Abstract
We examine the effect of bone marrow transplantation (BMT) on retinal cell turnover by performing simultaneous cell tracking of native microglia and engrafting donor bone marrow-derived cell (BMDC) populations in the retinae of live mice using a custom-built multi-color confocal scanning laser ophthalmoscope (SLO) specifically developed for murine retinal imaging. CX3CR1GFP/+ mice whose retinal microglia express the green fluorescent protein (GFP) were exposed to a lethal dose of gamma radiation and subsequently rescued with bone marrow cells from universal DsRed donor mice. Over a time course of four months after the irradiation and BMT, progressive loss of GFP+ microglia was accompanied by delayed engraftment of DsRed+ BMDC. Morphologic examination revealed that the remaining GFP+ microglia were ramified, while engrafting DsRed+ cells exhibited both ramification and dendriform shape. Leukocyte endothelial interaction, normally absent in healthy retinal vasculature, was observed even after three months, indicating sustained inflammation long after the radiation exposure. Fluorescein angiography demonstrated that the blood-retina barrier is compromised early after irradiation. In vivo imaging provides a powerful means to study dynamic cellular processes over a broad range of timescales from seconds to months that have previously not been accessible by ex vivo analysis.
Introduction
Bone marrow transplantation (BMT) is an established clinical procedure for the treatment of hematologic malignancies such as leukemia and multiple myeloma.Citation1 In this procedure, patients receive high dose chemotherapy or gamma irradiation that aims to eradicate malignant cells but also destroys normal hematopoietic cells. The latter population is then rescued by infusion of bone marrow cells to reestablish normal hematopoiesis. Although the main target of gamma irradiation is the bone marrow, injury to other tissues including the central nervous system (CNS) and the retina have also been reported.Citation2-Citation6 Closer examination of the cellular response in the CNS, including the retina, following radiation and BMT is crucial for the development of new strategies for mitigating radiation-induced injury to these tissues. BMT is also commonly used as a research tool to create chimeric animal models whose bone marrow derived cells (BMDCs) carry a reporter gene or other constructs that can be traced in the host. In this setting, the potential injury response to radiation and BMT needs to be taken into account in the overall analysis of BMDCs in chimeric animal models.
BMDCs that enter the circulation have the ability to traffic to most tissues in the body, but it is unclear that they can penetrate an intact blood-brain barrier/blood-retina barrier (BBB/BRB). When these barriers are breached, as in disease or injury conditions, BMDCs can move into the brain and retina where they are not found under normal conditions.Citation7 BMDCs have been observed (by histology) to cross the BBB/BRB following radiation and BMT with fluorescent donor cells.Citation8-Citation10
It was shown previously, that some BMDCs can enter the retina and assume a microglial-like phenotype.Citation10 Microglia are the immune sentinels of the CNS that reside within the normal, intact BBB/BRB.Citation11-Citation13 They are thought to be long-lived cells capable of local self-renewal with little or no turnover under homeostatic conditions.Citation14 Suggestions that BMT causes microglia turnover came from the observation that at least some of the transplanted BMDCs entering the CNS or retina express the microglial marker Iba-1, indicating that these newly arrived cells can replace the resident microglia population.Citation8-Citation10
Here, we report techniques that enable longitudinal in vivo tracking of both the (disappearing) resident microglial and the (engrafting) BMDC populations, that will help gain a more complete picture of cellular radiation response. The inner retina as an optically directly accessible gray matter compartment of the CNS lends itself to in vivo imaging and cell tracking, allowing disease progression in individual animals to be followed serially over time. We have developed a multi-color scanning laser ophthalmoscope (SLO) specifically for confocal imaging of the mouse retina with the ability to simultaneously acquire up to three fluorescence channels at video rate. The dynamics of the native microglia population and the engrafting BMDCs after BMT were investigated. For this purpose, heterozygous CX3CR1GFP/+ mice that express GFP in retinal microglia were exposed to a lethal dose of gamma radiation and rescued with a bone marrow transplant from universal DsRed donor mice. The numbers of native GFP+ cells and donor DsRed+ BMDCs in the retina were quantified by in vivo imaging before and over a time course of four months after the irradiation and BMT. We observed progressive loss of GFP+ microglia with delayed engraftment of DsRed+ BMDCs. The total cell number was below the baseline value of resident microglial cells for most of the observation period, even after four months. Leukocyte endothelial interaction, essential for circulating cells to recognize their homing site, was observed throughout this period, suggesting prolonged inflammation in the retina. Fluorescein angiography demonstrated that the integrity of the blood-retina barrier is compromised after irradiation.
Results
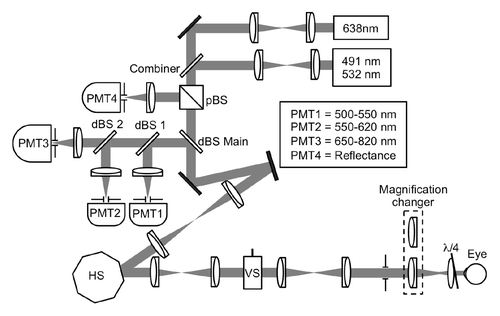
Our laboratory has developed a scanning laser ophthalmoscope (SLO) specifically for mouse retinal imaging based on the video-rate confocal microscope introduced by Veilleux et al.Citation15,Citation16 (see Materials and Methods section for design details). Briefly, multiple laser sources and a multi-edge dichroic beam splitter allowed up to three channels to be acquired simultaneously (). Pinholes 50 µm in diameter, corresponding to 3 to 4 Airy discs, resulted in a depth of focus of approximately 40 µm, thus allowing a thick optical section of retinal tissue to be imaged at once, without the need for axial movement. In its current configuration, the SLO does not use adaptive optics.Citation17,Citation18 GFP and DsRed were excited with the 491 and 532 nm excitation lasers. Their fluorescence was detected through 525/50 and 593/40 (both Semrock, Rochester, NY) bandpass filters and assigned green (GFP) and red (DsRed) color in the RGB images, respectively. Alexa647 for staining vasculature was excited by the 638 nm laser, detected through a 650 nm longpass filter and assigned the blue channel.
