Abstract
Signaling by cytokines such as the interferons (IFNs) involves Janus kinases (JAKs) and signal transducer and activator of transcription (STAT) transcription factors. The beauty of the classical model of JAK-STAT signaling is its simplicity in that JAK-activated STATs in the nucleus are responsible for specific gene activation. The fact that many ligands, growth factors, and hormones use the same STAT transcription factors, but exert different functions at the level of the cell, tissue, and organ would suggest significant shortcomings in the classical model. Our studies have resulted in the development of a non-canonical, more complex model of IFN signaling that bears a striking resemblance to that of steroid hormone (SH)/steroid receptor (SR) signaling. Thus, both types I and II IFN signaling involves nuclear translocation of complexed ligand, receptor, activated JAKs, and activated STATs to the promoters of the genes that are specifically activated by the IFNs, where they are involved in specific gene activation and epigenetic remodeling. Receptor intracellular domains play an important role in binding the C-terminus of the IFNs, which is the basis for our development of IFN mimetics. The IFN mimetics are not recognized by poxvirus decoy receptors, since the decoy receptors compete for extracellular binding and not intracellular binding. Further, the type I IFN mimetics provide therapeutic protection against experimental allergic encephalomyelitis (EAE), a model of multiple sclerosis, without the side effects. Extracellular receptor binding by intact IFN is the primary reason for undesirable side effects of flu-like symptoms, bone-marrow suppression, and weight loss. The non-canonical model of IFN signaling thus provides insight into the specificity of such signaling and a mechanism for development of IFN mimetics. It is our contention that this model applies to other cytokines.
Introduction
Interferon (IFN) was discovered in 1957 as an innate host defense response against viral infections.Citation1 The implication of this discovery was not fully realized for nearly 20 years as IFNs have been shown to be immune regulatory cytokines as well as antiviral proteins. We and others independently made the definitive observations that IFNα/β negatively regulated the in vitro antibody response, thus providing the first evidence that IFNs were not only antivirals but also immunoregulators.Citation2 IFNα/β was shortly thereafter shown to induce suppressor cells (regulatory cells), which in turn produced a soluble suppressor factor(s) that mediated the immunosuppression.Citation3 During this time, small scale clinical studies showed that IFNα and IFNβ had some therapeutic efficacy in the treatment of relapsing/remitting multiple sclerosis (MS) as well as some cancers.Citation4 These early IFN studies served as precursors to the discovery and characterization of other cytokines as well as to studies at the level of signal transduction that provided insight into the mechanism of cytokine signaling.
In the classical model of cytokine signaling, ligand activates the cell solely via interaction with the extracellular domain of the receptor complex. This in turn results in the activation of receptor or receptor-associated tyrosine kinases, primarily of the Janus or JAK kinase family, leading to phosphorylation and dimerization of the STAT transcription factors, which then disassociate from the receptor cytoplasmic domain and translocate to the nucleus.Citation5 This view ascribes no further role to the ligand, JAKs, or the receptor in the signaling process. Further, there is the suggestion that the STAT transcription factors possess intrinsic nuclear localization sequences (NLSs) that are responsible for nuclear translocation of STATs for specific gene activation.Citation6
It has recently been acknowledged, however, that the classical model of JAK-STAT signaling was over-simplified in its original form, and that other ubiquitous pathways, including MAP kinase, PI3 kinase, CaM kinase II, and NFκB cooperate with or act in parallel to JAK-STAT signaling to regulate IFNγ effects on the cell.Citation7 At the STAT level, there is evidence of a functional interaction between different STATs in gene activation/suppression, which provides more insight into STAT mediation of cytokine signaling.Citation8 It is not clear, however, as to how these STAT interactions at the level of DNA binding translate into specific gene activation by the inducing cytokine. It suggests some sort of intrinsic built-in specificity of STATs beyond response element recognition. With the long interest and focus on STATs, one would have expected that such an intrinsic specificity-determining structure would have been discovered by now if it existed.
There are also studies of differential effects of IFNβ in a small cohort of patients with relapsing/remitting MS, where differential activation of p38, NFκβ, and STATs 1, 2, and 3 were observed in the context of apoptosis in monocytes and granulocytes.Citation9 These studies bear some resemblance to those summarized above concerning IFNγ signaling.
We will focus in this review on a non-canonical model of IFN signaling that has implication for insight into the mechanism of type I IFN therapy of multiple sclerosis (MS), particularly in the context of therapy vs. undesirable side effects associated with IFN treatment. Related to this, we will show that it is possible to dissociate type I IFN therapy in the mouse model of MS, experimental allergic encephalomyelitis (EAE), from the side effects of such therapy.Citation10 Such dissociation has been made possible as a result of our discovery of a non-canonical pathway of IFN signaling. The model involves the JAK-STAT pathway where STAT is but one of a number of important players in a series of complex events that shed light on specific gene activation as well as the associated epigenetic events. Thus far, the classical model of JAK-STAT signaling has not been helpful in linking activated STATs and activated JAKs in the nucleus for gene activation. The non-canonical model makes such a linkage.
