Abstract
Introduction: The alveolar capillary bed, which appears essential for the maintenance of alveolar septa, is altered in pulmonary emphysema. Until recently, techniques that allow its analysis in vivo in spontaneously breathing conditions were lacking. Fibered confocal fluorescence microscopy (FCFM) is a new technique that enables distal lung microstructures imaging in vivo. FCFM can be coupled with I.V fluorescein injection to image the pulmonary capillary network. The aim of this study was to assess the lung microcirculation in vivo using FCFM and I.V fluorescein in rats with experimental emphysema.
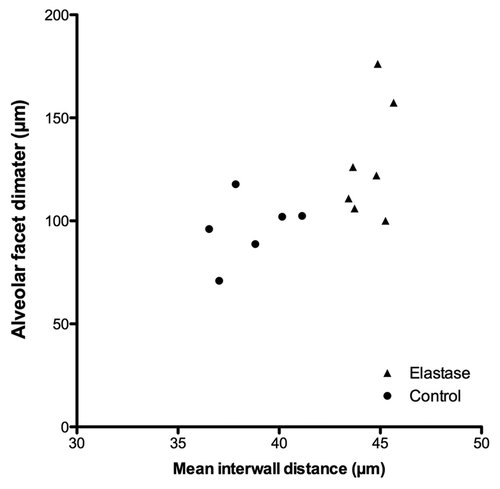
Results: In vivo pulmonary microcirculation imaging was possible in 7/7 elastase animals and in 6/7 controls. Using FCFM, intercapillary distances and alveolar facets diameters were found significantly higher in the elastase group compared with controls (49.5 vs. 41.8 µm p < 0.001, and 118.5 vs. 95.1 µm p < 0.001, respectively). Ex vivo mean interwall distance (MIWD) was correlated with the alveolar facets diameters measured in vivo (rs = 0.65 ; p = 0.016).
Methods: 14 Sprague-Dawley rats were assigned to intratracheal instillation of porcine pancreatic elastase (n = 7) or saline (n = 7). The subpleural microcirculation was assessed using FCFM in spontaneously breathing rats, through a 2mm thoracic window using a continuous aspiration system, after I.V. injection of fluorescein-dextran. FCFM sequences were recorded and the image analysis was performed separately by two observers, blindly to the animal group. Fluorescence intensity (FI), maximal intercapillary distances, and alveolar facets diameters measured with FCFM were compared between groups, and to ex vivo lung morphometric measurements (MIWD).
Conclusion: FCFM allows the quantitative assessment of the microcirculation alterations due to emphysema in vivo.
Introduction
Pulmonary emphysema is anatomically defined as the abnormal and permanent destruction of the distal airways up to the terminal bronchiole, with the destruction of the walls and no evident fibrosis.Citation1 The pathogenesis of pulmonary emphysema is complex, involving abnormal inflammatory response to inhalation of noxious particles and/or gases,Citation2 and protease/antiprotease imbalance.Citation3 Recently, the disruption of a lung cellular and molecular maintenance program involving alterations of the pulmonary capillary bed was proposed as one of the mechanisms leading to emphysema.Citation4-Citation7 Early ex vivo studies have described the loss of the pulmonary capillary bed in emphysematous lungs,Citation8 and COPD patients have a reduced pulmonary capillary length and density.Citation9 The quantitative assessment of emphysema has been largely studied with the development of software techniques for the analysis of CT or MRI images.Citation10-Citation15 However, there is currently no mean to assess the modifications of the human pulmonary microcirculation in vivo with the progression of the disease, even if new technologies such as optical microangiography (OMAG) emerge for the study of pulmonary microcirculation in animals,Citation16 or other organs, skin and retina, in humans.Citation17,Citation18
Fibered confocal fluorescence microscopy (FCFM), also called probe-based confocal laser endomicroscopy (pCLE), is a new minimally invasive endoscopic technique that enables to image a living tissue at the microscopic level. The procedure makes it possible to explore the proximal bronchi as well as alveolar regions in real time during bronchoscopy in patients under topical anesthesia.Citation19-Citation22 We previously demonstrated that the main endogenous fluorophore, observed with pCLE at 488 nm excitation wavelength, is the elastin, which is a major component of the distal lung interstitial network, and is present in the axial backbone of the alveolar ducts and alveolar entrances.Citation23,Citation24 In this human study, the elastic fibers and the microvessels could be measured during the procedure, and the first morphometric analysis of the normal acinar and alveolar structures in vivo have been reported.Citation20 However, pulmonary capillaries cannot be observed without intravascular contrast.Citation20
The aim of this experimental study is to investigate whether FCFM is able to assess and quantify emphysema in vivo, on the basis of the loss of the pulmonary capillary network in elastase-induced emphysema.