Figure 1. Schematic of the mouse SLO. Up to three channels of the reflectance channel and three fluorescence channels can be acquired simultaneously at video-rate to enable imaging of distinct cell populations. Each channel consists of a PMT protected by a confocal pinhole and a bandpass filter. dBS = dichroic beams splitter; pBS = polarizing beam splitter cube; HS = horizontal scanner (polygon); VS = vertical scanner (galvanometer)
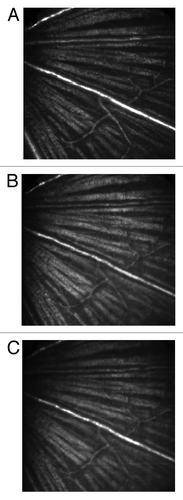
Careful alignment of the three laser sources and their telescopes was undertaken to minimize chromatic focusing error among the three wavelengths. To verify the results of the system alignment, the inner retina was imaged in reflectance mode with each of the three lasers in the same mouse. The results indicate that the three lasers image the same optical section in the mouse retina ().
Figure 2. Reflectance images taken with (A) 638 nm, (B) 532 nm and (C) 491 nm lasers demonstrate that the three lasers of the SLO share a common optical section in the retina. Field of view was 15° (approximately 400 µm).
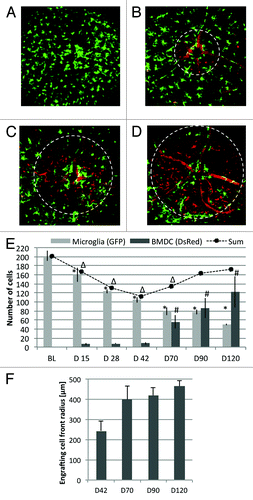
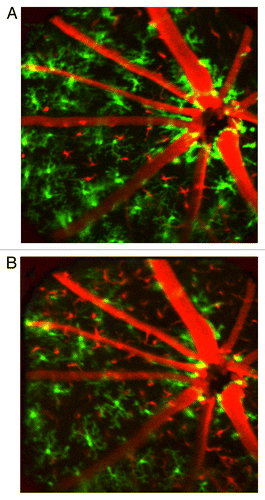
GFP+ resident microglia and DsRed+ BMDCs were tracked over a time course of four months after BMT from universal DsRed donors into lethally irradiated CX3CR1GFP/+ recipient mice (n = 3). show representative images of one mouse followed over the 120-d experimental period. Each image shows a field of view of 30° (approximately 800 µm) centered on the optic nerve head. Qualitatively, progressive loss of endogenous GFP+ microglia and influx of transplanted DsRed+ cells were observed over time. Most DsRed+ cells circulated in the vasculature because all newly generated hematopoietic cells were derived from the DsRed+ donor, but closer examination shows that some of the DsRed+ cells were stationary outside the vasculature (in the retina parenchyma). Quantitatively, the baseline number of native microglia was 202 ± 12 cells. This number decreased by 20% at the first measured time point 15 d after irradiation and transplantation (). By 42 d, the number of microglia had declined to 50% of the baseline number measured prior to irradiation. Over the same time course, negligible numbers (on average less than 10 cells per field of view) of extravasating BMDCs were detected, resulting in a relatively depleted hematopoietic cell population in the retina. Substantial engraftment (56 ± 15 cells per field of view) was first observed at day 70. At the end of the observation period at day 120, the number of native GFP+ microglia decreased to 25% of the baseline level, while the DsRed+ cells continued to increase, to 122 ± 34 cells per field of view. Due to delayed BMDC engraftment, the total number of hematopoietic cells, that is, the sum of all remaining GFP+ microglia and DsRed+ BMDCs, was relatively depleted and approached 85% of the baseline number of microglia after four months. Engrafting BMDCs were initially observed near the optic nerve head; the radius of the BMDC repopulation front increased over time ().
Figure 3. Representative images of the retina of one mouse followed over the 120-d experimental period. Green = native GFP+ microglia, Red = DsRed+ BMDCs. (A) Baseline taken prior to lethal irradiation and bone marrow transplantation. (B) Day 42, (C) day 70 and (D) day 90 after irradiation and bone marrow transplant. Field of view was 30° (approximately 800 µm). Loss of native, GFP+ microglia and influx of DsRed+ BMDCs was apparent. Dashed circles in panels B-D mark the size of the engrafting BMDC population. (E) Quantification of cell populations shows that the engraftment of BMDCs lags behind the loss of the native microglia even after 4 mo. The sum of both cell populations demonstrates relative depletion of hematopoietic cells in the retina. (Error bars are standard error. *, # and ∆ are statistical significance relative to baseline value for resident microglia, BMDC and total cells, respectively. T-test < 0.05) (F) Radius of the engrafting BMDC front measured from the optic nerve head. The population expands from the optic nerve with time.