The basics of the classical model of IFN signaling as well as that of other ligands that use the JAK-STAT pathway are quite simple and appealing. For example, see for IFNγ. Ligand interacts with the receptor extracellular domain, which somehow transmits a signal through the receptor hydrophobic transmembrane domains to the cytoplasmic domain. This causes allosteric changes in the cytoplasmic domain that result in JAK auto-activation (phosphorylation) and subsequent activation of the relevant STATs. The activated STATs form dimers, dissociate from the receptor and translocate to the nucleus via intrinsic nuclear localization sequences (NLS) where they determine the specificity of gene activation by the particular ligand. This occurs even in the many cases where functionally different ligands activate the same STATs.Citation11 The model is assumed to provide insight into important things like specific epigenetics associated with gene activation such as histone phosphorylations, histone methylations, dimethylations, or acetylations. The obvious question is where is such insight contained within the model?
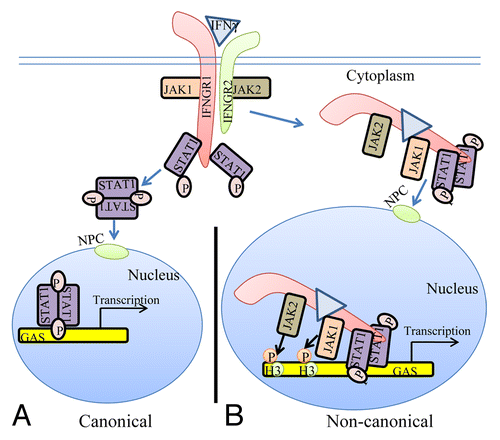
Figure 1. The classical and non-canonical models of IFNγ signaling. (A) In the classical model of IFNγ signaling, IFNγ crosslinks the IFNGR1 receptor subunit that results in allosteric changes in receptor cytoplasmic domain causing the movement of JAK2 from receptor subunit IFNGR2 to IFNGR1. The JAKs autophosphorylate and then phosphorylate IFNGR1 cytoplasmic domain. This results in binding, phosphorylation, and dimer formation of STAT1α. The dimeric STAT1α dissociates from receptor and undergoes nuclear translocation via an intrinsic NLS for specific gene activation. (B) The non-canonical model of IFNγ signaling involves IFNγ binding to receptor extracellular domain, followed by movement to IFNGR1 cytoplasmic domain in conjunction with endocytosis. The cytoplasmic binding increases the affinity of JAK2 for IFNGR1, which is the basis for its movement to IFNGR1. This results in autoactivation of the JAKs, phosphorylation of IFNGR1 cytoplasmic domain, and the binding and phosphorylation of STAT1α at IFNGR1. The complex of IFNGR1/STAT1α/JAK1/JAK2 undergoes active nuclear transport where the classic polycationic NLS of IFNγ plays a key role for this transport to genes in the nucleus that are specifically activated by IFNγ. Furthermore, the JAKs associated with the specific promoters were shown to be involved in epigenetic modifications. Details of the non-canonical model are presented in the text. GAS, IFN gamma activated sequence; H3, histone H3; NPC, nuclear pore complex.
It has been known for some time that internalized types I and II IFNs possessed anti-viral activity.Citation12,Citation13 Further, it has been shown that cells treated with IFNs showed nuclear transport of IFN and receptors.Citation10,Citation14,Citation15 In fact, all of the ligand signaling systems that use the JAK-STAT signaling pathway have, where examined, been shown to possess classic polycationic NLSs in their ligands and/or receptors and to undergo active nuclear translocation.Citation11 This also applies to well-known tyrosine kinase receptors such as epidermal growth factor (EGF) receptor,Citation16 fibroblast growth factor (FGF) receptor,Citation17 and platelet derived growth factor (PDGF) receptor.Citation18 To date none of these observations have been allowed into the conventional lexicon of ligand/receptor signaling either through signaling by receptor-associated tyrosine kinases or by receptor tyrosine kinases.
The classical model of JAK-STAT signaling by IFNs and other cytokines thus takes an Occam’s Razor approach to signaling events in that it is treated as if it explains all the pertinent aspects of specific gene activation by cytokines in the most concise manner.Citation5 The flip side of Occam’s Razor is Occam’s Broom, a term coined by Sidney Brenner in reference to the willful overlooking of data that does not fit into a particular model.Citation19 It is our view that Occam’s Broom is operating in all of its glory in attempts to not modify the classical model of JAK-STAT signaling substantively and in accepting it in present form as explaining the mechanism of specific gene activation by cytokines such as the IFNs in the essentials. We present here our non-canonical model of IFN signaling to more completely understand and mechanistically explain the complexity of events of specific gene activation including the associated epigenetics.
Basics of the Non-Canonical Model
It was previously shown that IFNγ and one of its receptor subunits, IFNGR1, are translocated to the nucleus together with activated STAT1α.Citation14,Citation15,Citation20 Active nuclear transport depended on a polycationic nuclear localization sequence (NLS) in the C-terminus of IFNγ, the nuclear import proteins importins α and β, and ATP/GTP as an energy source.Citation14,Citation21 The nuclear targets of IFNγ and IFNGR1 were also identified.Citation15,Citation22 By chromatin immunoprecipitation (ChIP) followed by PCR, IFNγ, its receptor subunit IFNGR1, and STAT1α were found to be associated with the IFNγ-activated sequence (GAS) element in the promoter of two genes stimulated by IFNγ. Examination of nuclear extracts from IFNγ treated WISH cells showed that IFNγ, IFNGR1, and STAT1α proteins were associated with the GAS promoter. The same associations were also demonstrated by electrophoretic mobility shift assay (EMSA). Transfection with a GAS-luciferase gene together with IFNGR1 and nonsecreted IFNγ resulted in enhanced promoter activity. Additionally, IFNGR1 fused to the yeast GAL-4 DNA binding domain resulted in enhanced transcription from the GAL-4 response element in IFNγ treated cells, suggesting the presence of a transactivation domain in IFNGR1. These nuclear studies suggest a transcriptional/co-transcriptional role for IFNGR1, which may provide insight into the specificity of IFNγ signaling. A model for these non-canonical IFNγ signaling events is presented in . The movement to nucleus of IFNGR1 occurs in a complex with IFNγ activated STAT1α and activated JAK1 and JAK2.Citation22 The activated JAKs provide a direct link between transcription factors and specific epigenetic events such as pJAK2 (activated) phosphorylation of tyrosine 41 on histone H3. Details concerning JAKs and epigenetics are dealt with below.
