.1016/j.immuni.2011.03.022; PMID: 21596592
- Egen JG, Rothfuchs AG, Feng CG, Winter N, Sher A, Germain RN. Macrophage and T cell dynamics during the development and disintegration of mycobacterial granulomas. Immunity 2008; 28:271 - 84; http://dx.doi.org/10.1016/j.immuni.2007.12.010; PMID: 18261937
- Baer K, Klotz C, Kappe SHIK, Schnieder T, Frevert U. Release of hepatic Plasmodium yoelii merozoites into the pulmonary microvasculature. PLoS Pathog 2007; 3:e171; http://dx.doi.org/10.1371/journal.ppat.0030171; PMID: 17997605
- Babbey CM, Ryan JC, Gill EM, Ghabril MS, Burch CR, Paulman A, et al. Quantitative intravital microscpy of hepatic transport. Intravital 2012; 1:1 - 10; http://dx.doi.org/10.4161/intv.21296
- Krzych U, Guebre-Xabier M, Schwenk R. Malaria and the liver: tolerance and immunity to attenuated Plasmodium sporozoites. In: Crispe IN, ed. T Lymphocytes in the Liver: Immunobiology, Pathology, and Host Defence. New York: John Wiley & Sons, Inc., 1999:163-95.
- Frevert U, Usynin I, Baer K, Klotz C. Nomadic or sessile: can Kupffer cells function as portals for malaria sporozoites to the liver?. Cell Microbiol 2006; 8:1537 - 46; http://dx.doi.org/10.1111/j.1462-5822.2006.00777.x; PMID: 16911567
- Sturm A, Amino R, van de Sand C, Regen T, Retzlaff S, Rennenberg A, et al. Manipulation of host hepatocytes by the malaria parasite for delivery into liver sinusoids. Science 2006; 313:1287 - 90; http://dx.doi.org/10.1126/science.1129720; PMID: 16888102
- Frevert U, Engelmann S, Zougbédé S, Stange J, Ng B, Matuschewski K, et al. Intravital observation of Plasmodium berghei sporozoite infection of the liver. PLoS Biol 2005; 3:e192; http://dx.doi.org/10.1371/journal.pbio.0030192; PMID: 15901208
- Nardin E. The past decade in malaria synthetic peptide vaccine clinical trials. Hum Vaccin 2010; 6:27 - 38; http://dx.doi.org/10.4161/hv.6.1.9601; PMID: 20173408
- Khan SM, Janse CJ, Kappe SH, Mikolajczak SA. Genetic engineering of attenuated malaria parasites for vaccination. Curr Opin Biotechnol 2012; 23:1 - 9; http://dx.doi.org/10.1016/j.copbio.2012.04.003; PMID: 22244690
- Sauerwein RW, Bijker EM, Richie TL. Empowering malaria vaccination by drug administration. Curr Opin Immunol 2010; 22:367 - 73; http://dx.doi.org/10.1016/j.coi.2010.04.001; PMID: 20434895
- Hoffman SL, Billingsley PF, James E, Richman A, Loyevsky M, Li T, et al. Development of a metabolically active, non-replicating sporozoite vaccine to prevent Plasmodium falciparum malaria. Hum Vaccin 2010; 6:97 - 106; http://dx.doi.org/10.4161/hv.6.1.10396; PMID: 19946222
- Crispe IN. Liver antigen-presenting cells. J Hepatol 2011; 54:357 - 65; http://dx.doi.org/10.1016/j.jhep.2010.10.005; PMID: 21084131
- Motoike T, Loughna S, Perens E, Roman BL, Liao W, Chau TC, et al. Universal GFP reporter for the study of vascular development. Genesis 2000; 28:75 - 81; http://dx.doi.org/10.1002/1526-968X(200010)28:2<75::AID-GENE50>3.0.CO;2-S; PMID: 11064424
- Faust N, Varas F, Kelly LM, Heck S, Graf T. Insertion of enhanced green fluorescent protein into the lysozyme gene creates mice with green fluorescent granulocytes and macrophages. Blood 2000; 96:719 - 26; PMID: 10887140
- Lindquist RL, Shakhar G, Dudziak D, Wardemann H, Eisenreich T, Dustin ML, et al. Visualizing dendritic cell networks in vivo.. Nat Immunol 2004; 5:1243 - 50; http://dx.doi.org/10.1038/ni1139; PMID: 15543150
- Khan ZM, Vanderberg JP. Role of host cellular response in differential susceptibility of nonimmunized BALB/c mice to Plasmodium berghei and Plasmodium yoelii sporozoites. Infect Immun 1991; 59:2529 - 34; PMID: 1855974
- Vaughan AM, Mikolajczak SA, Wilson EM, Grompe M, Kaushansky A, Camargo N, et al. Complete Plasmodium falciparum liver-stage development in liver-chimeric mice. J Clin Invest 2012; 122:3618 - 28; http://dx.doi.org/10.1172/JCI62684; PMID: 22996664
- Nacer A, Movila A, Baer K, Mikolajczak SA, Kappe SHI, Frevert U. Neuroimmunological blood brain barrier opening in experimental cerebral malaria. PLoS Pathog 2012; 8:e1002982; http://dx.doi.org/10.1371/journal.ppat.1002982; PMID: 23133375
- Farrell GC, Teoh NC, McCuskey RS. Hepatic microcirculation in fatty liver disease. Anat Rec (Hoboken) 2008; 291:684 - 92; http://dx.doi.org/10.1002/ar.20715; PMID: 18484615
- Liu X, Thorling CA, Jin L, Roberts MS. intravital multiphoton imaging of rhodamine 123 in the rat liver after intravenous dosing. Intravital 2012; 1:1 - 6; http://dx.doi.org/10.4161/intv.21450
- Jenne CN, Wong CH, Petri B, Kubes P. The use of spinning-disk confocal microscopy for the intravital analysis of platelet dynamics in response to systemic and local inflammation. PLoS One 2011; 6:e25109; http://dx.doi.org/10.1371/journal.pone.0025109; PMID: 21949865
- Warnick RE, Fike JR, Chan PH, Anderson DK, Ross GY, Gutin PH. Measurement of vascular permeability in spinal cord using Evans Blue spectrophotometry and correction for turbidity. J Neurosci Methods 1995; 58:167 - 71; http://dx.doi.org/10.1016/0165-0270(94)00172-D; PMID: 7475223
- Nag S. Blood-brain barrier permeability using tracers and immunohistochemistry. In: Nag S, ed. The Blood-Brain Barrier. Totowa, NJ: Humana Press, 2003:133-44.