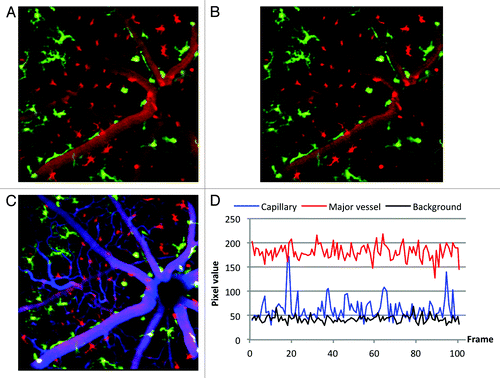
Because most DsRed+ cells circulated in the vasculature, images taken in the inner retina close to the microvasculature frequently showed endogenous GFP+ microglia and DsRed+ BMDCs, but also vasculature () that appears DsRed+ due to the movement of circulating hematopoietic cells during the image acquisition time (1/3 sec). To quantify BMDC engraftment and to visualize engrafting cells’ morphology, it was necessary to distinguish stationary DsRed+ cells that have exited the blood vessels and entered the retinal parenchyma from those moving in the capillaries (). To this end, real-time movies were recorded at 30 frames per second and its frames aligned to minimize motion artifacts. The mean and standard deviation for each pixel in a registered temporal stack was then computed, and a new image generated by subtracting the standard deviation from the mean. After subtracting the standard deviation in the DsRed channel, the visibility of vasculature is suppressed and morphology of stationary cells becomes more apparent(). To validate the computational method, the vasculature of three mice was co-labeled with Alexa647 conjugated to 70 kDa dextran 70 d after irradiation and BMT. The dye was injected on stage, so that one movie could be acquired each before and after the dye injection. The simultaneous acquisition of GFP, DsRed and Alexa647 fluorescence () demonstrates that the standard deviation computation is a valid method to suppress fluorescence signals of circulating DsRed+ cells, in order to enhance visibility of the extravasating BMDCs. Note that the operation removes primarily capillary structures where the transit of individual DsRed cells generates large fluctuation in the signal intensity. Larger vessels contain more DsRed cells, with corresponding larger mean and smaller fluctuation (). As a result, the subtraction does not remove the larger vessels from the image.
Figure 4. Images from co-registered z-stacks of the same retina 70 d after irradiation and BM transplant demonstrating, validating the standard deviation subtraction technique. Field of view was 20° (approximately 525 µm). (A) A 50-frame average image shows remaining GFP microglia and donor DsRed BMDCs in the inner retinal layer. Frame averaging introduces confusion between vasculature and extravasating BMDCs. (B) The same image as in A, but with the standard deviation subtracted in the DsRed channel. Visibility of vasculature is suppressed and stationary cells are clearly visible. (C) Alexa647 was injected into the same mouse during the same imaging session to label vasculature. The angiography demonstrates that cells identified as stationary by the standard deviation method are outside the vasculature. (D) Typical pixel values of the DsRed channel for a capillary, a major blood vessel and background plotted as a function of frame number taken from a 100 sec long real-time movie acquisition.
Suppressing visibility of capillaries enabled the detection of the earliest extravasating BMDCs and allowed to observe their morphological features. Initial engraftment reproducibly occurred near the optic nerve head (). Engrafting BMDCs were characterized by both ramified and dendriform morphology. Most remaining native microglia displayed ramified (i.e., “resting”) morphology.
Figure 5. Images taken (A) 42 and (B) 60 d after irradiation and bone marrow transplantation demonstrate that engraftment of bone marrow derived cells first occurs near the optic nerve head. Engrafting cells display both ramified and dendriform morphology.
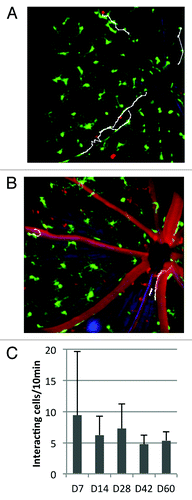
One of the most powerful aspects of in vivo imaging is the ability to detect dynamic interactions between cells and their environment. Thus, in addition to longitudinal cell tracking, in vivo imaging also enables “zooming in” on the short-term dynamics at each time point to visualize cell behavior and cellular interactions that are not available using histological methods. Circulating BMDCs that temporarily interact with the vascular endothelium can be observed by acquiring time-lapse images. shows the result of manually tracking DsRed+ leukocytes after time-lapse imaging, where one 10-frame average image was acquired every 30 sec over a period of ten minutes. Repeated time-lapse imaging demonstrates that leukocyte endothelial interaction, normally absent in healthy retinal vasculatures, occurred throughout the observation period in time-lapse imaging, even two months after the irradiation. Enumeration of the interacting cells demonstrates that after an initial peak at day 7 the number of interacting cells remains at approximately 5 cells per 10 min observation period for the duration of the experiment. The interacting leukocytes moved with an average velocity on the order of 10 µm/min. The duration of interaction lasted up to 600 sec.
Figure 6. Leukocyte-endothelial interactions (LEI) in the irradiated retina (A) 7 and (B) 60 d after irradiation and BMT and enumeration of interacting leukocytes at several time points throughout the experimental period. Most interacting cells moved unidirectionally toward the optic nerve head. Change in direction of the interacting cells was observed only on and after day 14. (C) demonstrates that leukocyte endothelial interaction persists throughout the observation period. Panels A and B are frames of a time-lapse imaging sequence where one image was taken every 30 sec for ten minutes. Interacting leukocytes were tracked manually in ImageJ and the tracking traces overlayed in white. Field of view was 20° (approximately 525 µm).
