Stem cells are a critical component of most multicellular organisms as they are responsible for maintaining tissue turnover throughout the lifetime of the organism. This is such a fundamental mechanism of life that it is conserved from worms to humans. Loss of stem cells is associated with severe diseases and loss of stem cell function is at the heart of aging. Understanding the mechanisms responsible to ensure stem cell function is critical to harness their potential to cure and prevent an extremely wide range of diseases. In fact, regenerative medicine has attracted vast enthusiasm as it promises to revolutionize our health expectations in a similar way that the discovery of antibiotics did to survive infections.
In 1978 Raymond Schofield introduced the concept of stem cell niche when he postulated that the existence and correct functioning of stem cells depends on their interaction with specific anatomical sites.Citation1 Based on experimental evidence that hematopoietic stem cells could migrate to the spleen following transplantation, but could only lead to successful transplants if harvested from the bone marrow, he hypothesized that stem cells must reside in these sites in order to self-renew i.e., to ensure that upon cell division at least one daughter cell is a stem cell equivalent to the mother cell. He proposed an instructive role for the niche that determines stem cell fate, from their rate of proliferation to the differentiation (or not) of their daughter cells. Years later, he was proved right when stem cell niches were identified in Drosophila gonads firstCitation2 and in several mammalian tissues afterwards.Citation3 Since then, stem cell biology has developed based on the paradigm that tissues follow a one-direction system in which stem cells proliferate to generate daughters that in part remain stem cells and in part replenish the pool of differentiating cells. The differentiating cells are responsible for maintaining tissue homeostasis and are regularly lost after a definite period of time. Most niches are comprised of different cell types organized in specific patterns to provide support for stem cells. The presence of this specialized environment poses two questions: (1) Do stem cells located in various positions within the niche have different fates? And (2) Can cells outside the niche re-acquire stem cell properties if they encounter niche signals?
Recently, the Greco and Van Rheenen groups used prolonged intravital time-lapse two-photon microscopy to follow the cellular dynamics of stem cells located in the hair follicle bulge/hair germ and at the bottom of intestinal crypts during physiological regeneration.Citation4,Citation5 These studies provide direct evidence of the correlation between stem cell position within the niche and its fate and demonstrate that cells located in the vicinity of, albeit outside the niche, can substitute for stem cells to repair the tissue following injury.
Rompolas et al. used an elegant approach combining genetic lineage tracing of single bulge cells or hair germ cells (using K19 creER and Lgr5 creER mouse lines, respectively) with two-photon intravital microscopy to monitor the fate of stem cells located in different areas of the niche. Using this methodology, they provide direct evidence that establishes a link between the initial position of stem cells in the niche and their fate. More specifically, while the cells located in the upper half of the bulge tend to remain quiescent and uncommitted, the cells in the lower half of the bulge respond to activating signals from the dermal papilla, undergo cell division, and become part of the outer root sheet (ORS), the most external cell layer forming the hair follicle. The ORS cell are in an intermediate state: some migrate downwards and continue differentiation to support hair growth, whereas the remaining fraction does not migrate downwards, survives the apoptosis-driven regression phase of the hair cycle and eventually is found in the new hair germ. From this new position, the cells receive differentiation stimuli and give rise to the cells that support of the subsequent hair cycle.
Using a similar multi-photon intravital microscopy approach, Ritsma et al. examined the fate of single intestinal stem cells in the Lgr5-Confetti mice. In the small intestine, stem cells are distributed between the Paneth cells and the mesenchyme, in clusters of 14–16 proliferative crypt base columnar (CBC) cells. Expression of Lgr5 is associated with these stem cells and, combined with the confetti reporter strategy,Citation6 can be used to trace the progeny of different stem cells because each stem cell expresses one of four fluorescent proteins upon cre-mediated recombination. Previously, it was proposed that the homeostatic maintenance of intestinal crypts is achieved via neutral competition between the dividing stem cells, where the daughter cells are competing for the limited niche space without any cells having a higher chance to win.Citation6 By monitoring several crypts over multiple days, Ritsma et al. showed that indeed CBC cells originating from both central and border region of the niche can contribute to progeny that extend into both regions; however, the cells originating from a central mother cell most often outcompete the cells at the niche border, i.e., have a survival advantage. This leads to passive displacement of border CBC cells into the trans-amplifying (TA) region above the niche, where terminal differentiation commences.
These two studies provide direct evidence for the correlation between a specific niche location and stem-cell fate and it is likely that the same selection mechanisms apply to both intestinal crypt and hair follicle bulge/hair germ cells. The next question now to be addressed is whether stem cells located in different niche sub-regions are capable of transiting reversibly between different positions and of assuming different fates based on specific instructive signals received from the niche cells. Another question is whether stem cells residing in different niche locations still hold the same potential, even though in fact they are more likely to give rise to differentiating or self-renewing progeny. And if all cells within the niche hold the same potential, how strict is the niche boundary, i.e., could cells outside the niche maintain stem cell potential? Studies in the Drosophila gonads showed that genetic ablation of germ cells but not of niche cells leads to follicular cells to move into the space previously occupied by the stem cells and to acquire their function.Citation7 Nobel prize-winning work from Shinya Yamanka and colleagues showed that differentiated mammalian cells can be engineered in vitro to revert as far back in development as to become equivalent to pluripotent embryonic stem cells.Citation8 And now, both Rompolas et al. and Ritsma et al. provide direct evidence that the same mechanism holds true in mammalian tissues in vivo.