- Baer K, Roosevelt M, Clarkson AB Jr., van Rooijen N, Schnieder T, Frevert U. Kupffer cells are obligatory for Plasmodium yoelii sporozoite infection of the liver. Cell Microbiol 2007; 9:397 - 412; http://dx.doi.org/10.1111/j.1462-5822.2006.00798.x; PMID: 16953803
- McCuskey RS, Urbaschek R, McCuskey PA, Urbaschek B. In vivo microscopic observations of the responses of Kupffer cells and the hepatic microcirculation to Mycobacterium bovis BCG alone and in combination with endotoxin. Infect Immun 1983; 42:362 - 7; PMID: 6352499
- Li C, McCuskey P, Kan Z, Yang DJ, Wright KC, Wallace S. In vivo and electron microscopic studies of rat liver after intravenous injection of polyamino acid microspheres. J Biomed Mater Res 1994; 28:881 - 90; http://dx.doi.org/10.1002/jbm.820280807; PMID: 7527046
- Eguchi H, McCuskey PA, McCuskey RS. Kupffer cell activity and hepatic microvascular events after acute ethanol ingestion in mice. Hepatology 1991; 13:751 - 7; http://dx.doi.org/10.1002/hep.1840130423; PMID: 2010170
- McCuskey RS, McCuskey PA, Eguchi H, Crichton EG, Urbaschek R, Urbaschek B. In vivo microscopy of the liver following acute administration of ethanol. Prog Clin Biol Res 1990; 325:341 - 50; PMID: 2300616
- Ivancev K, Lunderquist A, McCuskey R, McCuskey P, Wretlind A. Effect of intravenously injected iodinated lipid emulsion on the liver. An experimental study correlating computed tomography findings with in vivo microscopy and electron microscopy findings. Acta Radiol 1989; 30:291 - 8; http://dx.doi.org/10.3109/02841858909174683; PMID: 2544217
- Post S, Gonzalez AP, Palma P, Rentsch M, Stiehl A, Menger MD. Assessment of hepatic phagocytic activity by in vivo microscopy after liver transplantation in the rat. Hepatology 1992; 16:803 - 9; http://dx.doi.org/10.1002/hep.1840160329; PMID: 1505924
- Cara DC, Kubes P. Intravital microscopy as a tool for studying recruitment and chemotaxis. Methods Mol Biol 2004; 239:123 - 32; PMID: 14573914
- McDonald B, Pittman K, Menezes GB, Hirota SA, Slaba I, Waterhouse CC, et al. Intravascular danger signals guide neutrophils to sites of sterile inflammation. Science 2010; 330:362 - 6; http://dx.doi.org/10.1126/science.1195491; PMID: 20947763
- McDonald B, Kubes P. Neutrophils and intravascular immunity in the liver during infection and sterile inflammation. Toxicol Pathol 2012; 40:157 - 65; http://dx.doi.org/10.1177/0192623311427570; PMID: 22105645
- Phillipson M, Kubes P. The neutrophil in vascular inflammation. Nat Med 2011; 17:1381 - 90; http://dx.doi.org/10.1038/nm.2514; PMID: 22064428
- Menezes GB, Mansur DS, McDonald B, Kubes P, Teixeira MM. Sensing sterile injury: opportunities for pharmacological control. Pharmacol Ther 2011; 132:204 - 14; http://dx.doi.org/10.1016/j.pharmthera.2011.07.002; PMID: 21763344
- Wong CH, Heit B, Kubes P. Molecular regulators of leucocyte chemotaxis during inflammation. Cardiovasc Res 2010; 86:183 - 91; http://dx.doi.org/10.1093/cvr/cvq040; PMID: 20124403
- Menezes GB, Lee WY, Zhou H, Waterhouse CC, Cara DC, Kubes P. Selective down-regulation of neutrophil Mac-1 in endotoxemic hepatic microcirculation via IL-10. J Immunol 2009; 183:7557 - 68; http://dx.doi.org/10.4049/jimmunol.0901786; PMID: 19917697
- Jacobs-Lorena VY, Mikolajczak SA, Labaied M, Vaughan AM, Kappe SH. A dispensable Plasmodium locus for stable transgene expression. Mol Biochem Parasitol 2010; 171:40 - 4; http://dx.doi.org/10.1016/j.molbiopara.2009.12.009; PMID: 20045029