Results
Observation of the subpleural microcirculation in vivo using FCFM and I.V. fluorescein
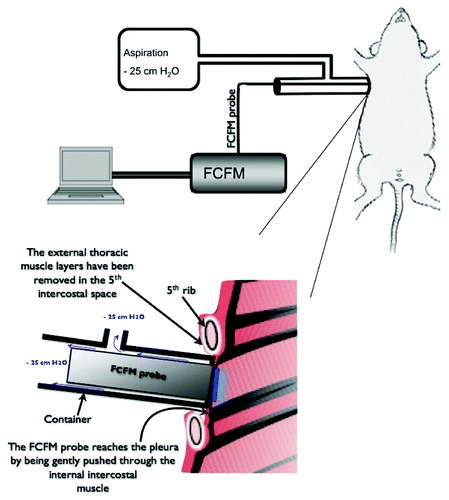
To achieve the subpleural microcirculation imaging, the miniprobe was introduced into the thorax through a 2 mm surgical opening in the right 5th intercostal space, and a customized aspiration system was used to hold the miniprobe in order to prevent the lung to collapse (). Special care was taken to avoid any mechanical damage onto the lung surface. After the smooth tip of the imaging probe was properly placed against the pleura, 0.8 ml/kg of 1% w/v fluorescein-dextran 150 kD was administered intravenously. The microscopic fluorescent imaging was then observed in real-time and recorded for further quantitative analysis. The imaging procedure was interrupted immediately if the lung collapsed
Figure 1. Fiberd confocal fluorescence microscopy (FCFM) associated to a continuous aspiration system to assess the subpleural microcirculation in spontaneously breathing animals. The FCFM probe is sealed into a cylindrical chamber that is connected to an aspiration device (vacuum pump) providing a continuous depression of 25 cm H2O into the chamber. The extremity of the chamber, where the probe-tip emerges, is sealed to the lateral thoracic wall. The chest had been surgically prepared by removing the thoracic muscles layers up the internal intercostal muscle. The probe is then applied through the last muscle onto the pleural surface.
Before fluorescein injection, subpleural 488 nm FCFM microimaging of normal and emphysematous lung did not show any fluorescent signal. In contrast, a strong and clear intravascular signal could be observed immediately after the fluorescein-dextran IV injection, with a maximal intensity obtained after 2 min, which remained stable during 15 min after the injection (Supplemental Materials). This allowed subpleural microvascular imaging in 7/7 animals in the elastase group and 6/7 in the control group, where the procedure was unsuccessful in one case because of lung collapse.
Two main patterns could be observed, corresponding to subpleural capillary network and extra-alveolar vessels (), and, later during the imaging acquisition, the edges of the subpleural alveolar facets (), framing the peripheral alveoli.
Figure 2. The subpleural capillary network observed with fiberd confocal fluorescence microscopy (FCFM) after IV injection of fluorescein. (A) The subpleural capillary network is easily recognized, and extra-alveolar vessels can be observed (*). (B) The intercapillary distance was measured on selected frames displaying typical capillary network. The ten maximal intercapillary distances were selected for the quantitative analysis, and are displayed as median ± interquartile range for each animal. Inter-individual variability, assessed by the inter-quartile range of the medians was [30–55] µm in the control group, and [42–66] µm in the elastase group. (C) The intercapillary distance was higher in the elastase-treated animals (median = 49.5µm [IQR (40.8 - 62.4)]) than in controls (median = 41.8µm [IQR (32.7 – 51.9)]) (p < 0.001, Mann-Whitney test).
![Figure 2. The subpleural capillary network observed with fiberd confocal fluorescence microscopy (FCFM) after IV injection of fluorescein. (A) The subpleural capillary network is easily recognized, and extra-alveolar vessels can be observed (*). (B) The intercapillary distance was measured on selected frames displaying typical capillary network. The ten maximal intercapillary distances were selected for the quantitative analysis, and are displayed as median ± interquartile range for each animal. Inter-individual variability, assessed by the inter-quartile range of the medians was [30–55] µm in the control group, and [42–66] µm in the elastase group. (C) The intercapillary distance was higher in the elastase-treated animals (median = 49.5µm [IQR (40.8 - 62.4)]) than in controls (median = 41.8µm [IQR (32.7 – 51.9)]) (p < 0.001, Mann-Whitney test).](/cms/asset/9415d21f-2e8b-453c-b4c8-14878bceac0a/kinv_a_10923471_f0002.gif)
Figure 3. The subpleural alveolar facets could be observed with fiberd confocal fluorescence microscopy (FCFM) after a few minutes of examination. (A) The ten maximal diameters were selected for the quantitative analysis. (B) The 10 measures per animal are displayed as median ± interquartile range. Inter-individual variability, assessed by the inter-quartile range of the medians was [84–106] µm in the control group, and [104–152] µm in the elastase group. (C) The alveolar facets diameters were significantly higher in the elastase-treated animals (median = 118.5µm [IQR (97.2 - 145.9)]) than in controls (median = 95.1µm [IQR (81.6 - 106.8)]) (p < 0.0001, Mann-Whitney test).
![Figure 3. The subpleural alveolar facets could be observed with fiberd confocal fluorescence microscopy (FCFM) after a few minutes of examination. (A) The ten maximal diameters were selected for the quantitative analysis. (B) The 10 measures per animal are displayed as median ± interquartile range. Inter-individual variability, assessed by the inter-quartile range of the medians was [84–106] µm in the control group, and [104–152] µm in the elastase group. (C) The alveolar facets diameters were significantly higher in the elastase-treated animals (median = 118.5µm [IQR (97.2 - 145.9)]) than in controls (median = 95.1µm [IQR (81.6 - 106.8)]) (p < 0.0001, Mann-Whitney test).](/cms/asset/b138351e-71aa-4f7b-aeaa-44177e8ade2b/kinv_a_10923471_f0003.gif)
The diameters of the alveolar facets were significantly higher in elastase-instilled animals than controls (median = 118.5 µm [IQR (97.2 - 145.9)] in the elastase group vs. 95.1 µm [IQR (81.6 - 106.8)] in the control group ; p < 0.0001, Mann-Whitney test) (). The measurements of the maximal intercapillary distance are illustrated in . It was significantly higher in the elastase-treated animals than in controls (median = 49.5µm [IQR (40.8 - 62.4)] in the elastase group vs. 41.8µm [IQR (32.7 – 51.9)] in the control group ; p < 0.001, Mann-Whitney test).