Cytokines such as IFNs are assumed to bind solely to the receptor extracellular domain, resulting in allosteric changes on the cytoplasmic domain that initiate signaling events. It was shown, however, that IFNγ bound first to IFNGR1 extracellular domain involving in part its N-terminus and then, during endocytosis, to IFNGR1 cytoplasmic domain via its C-terminus.Citation14 The intracellular binding was blocked by an intracellular excess of a peptide representing the cytoplasmic binding site on IFNGR1 for the C-terminus of IFNγ. Moreover, such cells were also blocked with respect to the tyrosine phosphorylation of STAT1α. Thus, internalized IFNγ appeared to be able to interact with the cytoplasmic domain of IFNGR1 in intact cells as part of the signal transduction events leading to STAT1α tyrosine phosphorylation. Cytosolic injection of antibodies to IFNγ C-terminal amino acids 95–132 blocked STAT1α nuclear translocation in response to extracellular IFNγ,Citation21 consistent with these observations. This further supports the idea that the C-terminus of endocytosed IFNγ access IFNGR1 cytoplasmic domain in the cytosol, although the mechanism is as yet unknown.
The intracellular IFNγ effects can be replicated by internalized C-terminus residues 95–132 of mouse IFNγ or residues 95–134 of human IFNγ.Citation23 Thus, peptides corresponding to these residues, mIFNγ(95–132) and hIFNγ(95–134) respectively, with a palmitate attached for cell penetration, function as IFNγ mimetics. The properties and uses of these IFNγ mimetics as well as type I IFN mimetics are described in detail below in the IFN mimetic section. It is noteworthy that there are no IFN or other cytokine mimetics based on extracellular recognition and cross-linking of receptor chains as per the classical model.
Recently, insight has been gleaned on the intracellular aspects of type I IFN signaling. It was shown by western blotting of nuclear extracts that type I IFN signaling involves activated TYK2 in the nucleus, similar to pJAK2 in the nucleus of IFNγ treated cells.Citation10 The nucleus of WISH cells contained constitutively expressed nonphosphorylated TYK2, but activated TYK2, pTYK2, as well as pJAK1 were found in the nucleus of cells only after treatment with type I IFNs IFNα or IFNτ. Both activated STAT1 and STAT2 were present in the nucleus of cells treated with type I IFNs. With IFNγ, only the receptor subunit IFNGR1 underwent nuclear translocation in IFNγ treated cells, but both receptor subunits IFNAR1 and IFNAR2 underwent nuclear translocation in type I IFN treated cells as determined by western blotting of nuclear extracts and confocal microscopy of GFP-receptor fusion proteins. The GFP-IFNτ fusion protein also underwent nuclear import, thus demonstrating that type I IFNs also translocated to the nucleus.
With all of these components of the type I IFN signaling system in the nucleus, there was interest in determining where they went in terms of promoters and whether they were associated with each other for some coordinate nuclear function. Therefore, ChIP-qPCR assays were performed to determine if the type I IFN players were specifically recruited to the promoter region of a gene activated by IFNα in cells.Citation10 The promoter region of the oligoadenylate synthetase 1 (OAS1) gene, which has an IFN sensitive response element (ISRE) and is involved in IFN antiviral activity was thus examined.Citation10 IFNAR1, IFNAR2, TYK2, pSTAT1, and H3pY41 were found at the OAS1 promoter, but not at the β-actin promoter, a gene that is not directly affected by type I IFNs. Consistent with the ChIP data, immunoprecipitation of IFNAR1 in nuclear extracts of IFNα treated cells followed by western blotting showed TYK2, pSTAT1, and H3pY41 associated with IFNAR1. Thus, the various players in type I IFN signaling were found associated in the nucleus of IFN treated cells specifically at the promoter of a key gene in IFN antiviral activity.
Given the specific epigenetic events that are associated with gene activation, ChIP analysis was used to monitor demethylation/acetylation of lysine 9 on histone H3.Citation10 Type I IFN treated cells showed decreased trimethylated lysine on H3, H3K9me3, in the OAS1 promoter region of cells. Acetylation of H3K9, H3K9ac, occurred concomitantly over the same time span. Demethylation/acetylation of H3K9 is associated with gene activation.Citation24,Citation25 Related to this, phosphorylation of H3 at Y41, H3pY41, increased as H3K9me3 decreased over the same time period, providing a functional linkage of activated JAKs in the above complex epigenetic event. By comparison, the constitutively activated β-actin gene, which is not affected by IFN, showed constitutive H3K9ac, no H3pY41, and no H3K9me3. The nuclear trafficking and activities at specific genes that are associated with treatment of cells with IFN suggest that the receptor/transcription factor/JAK complex plays a key role in specific gene activation, including the related heterochromatin modifications.