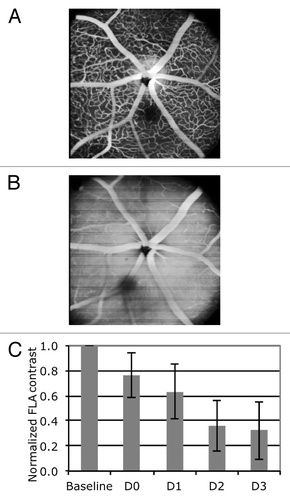
One possible route of microglial loss or BMDC extravasation is through the blood-retina barrier that is formed by vascular endothelial cells and their tight junctions. However, the BRB is designed to prevent free exchange of cells and materials between the vasculature and the retinal parenchyma. To investigate the integrity of the BRB after irradiation, fluorescein angiography was performed. Fluorescein leaks out of the blood vessels in regions where the BRB is compromised. As a measure of leakage, the image contrast between retinal capillaries and parenchyma was quantified before and for three days after the irradiation. Fluorescein leakage was observed and image contrast decreased to 35% of pre-irradiation level 3 d after irradiation, demonstrating that BRB integrity is compromised early after irradiation.
Discussion
The inner retina represents an optically accessible gray matter compartment of the CNS that lends itself to in vivo imaging and cell tracking by fluorescence microscopy, where an expanding number of cell types can be fluorescently labeled (in the rodent retina). Live animal tracking enables both the long-term dynamics such as disease progression and the short-term dynamics such as cell-cell interaction to be monitored and quantified in individual animals.Citation19-Citation23
Because an SLO relies on the mouse eye as the objective lens, the image quality depends heavily on the mouse eye optical parameters. The numerical aperture of the mouse eye has been reported to be as high as 0.5, approximately 2.5 times as large as that of the fully dilated human eye.Citation24,Citation25 Thus, in an ideal system, imaging in the mouse eye with an SLO could provide improvements of 2.5 × and 6 × in lateral and axial resolution, respectively, compared with that in the human eye. Adaptive optics has been applied to the rodent eye in order to approach diffraction limited image resolution of approximately 0.7 µm in the retina.Citation17,Citation25-Citation27 While adaptive optics imaging is crucial for studies that aim to image fine details of cellular morphology in a small field of view, the study presented here focused on the quantification of cells and their interaction with the environment in large areas of the retina. Depending on the magnification, our field of view in the retina ranges from 525–800 µm, corresponding to a pixel resolution of 1.1–1.6 µm/pixel in a 500 pixel × 500 pixel image, which was sufficient to visualize microglial morphology. In order to image thick optical sections of the retina, thereby eliminating the need of axial translation during time lapse imaging, our SLO underfills the pupil with an incident beam diameter of 1.1 mm, close to the optimum of decreasing aberrations but increasing Strehl ratio.Citation25 The depth of focus in this configuration is approximately 40 µm, allowing the ganglion cell complex (consisting of retinal nerve fiber layer, ganglion cell layer and inner plexiform layer) where most microglial cells reside to be captured without the need for axial focus shifting.Citation28
With this system, we have simultaneously tracked and quantified both the endogenous GFP+ and the DsRed+ BM-derived donor microglial populations in the retinae of irradiated live mice. Progressive loss of the native microglia was observed throughout the entire observation period of four months. At the end of the follow up experiments, 25% of the native microglia (relative to the baseline) remained in the retina. Curiously, the microglia that remained in the retina were ramified, that is, they appeared to be “resting” rather than “activated.” In fact, hardly any amoeboid microglia were found, raising the question what the mechanism of the microglia cell disappearance is. One possibility for the loss of resident microglia is due to cell death, although additional studies will be needed to clarify whether cell death results from direct radiation damage. However, one would expect surviving nearby microglia to be activated as they help to clear debris from dead cells. Alternatively, migration of microglia to an exit point, such as blood vessels or the subarachnoid space surrounding the optic nerve, could be another means of microglial depletion. Again, microglial migration requires the cells to take on amoeboid shape, since ramification is not compatible with migration. Because imaging took place every 2 to 4 weeks and during this time at most 40 cells were lost on average (), it is conceivable that phagocytic or migratory events were missed entirely. Similarly, extravasation of BMDCs was not directly observed. However, the first BMDCs that appear to be stationary and therefore considered extravasated were reproducibly observed near the optic nerve head. At subsequent imaging time points, the radius of the front of engrafting BMDCs expanded away from the optic nerve head (). Similarly, resident microglial cells were also initially lost near the optic nerve head. Since the zone of engrafting BMDCs seems to coincide with the zone of highest microglia loss, we suspect the optic nerve head may be the dominant site of exit and entry of these cell populations.
The quantification of cells also showed that appearance of BMDCs lags behind the loss of the resident cells. Substantial engraftment was observed at and following day 70. The BMDC population approximately doubled between days 70 and 120. However, the sum of the BMDCs and the remaining endogenous microglia remains smaller than the baseline number (). While this result is not statistically significant given our small sample size here, the delayed engraftment results in a relatively, at least transiently, depleted retinal immune cell population that may account for reports in the literature of BM transplant patients who develop infections in the posterior chamber within months of their procedures.Citation2-Citation4
In addition to cell counting, examination of cellular morphology can aid in determining the cell type and the activation status of cells. However, morphological detail of engrafting cells can sometimes be masked by other transplanted cells moving through the blood vessels in the field of view. That movement within blood vessels leads to a higher standard deviation in registered temporal image stacks. Computing the standard deviation of moving cells has originally been introduced to highlight capillaries in imaging of human retina without the need for fluorescence contrast agent.Citation29,Citation30 In our case, DsRed+ cells moving in blood vessels introduce the appearance of structure that can be confused with engrafting cells. Therefore, we computed the standard deviation of registered temporal image stacks and subtracted it from the mean. In the resulting images the structural information is reduced to cellular features, except in large blood vessels where the standard deviation is close to zero. Counterstaining the vasculature with Alexa647 conjugated to dextran confirmed that the computation isolates stationary cells that are located outside of the vasculature. The resulting structural examination of the BMDCs reveals a spectrum of morphological features that includes ramified but also a high number of dendriform cells ( and ). While ramified cells likely are microglia, dendriform cells can be either activated microglia or monocytes that are extravasating into the retinal parenchyma. Increased recruitment of monocytes may indicate ongoing inflammation.