The intravascular fluorescence intensity was normally distributed in both elastase and control groups, and was significantly lower in treated animals as compared with controls (mean ± SD were 764.5 ± 341 vs. 926 ± 258 arbitrary units; p = 0.004, unpaired t test, ).
Figure 4. The fluorescence intensity (FI) was measured on ten frames representative of the capillary network, excluding frames displaying large extra-alveolar microvessels. (A) The 10 measures per animal are displayed as median ± interquartile range. Inter-individual variability, assessed by the inter-quartile range of the medians was [789–1120] arbitrary units (A.U.) in the control group, and [424–951] A.U. in the elastase group. (B) The mean FI was significantly lower in the elastase-treated animals (mean ± SD = 764 ± 341 A.U.) than in controls (mean ± SD = 926 ± 258 A.U.) (p = 0,004, unpaired t-test)
![Figure 4. The fluorescence intensity (FI) was measured on ten frames representative of the capillary network, excluding frames displaying large extra-alveolar microvessels. (A) The 10 measures per animal are displayed as median ± interquartile range. Inter-individual variability, assessed by the inter-quartile range of the medians was [789–1120] arbitrary units (A.U.) in the control group, and [424–951] A.U. in the elastase group. (B) The mean FI was significantly lower in the elastase-treated animals (mean ± SD = 764 ± 341 A.U.) than in controls (mean ± SD = 926 ± 258 A.U.) (p = 0,004, unpaired t-test)](/cms/asset/ac717528-daf9-4f8a-b7eb-77b75e17bd9c/kinv_a_10923471_f0004.gif)
Ex vivo measurements of distal airspace
Examples of airspace enlargement from ex vivo microscopic slides are shown in . The MIWD was higher in the elastase group compared with controls (44.8 vs. 38.3 µm) (p = 0.001, Mann Withney test) (). There was a good correlation between the MIWD and the mean diameter of the alveolar facets (rs = 0.65, p = 0,016) (). No correlation could be found between the MIWD and the fluorescence intensity (rs = -0.36 ; p = 0.22), and the mean maximal intercapillary distance (rs = -0.24 ; p = 0.43).
Discussion
Historical ex vivo studies have demonstrated the alteration of the pulmonary microcirculation during emphysema. Liebow first described in 1959 the loss of the capillary bed in the human emphysematous lung.Citation8 His findings were further confirmed by Reid and Heard,Citation25 who observed a decrease in the arteriolar branching in emphysematous tissue, in relation to the severity of the parenchymal destruction. Later, unbiased morphometric methods ex vivo showed the decreased length and length density of the capillary network in emphysematous patients.Citation9
In the present study, the use of fibered confocal fluorescence microscopy (FCFM) with an exogenous fluorophore allowed to assess the lung microcirculation changes in vivo, in spontaneously breathing animals. The technique was able to accurately assess the alteration of the subpleural microvascular network in elastase-induced emphysema. These data were consistent with ex vivo morphometric analysis based on the mean interwall distance measurements. The FCFM measurements from the control group showed a median maximal diameter of the alveolar facets of 95 µm [IQR (82 – 107)]. These results are similar to the findings of a previous study using intravital laser confocal microscopy in vivo.Citation26 Using FCFM the alveolar facet diameters appear higher than the alveolar diameter usually found in rats ex vivo (70 – 80 µm).Citation27,Citation28 An explanation could be that the published alveolar diameter is derived from averaged measurements per field of view, whereas our results are based on subpleural maximal alveolar diameters. It is admitted that histostereology is the gold-standard for the assessment of pulmonary anatomy at the microscopic level,Citation29 but histostereology relies on ex vivo and time-consuming tissue preparations. Our results showed that the alveolar facet diameter was correlated with the ex vivo morphometric analysis, therefore validating the technique to assess emphysema in vivo.
Compared with controls, in vivo exploration of emphysema by FCFM showed a 25% increase of alveolar facet maximal diameter, as well as a smaller (10%) but significant increase of the maximal intercapillary distance. This is may be due to the fact that the subpleural capillary bed is scarcer as compared with more central parts of the lungs. Along the same line, no correlation could be found between the mean interwall distance measured on histological preparations and the intercapillary distance in vivo. This probably also reflects the heterogeneity of the emphysema-related pulmonary lesions, which could not be assessed through a small thoracic window. Heterogeneity of emphysema may be particularly pronounced in the elastase intratracheal instillation model, with predominant distribution in the central parts of the lungs as a result of differences in elastase concentrations between central and peripheral airways, as compared with other experimental models.Citation30
The fluorescence intensity was significantly lower in the emphysematous rats compared with controls, corresponding to a looser microcirculation network. This result was consistent with the increased intercapillary distance in emphysematous rats. Even if the fluorescence intensity assessment was successfully used with the FCFM associated software in other animal and human studies,Citation31-Citation33 another parameter, more accurate, to assess the lung microcirculation would be blood flow modifications. Recent progress in intravital microscopy recently allowed to image changes in pulmonary blood flow. Hanna et al. used a closed-chest pulmonary window that allowed real-time images to be captured by fluorescence and multispectral absorption microscopy.Citation34 However blood-flow measurements in this study were not performed, as, if the impact of pressure artifacts on vessel morphology were limited by the technique, its consequences on blood flow could not be appreciated.