Activated JAKs and STATs in the Nucleus: Evidence for Coordination
Activated JAK2 has recently been reported to be present in the nucleus and was shown to perform the epigenetic function of phosphorylation of tyrosine 41 on histone H3.Citation26 It is highly unlikely that the activated JAK2 is acting randomly in the nucleus, so how and with what are its epigenetic functions coordinated? Of particular interest is how is activated JAK activity coordinated with that of activated STATs in the nucleus? In the study, constitutively activated mutated JAK2 JAK2V617F was found in the nucleus and shown to phosphorylate histone H3 on tyrosine 41 (H3pY41), which led to dissociation of heterochromatin protein 1α (HP1α) from H3.Citation26 The resultant heterochromatin remodeling was associated with exposure of euchromatin for gene activation. Wild-type JAK2 was shown to be constitutively present in the nucleus of cells also, but unlike JAK2V617F, was only activated when K562 cells were treated with the growth factors PDGF or leukemia inhibitory factor, or when BaF3 cells were treated with the cytokine IL-3.Citation26 It would seem obvious that the nuclear H3 phosphorylations are not random, but must be under the control of factors associated with the activating cytokine. It is difficult to address this issue in the context of the classical JAK/STAT model as it says nothing about activated JAKs in the nucleus.
The question of JAK-STAT coordination in the nucleus was addressed in IFN studies by treating cells with IFNγ and tracking activated JAK2 in the nucleus.Citation22 Using ChIP analysis, it was shown that activated JAK2 (pJAK2) and H3pY41 were associated with the GAS promoter element at the IRF-1 gene, a gene that is activated by IFNγ.Citation22 pJAK1 was also associated with the IRF-1 GAS element. None of these factors were associated with the promoter of the β-actin gene, a gene not affected by IFNγ. A similar result was observed with TYK2 in IFNα treated cells where TYK2 and H3pY41 were present at the promoter of the OAS 1 gene, a gene activated by type I IFNs, but were absent from the promoter of the β-actin gene.Citation10 It is important to note that ChIP analysis also showed the presence of STAT1 at the IRF1 and OAS1 promoters of IFNγ and/or IFNα treated cells, but not at the β-actin promoter. This would suggest that the activated JAKs and the STATs track to the same promoters, which would suggest that their nuclear activities are coordinated.
The fact that the hematological disorders associated with JAK2V617F show characteristic phenotypic similarities would suggest that the epigenetic activity of JAK2V617F also occurs in association with the relevant hematological receptor. It is of interest therefore that it has been shown that JAK2V617F activation required the association of the mutant JAK2 with a homodimeric type I cytokine receptor.Citation27,Citation28 Specifically, either erythropoietin receptor, thrombopoeitin receptor, or granulocyte colony-stimulating receptor was required for hormone/growth factor-independent activation of JAK2V617F. This raises the question of whether there are receptor/JAK2V617F complexes in the vicinity of promoters of genes that are activated in cancers caused by or associated with JAK2V617F. All of this has implications for how particular tyrosine kinases cause or are associated with specific cancers and provides insight into the phenotypes of such cancers.
IFN Mimetics Development and Use as Therapeutics against Poxvirus and EAE, a Mouse Model of Multiple Sclerosis
As indicated above, we have exploited the non-canonical model of IFN signaling to develop IFNγ mimetics where the internalized mimetic, lipo-IFNγ(95–132) for example, is only recognized by the cytoplasmic domain of receptor subunit IFNGR1.Citation29 A stringent test of the mimetic in terms of antiviral activity was observed with a poxvirus, vaccinia virus, which is used worldwide to vaccinate against smallpox infections, and is a prototype of the poxvirus family.Citation30 These viruses are particularly effective in neutralizing host innate antiviral defense mechanisms, such as the IFN system, because they produce soluble secreted proteins that bind to and prevent IFNα, IFNβ, and IFNγ from binding to their respective receptors on the cell membrane.Citation30,Citation31 An important virulence factor of vaccinia virus is the B8R protein, which is a homolog of the extracellular domain of the IFNγ receptor and can therefore bind to intact IFNγ and prevent its interaction with the receptor.Citation31 It was hypothesized that the IFNγ mimetics would bypass the poxvirus virulence factor B8R protein that binds to intact IFNγ, thus preventing its interaction with the receptor. Human and murine IFNγ mimetic peptides were introduced into an adenoviral vector for intracellular expression. Murine IFNγ mimetic peptide, lipo-mIFNγ(95–132), was also expressed via chemical synthesis with attached palmitic acid for penetration of cell plasma membrane. In contrast to the intact human IFNγ, the mimetics did not bind poxvirus B8R protein. Expression of B8R protein in WISH cells did not block the antiviral effect of the mimetics against EMC virus or vesicular stomatitis virus, while the antiviral activity of human IFNγ was neutralized. Consistent with the antiviral activity, the upregulation of MHC class I molecules on WISH cells by the IFNγ mimetics was not affected by B8R protein, while IFNγ induced upregulation was blocked. Finally, the mimetics, but not IFNγ, inhibited vaccinia virus replication in African green monkey kidney BSC-40 cells. The small peptide mimetics of IFNγ can avoid the B8R virulence factor for poxviruses and thus are potential candidates for antivirals against smallpox virus.Citation23,Citation32,Citation33
It was further shown that lipo-mIFNγ(95–132) at 2000 units protected C57BL/6 mice against an overwhelming lethal vaccinia virus infection ().Citation32,Citation33 Control mice injected with a non-cell penetrating IFNγ peptide or PBS died at 6–9 d post infection, but intraperitoneal injection of the mimetic as late as 6 d post infection resulted in 40% protection. Administration of mimetic by the oral route also completely protected mice against the intranasal route of a lethal dose of vaccinia virus challenge. In addition to the direct antiviral effects, the mimetic also possessed adjuvant effects in boosting humoral and cellular immunity. This combination of antiviral and adjuvant effects by the IFN mimetic probably played a role in its potent anti-vaccinia properties. IFNγ is generally not extensively used as a therapeutic, the reason for which is not well understood. It should be noted that the presence of receptors on a large number of cells could serve as a “sink”, thus affecting access of IFNγ to sites and cells for which it was intended. The IFNγ mimetics do not recognize the receptor extracellular domain and thus could possibly have better access to intended targets.