Consistent with the notion of inflammation, leukocyte endothelial interaction was observed after the irradiation and bone marrow transplant. Leukocyte endothelial interaction is a result of adhesion molecule mediated signaling that enables circulating leukocytes to roll, arrest and eventually extravasate near the site of an inflammation.Citation31 Adhesion molecule upregulation and leukocyte endothelial interaction are considered to last approximately one week after injury.Citation32,Citation33 While we observed a peak of interacting leukocytes seven days after irradiation, our results also show that leukocyte endothelial interaction continues throughout the observation period. The interaction of leukocytes and endothelial cells is frequently accompanied by a disruption of the blood-retinal barrier. Fluorescein angiography here demonstrated that the blood retinal barrier was compromised during the first few days after irradiation (). In the protected environment of the CNS and the retina, leukocyte endothelial interaction and leakage through the blood-retina barrier are not observed under physiological condition, but are considered to be signs of inflammation.Citation34,Citation35 To note, it has been proposed that a subset of resident monocytes also patrol the intact vasculature of the mesentery and brain under physiological conditions without extravasating.Citation36,Citation37 Hence, it is possible that a fraction of LEI at later timepoints were in fact physiological LEI of such resident monocytes. Further studies will be needed to determine this. In conclusion, our results suggest that some form of inflammatory response exists immediately after irradiation and BMT, and potentially persists for months afterward.
Figure 7. Fluorescein angiography (FLA) at (A) baseline situation prior to irradiation and (B) two days after irradiation demonstrate massive leakage, manifesting itself by obvious loss of image contrast. (C) Evaluation of the image contrast shows that contrasts decreases to approximately 35% of its baseline value within three days after the irradiation.
In summary, in vivo imaging provides long-term and short-term dynamic information on the behavior and interactions of cells that cannot be gathered with ex vivo methods. We have developed a method to track and quantify the endogenous microglia and engrafting BMDC populations simultaneously over months in the living mouse retina by in vivo retinal imaging. Engraftment of DsRed+ BMDC after lethal irradiation and bone marrow transplant in CX3CR1GFP/+ mice is accompanied by loss of the resident GFP+ microglia. Leukocyte endothelial interaction, thought to be absent under homeostatic conditions and commonly associated with CNS inflammation, was observed even months after the irradiation. Experiments to directly correlate the effects of ionizing radiation on retinal vascular integrity, microglia and BMDCs in dependence of the irradiation dose directly delivered to the head are currently under way.
Materials and Methods
Scanning laser ophthalmoscope (SLO)
The current version of the SLO has three laser sources at 635 nm (L4 635S-24/OSYS, Micro Laser Systems, Inc. Garden Grove, CA, USA) and at 491 and 532 nm (Dual Calypso, Cobolt AB, Vretenvägen, Sweden). The laser beams are combined by a 580 nm longpass dichroic beam splitter (Semrock, Rochester, NY) and directed through a central, triple-edge dichroic beam splitter (Di01-T488/532/638, Semrock, Rochester, NY). A 36-facet polygon scans the beam horizontally at 17,280 lines per second and a galvanometer provides the vertical scan. These pupils of the scan engine are conjugated and relayed into the iris of the mouse eye. In an SLO, the cornea and lens of the eye serve as the objective and the image quality critically depends on the optical characteristics of the eye. In our SLO, the tube lens is a 60D Volk lens. The mouse was held in a tube mounted on a six-axis stage that allowed precise positioning of the mouse eye pupil into the system pupil formed by the Volk lens. The mouse and the Volk lens were moved together relative to the last lens to adjust the focus within the retina. The last lens is interchangeable to allow changing the field of view between 15° and 45° (approximately 400 µm to 1200 µm), covering the central to mid-periphery regions of the mouse retina. For the experiments reported here, the SLO did not utilize adaptive optics.
Reflectance imaging is accomplished by means of polarization rotation with a quarter-wave plate placed between the Volk lens and the mouse eye; a polarizing beam splitter cube separates backscattering from the retina from the incident light and directs it into the reflectance channel. Fluorescence emitted in the retina is separated from the excitation light and directed into the fluorescence detection arm by the main, triple-edge dichroic beam splitter. Inside the fluorescence detection arm 560 nm and 650 longpass dichroic beam splitters (FF560-Di01 and FF650-Di01, Semrock, Rochester, NY) separate light into three distinct fluorescence detectors (Red = 650 - 825 nm; Green = 550 - 650 nm; Blue = 500–550 nm) that can be further narrowed by appropriate bandpass filters. Three channels can be acquired simultaneously, enabling the observation of up to three distinct cell populations in real-time at video-rate. Light in each channel is focused by a 75 mm achromatic lens and detected with photomultiplier tubes (PMTs) (R3896, Hamamatsu, Japan) through a 50 µm pinhole (corresponding to 3.2 to 4.1 times the Airy disc size).