To our knowledge, this study is the first to focus on pulmonary microcirculation in emphysema in vivo. Several ex vivo animal studies on emphysema have analyzed the peripheral capillary alterations.Citation30,Citation35 Whereas they have consistently found a decrease in the subpleural capillary density, they were limited by potential artifacts due ex vivo inflation of the lung,Citation30 which was not the case in our in vivo study.
Until now, studies on the pulmonary microcirculation had used intravital microscopy in healthy isolated perfused lungCitation36 and fluorescent microspheresCitation27 or intravital confocal microscopy and fluorescein contrast in acute respiratory distress syndrome in rats,Citation26,Citation37 or in models of hypoxia or drug-induced vasoconstrictive responses in mice.Citation38 These studies relied on mechanical ventilation, which also could disturb the architecture of the subpleural microcirculation.Citation39 Recent development of intravital microscopy using two-photon microscopy (TPM) was successfully applied to the pulmonary microcirculation analysis.Citation40,Citation41 The main advantage of this technique is the possibility to increase the depth of focus into the tissue. However, the technique has some limitations related to motion artifacts due to a slow acquisition rate. Using customized TPM system, respiratory gating, and post-acquisition image processing, Presson and al. showed that the pulmonary microcirculation could be imaged in living animals, without respiratory and cardiac motion artifacts, with a comparable precision as in pump-perfused animals.Citation41
These techniques offer very high-resolution and stable images of the pulmonary microcirculation with limited motion-artifacts. However, they require high-technicity equipment and a long preparation time.Citation41
FCFM has been previously used in two animal experimental studies on isolated perfused lungCitation42 and in vivo in a model of acute lung injury.Citation43 Compared with these studies, our in vivo FCFM approach makes it possible to study spontaneously breathing animals, a condition that may be crucial in emphysema, even if the aspiration system may affect the in vivo morphometric measurements. In the future, this approach could also be applied to longitudinal studies, therefore allowing therapeutics assessment in emphysema. For instance, studies have shown that the inhibition of VEGF receptors causes emphysema in adult rats,Citation5 a mechanism that could be targeted in vivo using specific fluorescent probes and confocal laser endomicroscopy.Citation44 FCFM could be used, for example, to assess the effect of therapies that would restore the VEGF-VEGFR-2 pathway for the treatment of emphysema in animal models.Citation45
Because of the fragility of the upper respiratory tract, the transbronchial acinar imaging was not possible using the confocal miniprobe dedicated to small animals. The transthoracic approach, however, can be applied to smaller animals, i.e mice, that are usually used in emphysema studies and other lung conditions. On the other hand, this limitation can also be overcome using a transbronchial exploration in bigger animal models. Along the same line, FCFM can be easily employed for human explorations, during bronchoscopy in spontaneously breathing patients under topical anesthesia.
Human studies using FCFM for distal lung explorations are still limited.Citation20 The technique has been recently used in a preliminary study in 13 patients, finding significant alveolar entrance enlargement and distortion or thickening of the remaining elastic fibers during emphysema.Citation46 Preliminary data using FCFM and IV fluorescein in humans also showed that imaging of pulmonary capillaries in vivo is possible,Citation47 in conjunction with elastin assessment. Along with these early results, our data indicate that FCFM could be used in the future for in vivo assessment of the capillary bed alterations in emphysema in humans.
In conclusion, this study demonstrates the possibility to assess the pulmonary microcirculation in vivo in spontaneously breathing small animals, and validates the technique for in vivo measurements of the distal lung structures. Even if it did not provide new fundamental data for the comprehension of the pathophysiological changes occurring in emphysema, or new therapeutic data, fundamental research on emphysema pathogenesis may benefit, in the future, from the possibilities of in vivo microvascular imaging using endomicroscopy. Future studies using FCFM may aim at describing and understanding other pulmonary conditions, such as interstitial lung diseases,Citation33,Citation48,Citation49 and new studies are also needed to evaluate the adaptation of the technique to longitudinal studies in emphysema or other experimental models focusing on vascular-related disorders.
Methods
Animals
Fourteen 14 week-old Sprague-Dawley male rats were used in this study. All animals were housed in a temperature-controlled room (21 ± 1°C.) and were maintained on a 12:12-h light:dark cycle with free access to water and food. Animals were assigned to intra-tracheal instillationCitation50 either of porcine pancreatic elastase (750 units/ kg; Elastin Products Company) diluted in 200 µL of physiological saline solution (n = 7), or of the same volume of physiological saline solution alone (n = 7). Elastase concentration was 130 IU / mg where 1 IU is the amount of enzyme able to catalyze 1 mg of elastin at pH 8.8 and 37°C. Elastase was prepared immediately before intratracheal instillation. All procedures were performed under general anesthesia with intraperitoneal injection of ketamine and chlorpromazine (50 mg/kg and 5 mg/kg, respectively).
Fibered confocal fluorescence imaging was performed 8 weeks after intratracheal instillation. All the animals were sacrificed immediately after the imaging procedure by lethal intravenous injection of 200 mg/kg thiopenthal, and the lungs were collected for histology.