Table 1. Summary of the effects of IFN mimetics and intact IFN
The pattern of nuclear signaling by type I IFNs is similar to that of IFNγ nuclear signaling.Citation10,Citation22,Citation34 Thus, in order to determine if IFNα1 and IFNβ possessed similar C-terminus function intracellularly while losing extracellular function, truncated IFNs IFNα1(69–189)R9 and IFNβ(100–187)R9 with 9 arginines (R9) for cell penetration were expressed in a bacterial system and purified.Citation10 As controls, these truncations were also expressed without R9. Both IFNα1(69–189)R9 and IFNβ(100–187)R9 possessed antiviral activity against EMC virus, while the same constructs without R9 for cell penetration lacked antiviral activity.Citation10 This is consistent with previous studies that showed that intracellularly expressed IFNα possessed antiproliferative and antiviral activity.Citation13
In addition to the B8R IFNγ decoy receptor, poxviruses also produce a type I IFN decoy receptor, B18R.Citation35 The type I IFN mimetics inhibited vaccinia virus growth in the presence of B18R, while the corresponding intact IFNs were ineffective (Ahmed and Johnson, in preparation). We have refined the requirements for IFNα1 mimetic activity and have shown that a shorter sequence, lipo-IFNα1(152–189), was as effective as IFNα1(69–189)R9 in inhibiting virus replication (Ahmed and Johnson, in preparation). It should be noted that the non-canonical model of IFN signaling provides a conceptual foundation such that the IFN mimetics described here are remarkably easy to produce. The question arises, therefore, as to the applicability of the non-canonical model to other cytokines and the potential benefit of development of mimetics of these cytokines using the IFN mimetic approach.
There are over 20 different isoforms of type I IFNs and they all function through the same heterodimeric receptor complex.Citation36 In addition to their similar antiviral activities, these IFNs vary with respect to anticellular and cytotoxic (apoptotic) effects. In this regard, IFNβ is the treatment of choice for relapsing/remitting multiple sclerosisCitation37,Citation38 Further, it has been shown that higher doses of IFNβ result in better therapeutic efficacy,Citation39 but undesirable toxic side effects of flu-like symptoms, liver damage, and bone marrow suppression limit the dose.Citation40 We showed that type I IFN toxicity (apoptosis) was due to differential extracellular IFN receptor recognition where greater receptor occupancy due to higher binding affinity contributed to the toxic effects.Citation41 This observation has been confirmed by others.Citation42
The IFNα1(69–189)R9 mimetic was tested for its ability to therapeutically treat SJL/J mice in EAE, a mouse model of MS, as well as for their toxicity relative to that of IFNβ. Immunization of mice with bovine myelin basic protein (MBP) where cellular infiltration into the CNS has occurred by day 12 was used to test the truncated IFNs.Citation10 SJL/J mice were injected i.p. with saline, IFNα1(69–189)R9 (15 μg/mouse), or the control peptide, IFNα1(69–189) (15 μg/mouse), every other day starting from day 12 post-immunization with MBP. The IFNα mimetic with the R9 reduced paralysis essentially completely, while the mice treated with saline or the mimetic lacking R9 developed paraplegia ().
For toxicity studies, mice were injected i.p. on alternate days with IFNβ, IFNβ(100–187)R9, or IFNα1(69–189)R9, all of the same antiviral activity (2000 U).Citation10 Injection of mice with IFNβ resulted in approximately 15% weight loss by day 10, while mice injected with the IFN mimetic gained weight, which is expected under normal growth conditions (). A similar pattern of bone marrow suppression occurred as reflected by peripheral lymphocyte count. IFNβ was also apoptotic, while the mimetics did not induce apoptosis. Thus, under conditions of the same antiviral activity, IFNβ was toxic and type I IFN mimetics lacked toxicity of weight loss, lymphopenia, and cellular toxicity. These mimetic results would suggest, as with binding studies mentioned above, that it is the IFN signal at the receptor extracellular domain that is responsible for their toxic effects, while antiviral and anti-EAE (MS) effects are associated with intracellular actions that are retained by the IFN mimetics. It is important to emphasize that the IFN mimetics are products of the non-canonical model of JAK-STAT signaling by IFN presented here.
Context and Conclusion
In a search for precedents and templates, we have found that our non-canonical model of IFN signaling bares remarkable similarity to that of steroid receptor (SR) signaling. Steroid hormone (SH) binds to SRs located in the cytoplasm or nucleus of the cell. In the absence of hormone, SR monomers are associated with heat shock proteins (HSPs) and usually possess some basal level of phosphorylation.Citation43 Upon binding of hormone, SRs dissociate from HSPs, dimerize and translocate to the nucleus where they bind to HREs (hormone-response elements) at genes that are activated by SHs. The complex of SH/SR recruits a series of co-activators to both regulate target gene transcription as well as the associated epigenetic events that accompany such activation. Site-specific phosphorylation of receptors occurs subsequent to hormone binding with varied kinetics, depending on the kinase and the target in the receptor complex.