Careful attention to the axial alignment of the imaging lasers was required to compensate for the chromatic aberrations of the mouse eye that have been reported to be approximately 7D across the visible wavelength range.Citation25 The instrument was initially aligned with the red laser as a reference beam. The lengths of the various telescopes were optimized to minimize divergence and times-diffraction-limit factor of the reference beam as measured with a beam propagation analyzer (ModeMaster, Coherent, Santa Clara, CA). The confocal pinhole of the reflectance channel was conjugated by placing a mirror in the last intermediate image plane of the system and optimizing the confocal throughput. In order to match the focal plane of the other laser wavelengths (488 nm and 532 nm) to that of the red laser, the respective source telescope was slightly adjusted to introduce a small beam divergence. The alignment of the three laser beams was optimized in three dimensions in an artificial eye, built from a 2 mm focal length lens (NA = 0.5, 2 mm clear aperture, Geltech 350150, Thorlabs, NJ) held in a brass housing with a target placed in the focal plane of the lens. The source telescope of the 491 and 532 laser was adjusted until best possible images of the target were acquired with all three wavelengths. As the final alignment step, the confocal pinholes of the three fluorescence channels were conjugated for each excitation wavelength.
Experimental design
Mice expressing GFP in microglia under the control of the fractalkine receptor promoter CX3CR1 (B6.129P-Cx3cr1tm1Litt/J) were purchased from Jackson Laboratory (Bar Harbor, ME). The fractalkine receptor is specifically expressed on microglia, a population of blood monocytes, NK and dendritic cells.Citation38 The mice were maintained as heterozygotes by crossing homozygous CX3CR1-GFP mice with the parental C57BL/6 strain to ensure proper function of the fractalkine receptor on microglia.Citation39 Mice were exposed to a single dose of 9 Gy gamma radiation with a Cesium source (Gammacell 40 Exactor, MDS Nordion, Ottawa, ONT, CAN). Lethally irradiated mice were rescued five hours after the exposure by bone marrow transplantation of 4 × 106 cells harvested from homozygous actin-DsRed donor mice (B6.Cg-Tg(CAG-DsRed*MST)1Nagy/J).
For imaging, the mice were held in a heated holding tube that integrated a nose cone for delivery of 1–2% isoflurane mixed in oxygen for inhalation anesthesia. The tube was mounted on a six-axis stage that aided the positioning of the mouse eye in the SLO imaging beam. The pupil was dilated with a drop of Tropicamide. A contact lens was placed on the mydriatic eye and a drop of GenTeal eye drops prevented the cornea from drying. In vivo images were recorded at baseline prior to the irradiation and at days 15, 28, 42, 70, 90 and 120 after the irradiation. At each time point, the numbers of resident GFP+ cells and DsRed+ bone marrow derived cells were evaluated.
All animal procedures were approved by the Massachusetts General Hospital animal care and use committee and were consistent with the ARVO statement for the Use of Animals in Ophthalmic and Vision Research.
Image analysis
Acquired images were processed in ImageJ for cell counting.Citation40 Multi-color images (RGB) were split into separate, 8-bit grayscale channels. Signals in GFP and DsRed channels were thresholded so that the microglial cell morphology was reduced to somata. Thresholded cells were counted using ImageJ’s particle analyzer tool. Residual cell structures or noise spikes that may not have been eliminated by the threshold were excluded from the analysis, by a) defining the minimum size to be counted as 16 square-pixels and b) prohibiting elongated shapes to be counted. Finally, the resulting counting mask was overlaid on the original image to visually control for the accuracy of the procedure.
The dimension of the engrafting BMDC front was determined in ImageJ as the size of the circle that encompasses most BMDCs that have a distinct morphology, such as ramified or dendriform cells, and were therefore certainly located outside of the blood vessels. The circle was approximately centered on the optic disc.
To identify interactions between bone marrow derived leukocytes with the blood vessel endothelial wall, time-lapse imaging was undertaken. One image was taken once every 30 sec for several minutes. The images of the resulting temporal stack were aligned to compensate for motion artifacts such as rotation or drifting of the eye. Cells moving in the major blood vessels were tracked manually in ImageJ using the MTrackJ plugin.
Fluorescein angiography
C57BL/6 mice were injected with 25 µl of 5% fluorescein solution, diluted in Phosphate Buffered Saline from 10% Fluorescein (USP, IMS Ltd, South El Monte, CA) via the tail vein while under isofluorane anesthesia and on the SLO stage. Immediately, images were taken at 30 sec intervals over a 3 min period to follow leakage of the fluorescein into the retinal parenchyma. As a measure of fluorescein leakage, the contrast between capillaries and non-vascular tissue was evaluated within segments delineated by the major blood vessels. Michelson contrast, defined as the ratio of the difference between maximum and minimum pixel values over their sum, was used for the measurement. Contrast value was normalized to baseline values taken before irradiation for each mouse.