All the animal procedures were completed in accordance to the institutional animal care and use committee (N-03-07-08/11/06-12).
Fibered confocal fluorescence microscopy procedure in vivo
The catheter-based confocal fluorescence imaging of the lungs was performed with a CellVizio® Lab (Mauna Kea Technologies) in spontaneously breathing animals anesthetized by intra-peritoneal injection of thiopenthal (100 mg/kg). The confocal fluorescence imaging system uses an excitation laser source at 488 nm and enables the real-time observation of fluorescent structures with an image flow rate at 9 to 12 images per second. The lateral resolution is 3.5 µm within a 600 × 600 µm field of view (FOV). Before each imaging procedure the system was calibrated for the 1.4 mm diameter Proflex S-1400® miniprobe (Mauna Kea Technologies), with the dedicated calibration kit according to the manufacturer recommendations.
Quantitative analysis of the subpleural vascular network using fluorescence endomicroscopy
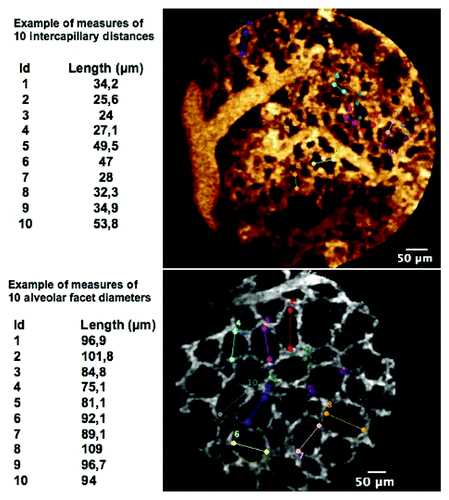
Quantitative measurements were performed concurrently by two observers (MS, LT), blindly to the animal group (elastase or saline), and from the histological results. In order to assess the density of the microvascular network, the entire FCFM sequences were analyzed. At least one representative frame showing the characteristic subpleural capillary network (capillaries were recognized based on an averaged diameter of 10 µm), as well as one frame displaying alveolar facets were selected for the analysis by each observer for each animal. The diameter of the ten largest subpleural alveolar facets and 10 measurements of the longest maximal intercapillary distance were analyzed, on each frame, using the Signal Quantification Toolbox of the Medviewer® software (Medviewer 1.1.1®, Mauna Kea Technologies) ().
Figure 7. Examples of measurements of (top) intercapillary distances, and (bottom) alveolar facets diameters.
The intensity of the intravascular fluorescent signal was assessed using the software’s Signal Quantification Toolbox, with the lower and upper level thresholds of the look-up table set to 0 and 8,000, respectively. Fluorescence intensity (FI) was defined as the median of pixel intensities over the entire selected image. For FI analysis, ten representing frames, with the highest fluorescence intensity, displaying the subpleural microcirculation were retrieved from the video file of each animal, blindly to the animal group. Frames displaying vessels with a diameter of 25 µm or more were not considered for the FI analysis. Median FI values, facet diameters and intercapillary distances were compared between elastase-treated animals and controls. The high rate of acquisition of FCFM (9 frames/second) as well as a slight pressure of the probe tip onto the surface of the pleura during respiration allowed limiting motion artifacts. In order to minimize related pressure artifacts, the slightest pressure onto the lung surface was applied, resulting in a discontinuous contact between the lung and the probe-tip during the respiration cycles. For quantitative measurements, this allowed to select images with the minimal compression effect from the last frames before the contact is lost.
Ex vivo lung tissue processing and histology
Animals were sacrificed immediately after the imaging procedure and the respiratory tract including the trachea was collected. A catheter was carefully introduced into the trachea and tightly ligatured to avoid any air or liquid leak. Lungs were immediately fixed in an ethanol and acetic acid mix solution (Hydrosafe®, Labonord) at a constant pressure of 25 cmH2O for 7 d. Thereafter the lungs were kept in the same fixative until they were processed for histomorphometric analysis.
Lung lobes were separated and cut in 6 sagittal fragments, except the accessory lobe, which was saved for further experiments. The samples were then embedded in paraffin, cut into 5µm slices, and stained with hematoxylin-eosin-safran (HES).
Ex vivo histomorphometric measurements
One HES-stained slide from each block (i.e., 6 slides per lung lobe) was used for the morphometric analysis. The slides were scanned (MiraxScan®, Zeiss) and observed with the dedicated software (MiraxViewer®, Zeiss) at x58 magnification. The magnified slices were integrally photographed, and the ImageJ softwareCitation51 was used to extract 1.89 mm2 FOVs. Sixty FOVs per animal were considered for the morphometric analysis, and were distributed as follows: 1 FOV per slide from the right upper lobe (i.e., 6 FOVs), 1 from the right middle lobe (i.e., 6 FOVs), 3 from the right lower lobe (i.e., 18 FOVs) and 5 from the left lung (i.e., 30 FOVs). Fields of view displaying extra-alveolar microvessels and/or bronchial structures were not considered for the analysis.
The automated quantification of the mean diameter of the alveolar structure was performed with a custom-written software (Matlab®, Mathworks). Briefly, the software drew 50 vertical and horizontal lines that intersected with the alveolar walls and ducts, on each of the 60 FOVs per animal. The mean length of the segments between the intersections, the mean interwall distance (MIWD),Citation52 was then computed and compared between elastase-treated animals and controls.