The kinases, although not the only components of the receptor associated co-activator complexes, are important for their action on members of the complex, as well as for specific epigenetic events of gene activation and thus act on histones as well as on members of the receptor complex. Many of the SH phosphorylation sites contain serine/threonine/proline motifs involving proline-specific kinases, such as the cyclin-dependent kinases and MAPKs.Citation43,Citation44 Tyrosine kinases such as Src have also been shown to participate in SR signaling in the nucleus. SRs similarly cross-talk with receptor tyrosine kinases such as EGFR. EGFR family members are an important target in some of the most prevalent and difficult cancers, such as non-small cell lung carcinoma.Citation45
In addition to their presence in the cytoplasm, a subset of SRs are also membrane-associated through an S-palmitoylation linkage to the inner side of the plasma membrane.Citation43,Citation46 The membrane-associated SR may be in some cases the same as cytoplasmic SR, but this is not universally agreed upon. Membrane SR is involved in activation of MAPK and P13K/Akt kinases and may undergo nuclear translocation like cytoplasmic SR.
There are also so-called primary SRCs (SR co-activators).Citation47 SRCs recruit secondary co-activators, such as the histone acetyltransferase p300/CBP, the histone methyltransferases PRMT1 (protein arginine N-methyltransferase 1) and CARM1 (co-activator-associated arginine methyltransferase 1), and the chromatin remodeling complex SWI/SNF. These secondary co-activators modify the chromatin and bridge the SR complex with the general transcription machinery.
A comparison of IFN signaling and SH signaling suggests the following similar features. Ligand associates with the receptor intracellularly. In the case of IFNγ, first there is extracellular binding to IFNGR1 and then intracellular binding in conjunction with the endocytosis. SH penetrates the plasma membrane and binds the cytoplasmic SR. In both cases the receptors function as transcription/co-transcription factors. Co-activators are associated with the ligand/receptor complex. Currently, much more is known concerning the SH/SR complex than the IFNγ/IFNGR1 or type I IFN/IFNAR complexes, but STATs and JAKs are associated in the cytoplasm and the nucleus. In both cases, the ligand-receptor-co-activator complex binds to response elements of genes that are specifically activated. Some of the co-factors, such as the kinases, are involved in specific epigenetic events for both systems. We do not feel that IFNs are a special case with respect to protein ligands with associated tyrosine kinase activity or with receptor tyrosine kinases, as EGFR and FGFR have similarities to the IFNs in receptor involvement in nuclear aspects of gene activation. We further feel that all of the cytokines, hormones, and growth factors that use the JAK-STAT pathway are likely to also share these similarities. In our view the template for all of this resides in the SH/SR system of specific gene activation.
| Abbreviations: | ||
| EAE | = | experimental allergic encephalomyelitis |
| MS | = | multiple sclerosis |
| EGF | = | epidermal growth factor |
| FGF | = | fibroblast growth factor |
| PDGF | = | platelet derived growth factor |
Disclosure of Potential Conflicts of Interest
The authors have no conflict of interest.
Acknowledgments
This work was supported by NIH grant R01 AI 056152 to HM Johnson.
References
- Isaacs A, Lindenmann J. Virus interference. I. The interferon. Proc R Soc Lond B Biol Sci 1957; 147:258 - 67; http://dx.doi.org/10.1098/rspb.1957.0048
- Johnson HM, Smith BG, Baron S. Inhibition of primary in vitro antibody response of mouse spleen cells by interferon preparations. J Immunol 1995; 114:403 - 9
- Johnson HM, Blalock JE. Interferon immunosuppression: mediation by a suppressor factor. Infect Immun 1980; 29:301 - 5; PMID: 6163706
- Knobler RL, Panitch HS, Braheny SL, Sipe JC, Rice GP, Huddlestone JR, Francis GS, Hooper CK, Kamin-Lewis RM, Johnson KP, et al. Systemic alpha-interferon therapy of multiple sclerosis. Neurology 1984; 34:1273 - 9; http://dx.doi.org/10.1212/WNL.34.10.1273; PMID: 6384817
- Brivanlou AH, Darnell JE Jr.. Signal transduction and the control of gene expression. Science 2002; 295:813 - 8; http://dx.doi.org/10.1126/science.1066355; PMID: 11823631
- Reich NC, Liu L. Tracking STAT nuclear traffic. Nat Rev Immunol 2006; 6:602 - 12; http://dx.doi.org/10.1038/nri1885; PMID: 16868551
- Gough DJ, Levy DE, Johnstone RW, Clarke CJ. IFNgamma signaling-does it mean JAK-STAT?. Cytokine Growth Factor Rev 2008; 19:383 - 94; http://dx.doi.org/10.1016/j.cytogfr.2008.08.004; PMID: 18929502
- Yang XP, Ghoreschi K, Steward-Tharp SM, Rodriguez-Canales J, Zhu J, Grainger JR, Hirahara K, Sun HW, Wei L, Vahedi G, et al. Opposing regulation of the locus encoding IL-17 through direct, reciprocal actions of STAT3 and STAT5. Nat Immunol 2011; 12:247 - 54; http://dx.doi.org/10.1038/ni.1995; PMID: 21278738
- Zula JA, Green HC, Ransohoff RM, Rudick RA, Stark GR, van Boxel-Dezaire AHH. The role of cell type-specific responses in IFN-β therapy of multiple sclerosis. Proc Natl Acad Sci U S A 2011; 108:19689 - 94; http://dx.doi.org/10.1073/pnas.1117347108; PMID: 22106296