Acknowledgments
This research was funded by the Department of Defense, project FA9550–10–1-0537. The authors thank Dr. Mark Cronin-Golomb of Tufts University for the generous loan of a laser beam propagation analyzer and Dr. Daniela Krause for help with the bone marrow transplantation. We thank Drs. Francesca Ferrara, Stefania Lymperi, Daniela Krause and Andrew Catic for help with gamma irradiations.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- Hari P, Pasquini MC, Vesole DH. Cure of multiple myeloma -- more hype, less reality. Bone Marrow Transplant 2006; 37:1 - 18; PMID: 16258534
- Moon SJ, Mieler WF. Retinal complications of bone marrow and solid organ transplantation. Curr Opin Ophthalmol 2003; 14:433 - 42; http://dx.doi.org/10.1097/00055735-200312000-00018; PMID: 14615651
- Chung H, Kim KH, Kim JG, Lee SY, Yoon YH. Retinal complications in patients with solid organ or bone marrow transplantations. Transplantation 2007; 83:694 - 9; http://dx.doi.org/10.1097/01.tp.0000259386.59375.8a; PMID: 17414700
- Coskuncan NM, Jabs DA, Dunn JP, Haller JA, Green WR, Vogelsang GB, et al. The eye in bone marrow transplantation. VI. Retinal complications. Arch Ophthalmol 1994; 112:372 - 9; http://dx.doi.org/10.1001/archopht.1994.01090150102031; PMID: 8129664
- Giuliari GP, Sadaka A, Hinkle DM, Simpson ER. Current treatments for radiation retinopathy. Acta Oncol 2011; 50:6 - 13; http://dx.doi.org/10.3109/0284186X.2010.500299; PMID: 20722590
- Chen M, Zhao J, Luo C, Pandi SP, Penalva RG, Fitzgerald DC, et al. Para-inflammation-mediated retinal recruitment of bone marrow-derived myeloid cells following whole-body irradiation is CCL2 dependent. Glia 2012; 60:833 - 42; http://dx.doi.org/10.1002/glia.22315; PMID: 22362506
- Mildner A, Schmidt H, Nitsche M, Merkler D, Hanisch UK, Mack M, et al. Microglia in the adult brain arise from Ly-6ChiCCR2+ monocytes only under defined host conditions. Nat Neurosci 2007; 10:1544 - 53; http://dx.doi.org/10.1038/nn2015; PMID: 18026096
- Simard AR, Rivest S. Bone marrow stem cells have the ability to populate the entire central nervous system into fully differentiated parenchymal microglia. FASEB J 2004; 18:998 - 1000; PMID: 15084516
- Ono K, Takii T, Onozaki K, Ikawa M, Okabe M, Sawada M. Migration of exogenous immature hematopoietic cells into adult mouse brain parenchyma under GFP-expressing bone marrow chimera. Biochem Biophys Res Commun 1999; 262:610 - 4; http://dx.doi.org/10.1006/bbrc.1999.1223; PMID: 10471372
- Xu H, Chen M, Mayer EJ, Forrester JV, Dick AD. Turnover of resident retinal microglia in the normal adult mouse. Glia 2007; 55:1189 - 98; http://dx.doi.org/10.1002/glia.20535; PMID: 17600341
- Vilhardt F. Microglia: phagocyte and glia cell. Int J Biochem Cell Biol 2005; 37:17 - 21; http://dx.doi.org/10.1016/j.biocel.2004.06.010; PMID: 15381143
- Nimmerjahn A, Kirchhoff F, Helmchen F. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science 2005; 308:1314 - 8; http://dx.doi.org/10.1126/science.1110647; PMID: 15831717
- Davalos D, Grutzendler J, Yang G, Kim JV, Zuo Y, Jung S, et al. ATP mediates rapid microglial response to local brain injury in vivo. Nat Neurosci 2005; 8:752 - 8; http://dx.doi.org/10.1038/nn1472; PMID: 15895084
- Ajami B, Bennett JL, Krieger C, Tetzlaff W, Rossi FMV. Local self-renewal can sustain CNS microglia maintenance and function throughout adult life. Nat Neurosci 2007; 10:1538 - 43; http://dx.doi.org/10.1038/nn2014; PMID: 18026097
- Veilleux I, Spencer JS, Biss DP, Côté D, Lin CP. In Vivo Cell Tracking With Video Rate Multimodality Laser Scanning Microscopy. IEEE J Sel Top Quant Elec 2008; 14: 10-18.
- Rajadhyaksha M, González S, Zavislan JM, Anderson RR, Webb RH. In vivo confocal scanning laser microscopy of human skin II: advances in instrumentation and comparison with histology. J Invest Dermatol 1999; 113:293 - 303; http://dx.doi.org/10.1046/j.1523-1747.1999.00690.x; PMID: 10469324
- Biss DP, Sumorok D, Burns SA, Webb RH, Zhou Y, Bifano TG, et al. In vivo fluorescent imaging of the mouse retina using adaptive optics. Opt Lett 2007; 32:659 - 61; http://dx.doi.org/10.1364/OL.32.000659; PMID: 17308593
- Alt C, Biss DP, Tajouri N, Jakobs TC, Lin CP. An adaptive-optics scanning laser ophthalmoscope for imaging murine retinal microstructure. Proc SPIE 2010; 7550:755019; http://dx.doi.org/10.1117/12.840583
- Cordeiro MF, Guo L, Luong V, Harding G, Wang W, Jones HE, et al. Real-time imaging of single nerve cell apoptosis in retinal neurodegeneration. Proc Natl Acad Sci U S A 2004; 101:13352 - 6; http://dx.doi.org/10.1073/pnas.0405479101; PMID: 15340151
- Eter N, Engel DR, Meyer L, Helb HM, Roth F, Maurer J, et al. In vivo visualization of dendritic cells, macrophages, and microglial cells responding to laser-induced damage in the fundus of the eye. Invest Ophthalmol Vis Sci 2008; 49:3649 - 58; http://dx.doi.org/10.1167/iovs.07-1322; PMID: 18316698