Rompolas et al. tested the stringency of niche-imposed cell fate through laser-induced cell ablation of cells in different niche comportments. They showed that after laser-induced ablation of either the bulge or hair germ, the niche consistently recovered the lost cells and proceeded to hair regeneration. However, this mechanism strictly requires the niche, in this case the dermal papilla, to remain intact and in direct contact with the surviving cells. Moreover, recordings of the recovery process revealed that upon loss of either stem cell pool, the neighboring epithelial cell population located above the bulge, that do not normally have a hair follicle fate, are mobilized and contribute to re-establishing the niche both anatomically and functionally as shown by their contribution to the subsequent hair growth cycle.
The fact that cells located in the vicinity of, albeit outside, the niche can repopulate the niche and reconstitute the stem cell pool is further demonstrated by Ritsma et al., who use a genetic approach to eliminate the entire stem cell population within the crypt studied, so that only TA cells remain and can rescue the crypt. Even though the recovery process is not 100% efficient, the authors are able to show that in many crypts TA cells proliferate and give rise to expanding, compact clones that re-occupy the niche space. Interestingly, also in this study the niche cells are preserved, in this case because they do not express the suicide gene.
These two studies provide two independent examples of how, as long as the supportive niche cells remain intact, stemness can be acquired by neighboring cells that would normally undergo terminal differentiation and this is a mechanism ensuring tissue repair following injury. Even though not as striking as the reprogramming of terminally differentiated cells to pluripotent stem cells, these are the first examples of how mammalian stem cell-based tissues may not follow such a strict uni-directional model of differentiation.
The defined structures of the niche in hair follicle and intestinal crypt as well as the availability of established lineage tracing and cell ablation approaches provided the researchers with the ideal experimental systems to study. The recent development of intravital microscopy protocols allowing prolonged and repeated imaging of these regions made such observations and interventions possible. The results obtained emphasize how direct observation of tissues over long periods of times holds the key to uncovering cellular dynamics otherwise impossible to demonstrate. Whether the existence of niche sub-regions and the correlation between position within the niche and stem cell fate is a characteristic of all adult stem cells remains to be further addressed in other tissues, and further development of lineage tracing and ablation approaches as well as imaging techniques will undoubtedly allow similar studies to be performed in other tissues in the next few years.
The findings obtained through intravital microscopy of hair follicle and crypt cells span well beyond the understanding of the principles regulating stem cell biology and may have consequences affecting our everyday life. One of the main challenges for using stem cells in the clinic is that they are available in limited numbers. Forcing stem cells to self-renew is the common strategy currently used or aimed for in order to expand the stem cell pool. That approach however not only has been proven challenging for many somatic stem cells, but is a double-edge sword, because of its intrinsic risk of exhausting the stem cell pool. These two recent studies suggest that stem cell loss can redefine the hierarchies between stem cells and differentiated cells upon injury and point at new approaches for repopulating the stem cell pool via reversion of partially differentiated cells, which are usually more abundant within a tissue than the stem cells themselves.
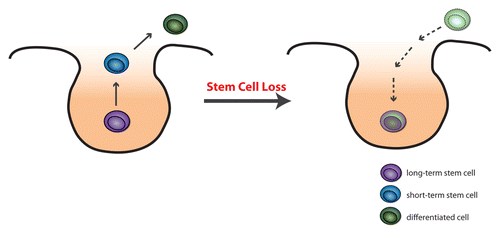
Acting at the niche level, either in vivo using niche-targeting drugs or in vitro by reproducing niche-derived supportive signals, as a mean to reach out to the stem cells can open new doors to new, powerful, and minimally invasive regenerative medicine approaches ().
Figure 1. Normally, all cells within the niche can act as stem cells, however those on the periphery of the niche have the higher chance of leaving the niche and differentiating. Conversely, should the stem cells be lost and the niche become vacant, differentiating cells can migrate into the niche and become new stem cells.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Citation: Rashidi NM, Lo Celso C. Flying back to the nest: Intravital microscopy reveals how the niche can induce stemness. IntraVital 2014; 3:e29653; 10.4161/intv.29653
References
- Schofield R. The relationship between the spleen colony-forming cell and the haemopoietic stem cell. Blood Cells 1978; 4:7 - 25; PMID: 747780
- Xie T, Spradling AC. A niche maintaining germ line stem cells in the Drosophila ovary. Science 2000; 290:328 - 30; http://dx.doi.org/10.1126/science.290.5490.328; PMID: 11030649
- Ferraro F, Lo Celso C, Scadden DT. Adult Stem Cells and their Niches. In: Meshorer E, The cell biology of stem cells. Springer; 2010.
- Ritsma L, Ellenbroek SI, Zomer A, Snippert HJ, de Sauvage FJ, Simons BD, Clevers H, van Rheenen J. Intestinal crypt homeostasis revealed at single-stem-cell level by in vivo live imaging. Nature 2014; 507:362 - 5; http://dx.doi.org/10.1038/nature12972; PMID: 24531760
- Rompolas P, Mesa KR, Greco V. Spatial organization within a niche as a determinant of stem-cell fate. Nature 2013; 502:513 - 8; http://dx.doi.org/10.1038/nature12602; PMID: 24097351
- Snippert HJ, van der Flier LG, Sato T, van Es JH, van den Born M, Kroon-Veenboer C, Barker N, Klein AM, van Rheenen J, Simons BD, et al. Intestinal crypt homeostasis results from neutral competition between symmetrically dividing Lgr5 stem cells. Cell 2010; 143:134 - 44; http://dx.doi.org/10.1016/j.cell.2010.09.016; PMID: 20887898
- Kai T, Spradling A. An empty Drosophila stem cell niche reactivates the proliferation of ectopic cells. Proc Natl Acad Sci U S A 2003; 100:4633 - 8; http://dx.doi.org/10.1073/pnas.0830856100; PMID: 12676994
- Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 2006; 126:663 - 76; http://dx.doi.org/10.1016/j.cell.2006.07.024; PMID: 16904174