- Ahmed CM, Noon-Song EN, Kemppainen K, Pascalli MP, Johnson HM. Type I IFN receptor controls activated TYK2 in the nucleus: implications for EAE therapy. J Neuroimmunol 2013; 254:101 - 9; http://dx.doi.org/10.1016/j.jneuroim.2012.10.006; PMID: 23110939
- Subramaniam PS, Torres BA, Johnson HM. So many ligands, so few transcription factors: a new paradigm for signaling through the STAT transcription factors. Cytokine 2001; 15:175 - 87; http://dx.doi.org/10.1006/cyto.2001.0905; PMID: 11563878
- Rutherford MN, Kumar A, Coulombe B, Skup D, Carver DH, Williams BR. Expression of intracellular interferon constitutively activates ISGF3 and confers resistance to EMC viral infection. J Interferon Cytokine Res 1996; 16:507 - 10; http://dx.doi.org/10.1089/jir.1996.16.507; PMID: 8836915
- Ahmed CM, Wills KN, Sugarman BJ, Johnson DE, Ramachandra M, Nagabhushan TL, Howe JA. Selective expression of nonsecreted interferon by an adenoviral vector confers antiproliferative and antiviral properties and causes reduction of tumor growth in nude mice. J Interferon Cytokine Res 2001; 21:399 - 408; http://dx.doi.org/10.1089/107999001750277871; PMID: 11440637
- Ahmed CM, Burkhart MA, Mujtaba MG, Subramaniam PS, Johnson HM. The role of IFNgamma nuclear localization sequence in intracellular function. J Cell Sci 2003; 116:3089 - 98; http://dx.doi.org/10.1242/jcs.00528; PMID: 12799413
- Ahmed CM, Johnson HM. IFN-γ and its receptor subunit IFNGR1 are recruited to the IFN-γ-activated sequence element at the promoter site of IFN-γ-activated genes: evidence of transactivational activity in IFNGR1. J Immunol 2006; 177:315 - 21; PMID: 16785527
- Wang YN, Yamaguchi H, Huo L, Du Y, Lee H-J, Lee H-H, Wang H, Hsu J-M, Hung MC. The translocon Sec61β localized in the inner nuclear membrane transports membrane-embedded EGF receptor to the nucleus. J Biol Chem 2010; 285:38720 - 9; http://dx.doi.org/10.1074/jbc.M110.158659; PMID: 20937808
- Bryant DM, Stow JL. Nuclear translocation of cell-surface receptors: lessons from fibroblast growth factor. Traffic 2005; 6:947 - 54; http://dx.doi.org/10.1111/j.1600-0854.2005.00332.x; PMID: 16138907
- Burwen SJ, Jones AL. The association of polypeptide hormones and growth factors with the nuclei of target cells. Trends Biochem Sci 1987; 12:159 - 62; http://dx.doi.org/10.1016/0968-0004(87)90074-0
- Dennett DC. Intuition pumps and other tools for thinking. Norton WW and Co., New York. 2013. Pp. 40-41.
- Larkin J 3rd, Johnson HM, Subramaniam PS. Differential nuclear localization of the IFNGR-1 and IFNGR-2 subunits of the IFN-gamma receptor complex following activation by IFN-gamma. J Interferon Cytokine Res 2000; 20:565 - 76; http://dx.doi.org/10.1089/10799900050044769; PMID: 10888113
- Subramaniam PS, Larkin J 3rd, Mujtaba MG, Walter MR, Johnson HM. The COOH-terminal nuclear localization sequence of interferon gamma regulates STAT1 alpha nuclear translocation at an intracellular site. J Cell Sci 2000; 113:2771 - 81; PMID: 10893192
- Noon-Song EN, Ahmed CM, Dabelic R, Canton J, Johnson HM. Controlling nuclear JAKs and STATs for specific gene activation by IFNγ. Biochem Biophys Res Commun 2011; 410:648 - 53; http://dx.doi.org/10.1016/j.bbrc.2011.06.047; PMID: 21689637
- Ahmed CM, Burkhart MA, Subramaniam PS, Mujtaba MG, Johnson HM. Peptide mimetics of gamma interferon possess antiviral properties against vaccinia virus and other viruses in the presence of poxvirus B8R protein. J Virol 2005; 79:5632 - 9; http://dx.doi.org/10.1128/JVI.79.9.5632-5639.2005; PMID: 15827178
- Berger SL. The complex language of chromatin regulation during transcription. Nature 2007; 447:407 - 12; http://dx.doi.org/10.1038/nature05915; PMID: 17522673
- Mehta NT, Truax AD, Boyd NH, Greer SF. Early epigenetic events regulate the adaptive immune response gene CIITA. Epigenetics 2011; 6:516 - 25; http://dx.doi.org/10.4161/epi.6.4.14516; PMID: 21266852
- Dawson MA, Bannister AJ, Göttgens B, Foster SD, Bartke T, Green AR, Kouzarides T. JAK2 phosphorylates histone H3Y41 and excludes HP1α from chromatin. Nature 2009; 461:819 - 22; http://dx.doi.org/10.1038/nature08448; PMID: 19783980
- Lu X, Levine R, Tong W, Wernig G, Pikman Y, Zarnegar S, Gilliland DG, Lodish H. Expression of a homodimeric type I cytokine receptor is required for JAK2V617F-mediated transformation. Proc Natl Acad Sci U S A 2005; 102:18962 - 7; http://dx.doi.org/10.1073/pnas.0509714102; PMID: 16365288
- Lu X, Huang LJ, Lodish HF. Dimerization by a cytokine receptor is necessary for constitutive activation of JAK2V617F. J Biol Chem 2008; 283:5258 - 66; http://dx.doi.org/10.1074/jbc.M707125200; PMID: 18158285
- Szente BE, Johnson HM. Binding of IFN γ and its C-terminal peptide to a cytoplasmic domain of its receptor that is essential for function. Biochem Biophys Res Commun 1994; 201:215 - 21; http://dx.doi.org/10.1006/bbrc.1994.1691; PMID: 8198577
- Moss B. Poxviridae: The viruses and their replication. In Fields Virology, 3rd ed. D. M. Knipe, and P.M. Howley, eds. Lippincott, Williams, and Wilkins, Philadelphia, PA. 2007; pp. 2905-2946.