- Paques M, Simonutti M, Augustin S, Goupille O, El Mathari B, Sahel JA. In vivo observation of the locomotion of microglial cells in the retina. Glia 2010; 58:1663 - 8; http://dx.doi.org/10.1002/glia.21037; PMID: 20578032
- Leung CK, Weinreb RN, Li ZW, Liu S, Lindsey JD, Choi N, et al. Long-term in vivo imaging and measurement of dendritic shrinkage of retinal ganglion cells. Invest Ophthalmol Vis Sci 2011; 52:1539 - 47; http://dx.doi.org/10.1167/iovs.10-6012; PMID: 21245394
- Alt C, Lin CP. In vivo quantification of microglia dynamics with a scanning laser ophthalmoscope in a mouse model of focal laser injury. Proc SPIE 2012; 8209:820907; http://dx.doi.org/10.1117/12.909141
- de la Cera EG, Rodríguez G, Llorente L, Schaeffel F, Marcos S. Optical aberrations in the mouse eye. Vision Res 2006; 46:2546 - 53; http://dx.doi.org/10.1016/j.visres.2006.01.011; PMID: 16516259
- Geng Y, Schery LA, Sharma R, Dubra A, Ahmad K, Libby RT, et al. Optical properties of the mouse eye. Biomed Opt Express 2011; 2:717 - 38; http://dx.doi.org/10.1364/BOE.2.000717; PMID: 21483598
- Geng Y, Greenberg KP, Wolfe R, Gray DC, Hunter JJ, Dubra A, et al. In vivo imaging of microscopic structures in the rat retina. Invest Ophthalmol Vis Sci 2009; 50:5872 - 9; http://dx.doi.org/10.1167/iovs.09-3675; PMID: 19578019
- Geng Y, Dubra A, Yin L, Merigan WH, Sharma R, Libby RT, et al. Adaptive optics retinal imaging in the living mouse eye. Biomed Opt Express 2012; 3:715 - 34; http://dx.doi.org/10.1364/BOE.3.000715; PMID: 22574260
- Nakano N, Ikeda HO, Hangai M, Muraoka Y, Toda Y, Kakizuka A, et al. Longitudinal and simultaneous imaging of retinal ganglion cells and inner retinal layers in a mouse model of glaucoma induced by N-methyl-D-aspartate. Invest Ophthalmol Vis Sci 2011; 52:8754 - 62; http://dx.doi.org/10.1167/iovs.10-6654; PMID: 22003119
- Burns SA, Zhangyi Z, Chui TYP, Song H, Elsner AE, Malinovsky VE. Imaging the Inner Retina Using Adaptive Optics. Invest Ophthalmol Vis Sci 2008; 49:4512
- Tam J, Martin JA, Roorda A. Noninvasive visualization and analysis of parafoveal capillaries in humans. Invest Ophthalmol Vis Sci 2010; 51:1691 - 8; http://dx.doi.org/10.1167/iovs.09-4483; PMID: 19907024
- Crane IJ, Liversidge J. Mechanisms of leukocyte migration across the blood-retina barrier. Semin Immunopathol 2008; 30:165 - 77; http://dx.doi.org/10.1007/s00281-008-0106-7; PMID: 18305941
- Yuan H, Goetz DJ, Gaber MW, Issekutz AC, Merchant TE, Kiani MF. Radiation-induced up-regulation of adhesion molecules in brain microvasculature and their modulation by dexamethasone. Radiat Res 2005; 163:544 - 51; http://dx.doi.org/10.1667/RR3361; PMID: 15850416
- Gaber MW, Yuan H, Killmar JT, Naimark MD, Kiani MF, Merchant TE. An intravital microscopy study of radiation-induced changes in permeability and leukocyte-endothelial cell interactions in the microvessels of the rat pia mater and cremaster muscle. Brain Res Brain Res Protoc 2004; 13:1 - 10; http://dx.doi.org/10.1016/j.brainresprot.2003.11.005; PMID: 15063835
- Jain P, Coisne C, Enzmann G, Rottapel R, Engelhardt B. Alpha4beta1 integrin mediates the recruitment of immature dendritic cells across the blood-brain barrier during experimental autoimmune encephalomyelitis. J Immunol 2010; 184:7196 - 206; http://dx.doi.org/10.4049/jimmunol.0901404; PMID: 20483748
- Pachter JS, de Vries HE, Fabry Z. The blood-brain barrier and its role in immune privilege in the central nervous system. J Neuropathol Exp Neurol 2003; 62:593 - 604; PMID: 12834104
- Auffray C, Fogg D, Garfa M, Elain G, Join-Lambert O, Kayal S, et al. Monitoring of blood vessels and tissues by a population of monocytes with patrolling behavior. Science 2007; 317:666 - 70; http://dx.doi.org/10.1126/science.1142883; PMID: 17673663
- Audoy-Rémus J, Richard J-F, Soulet D, Zhou H, Kubes P, Vallières L. Rod-Shaped monocytes patrol the brain vasculature and give rise to perivascular macrophages under the influence of proinflammatory cytokines and angiopoietin-2. J Neurosci 2008; 28:10187 - 99; http://dx.doi.org/10.1523/JNEUROSCI.3510-08.2008; PMID: 18842879
- Jung S, Aliberti J, Graemmel P, Sunshine MJ, Kreutzberg GW, Sher A, et al. Analysis of fractalkine receptor CX(3)CR1 function by targeted deletion and green fluorescent protein reporter gene insertion. Mol Cell Biol 2000; 20:4106 - 14; http://dx.doi.org/10.1128/MCB.20.11.4106-4114.2000; PMID: 10805752
- Liang KJ, Lee JE, Wang YD, Ma W, Fontainhas AM, Fariss RN, et al. Regulation of dynamic behavior of retinal microglia by CX3CR1 signaling. Invest Ophthalmol Vis Sci 2009; 50:4444 - 51; http://dx.doi.org/10.1167/iovs.08-3357; PMID: 19443728
- Available at http://rsb.info.nih.gov/ij; developed by Wayne Rasband, National Institutes of Health, Bethesda, MD.