Statistical analysis
The statistical analysis was performed with the GraphPad Prism® software (GraphPad Software, Inc.). The unpaired t test and Mann-Whitney test were used when appropriate to compare the fluorescence intensity median values, intercapillary distances, alveolar facet diameters, and the MIWD values between animal groups. The Spearman test was used to correlate FCFM measurements and MIWD. Statistical significance was set at a p value < 0.05.
Additional material
Download Zip (3 MB)Acknowledgments
This study was supported by ADIR Association.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Supplemental Materials
Supplemental materials may be found here: www.landesbioscience.com/journals/intravital/article/23471
References
- The definition of emphysema. Report of a national heart, lung, and blood institute, division of lung diseases workshop. Am Rev Respir Dis 1985; 132:182 - 5; PMID: 4014865
- Rabe KF, Hurd S, Anzueto A, Barnes PJ, Buist SA, Calverley P, et al, Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med 2007; 176:532 - 55; http://dx.doi.org/10.1164/rccm.200703-456SO; PMID: 17507545
- Shapiro SD, Ingenito EP. The pathogenesis of chronic obstructive pulmonary disease: advances in the past 100 years. Am J Respir Cell Mol Biol 2005; 32:367 - 72; http://dx.doi.org/10.1165/rcmb.F296; PMID: 15837726
- Kasahara Y, Tuder RM, Cool CD, Lynch DA, Flores SC, Voelkel NF. Endothelial cell death and decreased expression of vascular endothelial growth factor and vascular endothelial growth factor receptor 2 in emphysema. Am J Respir Crit Care Med 2001; 163:737 - 44; PMID: 11254533
- Kasahara Y, Tuder RM, Taraseviciene-Stewart L, Le Cras TD, Abman S, Hirth PK, et al. Inhibition of VEGF receptors causes lung cell apoptosis and emphysema. J Clin Invest 2000; 106:1311 - 9; http://dx.doi.org/10.1172/JCI10259; PMID: 11104784
- Morissette MC, Parent J, Milot J. Alveolar epithelial and endothelial cell apoptosis in emphysema: what we know and what we need to know. Int J Chron Obstruct Pulmon Dis 2009; 4:19 - 31; PMID: 19436685
- Voelkel NF, Vandivier RW, Tuder RM. Vascular endothelial growth factor in the lung. Am J Physiol Lung Cell Mol Physiol 2006; 290:L209 - 21; http://dx.doi.org/10.1152/ajplung.00185.2005; PMID: 16403941
- Liebow AA. Pulmonary emphysema with special reference to vascular changes. Am Rev Respir Dis 1959; 80:67 - 93; PMID: 13670406
- Wiebe BM, Laursen H. Lung morphometry by unbiased methods in emphysema: bronchial and blood vessel volume, alveolar surface area and capillary length. APMIS 1998; 106:651 - 6; http://dx.doi.org/10.1111/j.1699-0463.1998.tb01395.x; PMID: 9725798
- Froese AR, Ask K, Labiris R, Farncombe T, Warburton D, Inman MD, et al. Three-dimensional computed tomography imaging in an animal model of emphysema. Eur Respir J 2007; 30:1082 - 9; http://dx.doi.org/10.1183/09031936.00000507; PMID: 17804451
- Gevenois PA, De Vuyst P, de Maertelaer V, Zanen J, Jacobovitz D, Cosio MG, et al. Comparison of computed density and microscopic morphometry in pulmonary emphysema. Am J Respir Crit Care Med 1996; 154:187 - 92; PMID: 8680679
- Madani A, De Maertelaer V, Zanen J, Gevenois PA. Pulmonary emphysema: radiation dose and section thickness at multidetector CT quantification--comparison with macroscopic and microscopic morphometry. Radiology 2007; 243:250 - 7; http://dx.doi.org/10.1148/radiol.2431060194; PMID: 17392257
- Madani A, Keyzer C, Gevenois PA. Quantitative computed tomography assessment of lung structure and function in pulmonary emphysema. Eur Respir J 2001; 18:720 - 30; http://dx.doi.org/10.1183/09031936.01.00255701; PMID: 11716178
- Uppaluri R, Mitsa T, Sonka M, Hoffman EA, McLennan G. Quantification of pulmonary emphysema from lung computed tomography images. Am J Respir Crit Care Med 1997; 156:248 - 54; PMID: 9230756
- Chen XJ, Hedlund LW, Möller HE, Chawla MS, Maronpot RR, Johnson GA. Detection of emphysema in rat lungs by using magnetic resonance measurements of 3He diffusion. Proc Natl Acad Sci U S A 2000; 97:11478 - 81; http://dx.doi.org/10.1073/pnas.97.21.11478; PMID: 11027348
- Dutly AE, Kugathasan L, Trogadis JE, Keshavjee SH, Stewart DJ, Courtman DW. Fluorescent microangiography (FMA): an improved tool to visualize the pulmonary microvasculature. Lab Invest 2006; 86:409 - 16; http://dx.doi.org/10.1038/labinvest.3700399; PMID: 16518405
- An L, Subhush HM, Wilson DJ, Wang RK. High-resolution wide-field imaging of retinal and choroidal blood perfusion with optical microangiography. J Biomed Opt 2010; 15:026011; http://dx.doi.org/10.1117/1.3369811; PMID: 20459256
- Qin J, Jiang J, An L, Gareau D, Wang RK. In vivo volumetric imaging of microcirculation within human skin under psoriatic conditions using optical microangiography. Lasers Surg Med 2011; 43:122 - 9; http://dx.doi.org/10.1002/lsm.20977; PMID: 21384393
- Thiberville L, Moreno-Swirc S, Vercauteren T, Peltier E, Cavé C, Bourg Heckly G. In vivo imaging of the bronchial wall microstructure using fibered confocal fluorescence microscopy. Am J Respir Crit Care Med 2007; 175:22 - 31; http://dx.doi.org/10.1164/rccm.200605-684OC; PMID: 17023733
- Thiberville L, Salaün M, Lachkar S, Dominique S, Moreno-Swirc S, Vever-Bizet C, et al. Human in vivo fluorescence microimaging of the alveolar ducts and sacs during bronchoscopy. Eur Respir J 2009; 33:974 - 85; http://dx.doi.org/10.1183/09031936.00083708; PMID: 19213792
- Thiberville L, Salaün M, Lachkar S, Dominique S, Moreno-Swirc S, Vever-Bizet C, et al. Confocal fluorescence endomicroscopy of the human airways. Proc Am Thorac Soc 2009; 6:444 - 9; http://dx.doi.org/10.1513/pats.200902-009AW; PMID: 19687217
- Newton R, Kemp S, Zoumot Z, Yang GZ, Darzi A, Shah PL. An unusual case of haemoptysis. Thorax 2010; 65:309 - 53, 353; http://dx.doi.org/10.1136/thx.2009.129890; PMID: 20388754
- Mercer RR, Crapo JD. Spatial distribution of collagen and elastin fibers in the lungs. J Appl Physiol 1990; 69:756 - 65; PMID: 2228886
- Weibel ER, Sapoval B, Filoche M. Design of peripheral airways for efficient gas exchange. Respir Physiol Neurobiol 2005; 148:3 - 21; http://dx.doi.org/10.1016/j.resp.2005.03.005; PMID: 15921964
- Reid JA, Heard BE. The capillary network of normal and emphysematous human lungs studied by injections of indian ink. Thorax 1963; 18:201 - 12; http://dx.doi.org/10.1136/thx.18.3.201; PMID: 14064613
- Mitsuoka H, Sakurai T, Unno N, Kaneko H, Suzuki S, Nakamura S, et al. Intravital laser confocal microscopy of pulmonary edema resulting from intestinal ischemia-reperfusion injury in the rat. Crit Care Med 1999; 27:1862 - 8; http://dx.doi.org/10.1097/00003246-199909000-00026; PMID: 10507611
- Lamm WJ, Bernard SL, Wagner WW Jr., Glenny RW. Intravital microscopic observations of 15-microm microspheres lodging in the pulmonary microcirculation. J Appl Physiol 2005; 98:2242 - 8; http://dx.doi.org/10.1152/japplphysiol.01199.2004; PMID: 15705726
- Presson RG Jr., Todoran TM, De Witt BJ, McMurtry IF, Wagner WW Jr.. Capillary recruitment and transit time in the rat lung. J Appl Physiol 1997; 83:543 - 9; PMID: 9262451
- Weibel ER, Hsia CC, Ochs M. How much is there really? Why stereology is essential in lung morphometry. J Appl Physiol 2007; 102:459 - 67; http://dx.doi.org/10.1152/japplphysiol.00808.2006; PMID: 16973815
- Schraufnagel DE, Schmid A. Capillary structure in elastase-induced emphysema. Am J Pathol 1988; 130:126 - 35; PMID: 3337208
- Morisse H, Heyman L, Salaün M, Favennec L, Picquenot JM, Bohn P, et al. In vivo molecular microimaging of pulmonary aspergillosis. Med Mycol 2012; In press http://dx.doi.org/10.3109/13693786.2012.729138; PMID: 23088299
- Morisse H, Heyman L, Salaün M, Favennec L, Picquenot JM, Bohn P, et al. In vivo and in situ imaging of experimental invasive pulmonary aspergillosis using fibered confocal fluorescence microscopy. Med Mycol 2012; 50:386 - 95; http://dx.doi.org/10.3109/13693786.2011.617788; PMID: 22004362
- Salaün M, Roussel F, Bourg-Heckly G, Vever-Bizet C, Dominique S, Genevois A, et al. In vivo probe-based confocal laser endomicroscopy in amiodarone-related pneumonia. Eur Respir J 2012; In press http://dx.doi.org/10.1183/09031936.00191911; PMID: 23018901
- Hanna G, Fontanella A, Palmer G, Shan S, Radiloff DR, Zhao Y, et al. Automated measurement of blood flow velocity and direction and hemoglobin oxygen saturation in the rat lung using intravital microscopy. Am J Physiol Lung Cell Mol Physiol 2012; In press http://dx.doi.org/10.1152/ajplung.00178.2012; PMID: 23161885
- Yamato H, Sun JP, Churg A, Wright JL. Cigarette smoke-induced emphysema in guinea pigs is associated with diffusely decreased capillary density and capillary narrowing. Lab Invest 1996; 75:211 - 9; PMID: 8765321
- Sato N, Suzuki Y, Nishio K, Suzuki K, Naoki K, Takeshita K, et al. Roles of ICAM-1 for abnormal leukocyte recruitment in the microcirculation of bleomycin-induced fibrotic lung injury. Am J Respir Crit Care Med 2000; 161:1681 - 8; PMID: 10806175
- McCormack DG, Mehta S, Tyml K, Scott JA, Potter R, Rohan M. Pulmonary microvascular changes during sepsis: evaluation using intravital videomicroscopy. Microvasc Res 2000; 60:131 - 40; http://dx.doi.org/10.1006/mvre.2000.2261; PMID: 10964587
- Tabuchi A, Mertens M, Kuppe H, Pries AR, Kuebler WM. Intravital microscopy of the murine pulmonary microcirculation. J Appl Physiol 2008; 104:338 - 46; http://dx.doi.org/10.1152/japplphysiol.00348.2007; PMID: 18006870
- Tanabe N, Todoran TM, Zenk GM, Aono J, Wagner WW Jr., Presson RG Jr.. Role of positive airway pressure on pulmonary acinar perfusion heterogeneity. J Appl Physiol 2000; 89:1943 - 8; PMID: 11053347
- Kreisel D, Nava RG, Li W, Zinselmeyer BH, Wang B, Lai J, et al. In vivo two-photon imaging reveals monocyte-dependent neutrophil extravasation during pulmonary inflammation. Proc Natl Acad Sci U S A 2010; 107:18073 - 8; http://dx.doi.org/10.1073/pnas.1008737107; PMID: 20923880
- Presson RG Jr., Brown MB, Fisher AJ, Sandoval RM, Dunn KW, Lorenz KS, et al. Two-photon imaging within the murine thorax without respiratory and cardiac motion artifact. Am J Pathol 2011; 179:75 - 82; http://dx.doi.org/10.1016/j.ajpath.2011.03.048; PMID: 21703395
- Namati E, Thiesse J, de Ryk J, McLennan G. Alveolar dynamics during respiration: are the pores of Kohn a pathway to recruitment?. Am J Respir Cell Mol Biol 2008; 38:572 - 8; http://dx.doi.org/10.1165/rcmb.2007-0120OC; PMID: 18096874
- Chagnon F, Fournier C, Charette PG, Moleski L, Payet MD, Dobbs LG, et al. In vivo intravital endoscopic confocal fluorescence microscopy of normal and acutely injured rat lungs. Lab Invest 2010; 90:824 - 34; http://dx.doi.org/10.1038/labinvest.2010.76; PMID: 20386539
- Foersch S, Kiesslich R, Waldner MJ, Delaney P, Galle PR, Neurath MF, et al. Molecular imaging of VEGF in gastrointestinal cancer in vivo using confocal laser endomicroscopy. Gut 2010; 59:1046 - 55; http://dx.doi.org/10.1136/gut.2009.202986; PMID: 20639250
- Marwick JA, Stevenson CS, Giddings J, MacNee W, Butler K, Rahman I, et al. Cigarette smoke disrupts VEGF165-VEGFR-2 receptor signaling complex in rat lungs and patients with COPD: morphological impact of VEGFR-2 inhibition. Am J Physiol Lung Cell Mol Physiol 2006; 290:L897 - 908; http://dx.doi.org/10.1152/ajplung.00116.2005; PMID: 16361360
- Newton RC, Kemp SV, Yang GZ, Elson DS, Darzi A, Shah PL. Imaging parenchymal lung diseases with confocal endomicroscopy. Respir Med 2012; 106:127 - 37; http://dx.doi.org/10.1016/j.rmed.2011.09.009; PMID: 22000588
- Thiberville L, Salaün M, Hauss PA, Lachkar S, Dominique S. In vivo microimaging of the alveolar capillary network during alveoscopy (abstract). Eur Respir Society Meeting 2009.
- Salaün M, Bourg Heckly G, Roussel F, Lachkar S, Hauss P-A, Thiberville L. Distal lung elastic network alterations in pulmonary fibrosis, a prospective controlled study using in-vivo confocal endomicroscopy {abstract}. Eur Respir J 2010; 377
- Salaün M, Roussel F, Hauss PA, Lachkar S, Thiberville L. In vivo imaging of pulmonary alveolar proteinosis using confocal endomicroscopy. Eur Respir J 2010; 36:451 - 3; http://dx.doi.org/10.1183/09031936.00194509; PMID: 20675784
- Poole DC, Mathieu-Costello O. Effect of pulmonary emphysema on diaphragm capillary geometry. J Appl Physiol 1997; 82:599 - 606; PMID: 9049743
- Rasband WS, Image J. U. S. National Institutes of Health, Bethesda, Maryland, USA, http://rsb.info.nih.gov/ij/, 1997-2009.
- Onclinx C, De Maertelaer V, Gustin P, Gevenois PA. Elastase-induced pulmonary emphysema in rats: comparison of computed density and microscopic morphometry. Radiology 2006; 241:763 - 70; http://dx.doi.org/10.1148/radiol.2413051456; PMID: 17114624
![Figure 5. The mean interwall distance (MIWD) was used to assess pulmonary emphysema ex vivo. It was significantly higher in the elastase group (B) (median = 44.8 [IQR (43.7 – 45.3)]) than in controls (A) (median = 38.3µm [IQR (36.9 – 40.4)]) (p = 0.001, Mann-Whitney test).](/cms/asset/3c874dbd-b1d0-431b-8994-439c42df8cc1/kinv_a_10923471_f0005.gif)