- Alcamí A, Smith GL. The vaccinia virus soluble interferon-gamma receptor is a homodimer. J Gen Virol 2002; 83:545 - 9; PMID: 11842249
- Ahmed CM, Martin JP, Johnson HM. IFN mimetic as a therapeutic for lethal vaccinia virus infection: possible effects on innate and adaptive immune responses. J Immunol 2007; 178:4576 - 83; PMID: 17372016
- Ahmed CM, Dabelic R, Martin JP, Jager LD, Haider SM, Johnson HM. Enhancement of antiviral immunity by small molecule antagonist of suppressor of cytokine signaling. J Immunol 2010; 185:1103 - 13; http://dx.doi.org/10.4049/jimmunol.0902895; PMID: 20543109
- Johnson HM, Noon-Song EN, Kemppainen K, Ahmed CM. Steroid-like signalling by interferons: making sense of specific gene activation by cytokines. Biochem J 2012; 443:329 - 38; http://dx.doi.org/10.1042/BJ20112187; PMID: 22452815
- Alcamí A, Symons JA, Smith GL. The vaccinia virus soluble alpha/beta interferon (IFN) receptor binds to the cell surface and protects cells from the antiviral effects of IFN. J Virol 2000; 74:11230 - 9; http://dx.doi.org/10.1128/JVI.74.23.11230-11239.2000; PMID: 11070021
- Stark GR, Kerr IM, Williams BR, Silverman RH, Schreiber RD. How cells respond to interferons. Annu Rev Biochem 1998; 67:227 - 64; http://dx.doi.org/10.1146/annurev.biochem.67.1.227; PMID: 9759489
- Burks J. Interferon-beta1b for multiple sclerosis. Expert Rev Neurother 2005; 5:153 - 64; http://dx.doi.org/10.1586/14737175.5.2.153; PMID: 15853486
- Castro-Borrero W, Graves D, Frohman TC, Flores AB, Hardeman P, Logan D, Orchard M, Greenberg B, Frohman EM. Current and emerging therapies in multiple sclerosis: a systematic review. Ther Adv Neurol Disord 2012; 5:205 - 20; http://dx.doi.org/10.1177/1756285612450936; PMID: 22783370
- Schwid SR, Thorpe J, Sharief M, Sandberg-Wollheim M, Rammohan K, Wendt J, Panitch H, Goodin D, Li D, Chang P, et al, EVIDENCE (Evidence of Interferon Dose-Response: European North American Comparative Efficacy) Study Group, University of British Columbia MS/MRI Research Group. Enhanced benefit of increasing interferon beta-1a dose and frequency in relapsing multiple sclerosis: the EVIDENCE Study. Arch Neurol 2005; 62:785 - 92; http://dx.doi.org/10.1001/archneur.62.5.785; PMID: 15883267
- Francis GS, Grumser Y, Alteri E, Micaleff A, O’Brien F, Alsop J, Stam Moraga M, Kaplowitz N. Hepatic reactions during treatment of multiple sclerosis with interferon-beta-1a: incidence and clinical significance. Drug Saf 2003; 26:815 - 27; http://dx.doi.org/10.2165/00002018-200326110-00006; PMID: 12908850
- Subramaniam PS, Khan SA, Pontzer CH, Johnson HM. Differential recognition of the type I interferon receptor by interferons τ and α is responsible for their disparate cytotoxicities. Proc Natl Acad Sci U S A 1995; 92:12270 - 4; http://dx.doi.org/10.1073/pnas.92.26.12270; PMID: 8618883
- Thomas C, Moraga I, Levin D, Krutzik PO, Podoplelova Y, Trejo A, Lee C, Yarden G, Vleck SE, Glenn JS, et al. Structural linkage between ligand discrimination and receptor activation by type I interferons. Cell 2011; 146:621 - 32; http://dx.doi.org/10.1016/j.cell.2011.06.048; PMID: 21854986
- Weigel NL, Moore NL. Kinases and protein phosphorylation as regulators of steroid hormone action. Nucl Recept Signal 2007; 5:e005; PMID: 17525795
- Vicent GP, Nacht AS, Zaurín R, Ballaré C, Clausell J, Beato M. Minireview: role of kinases and chromatin remodeling in progesterone signaling to chromatin. Mol Endocrinol 2010; 24:2088 - 98; http://dx.doi.org/10.1210/me.2010-0027; PMID: 20484412
- Wang YN, Hung MC. Nuclear functions and subcellular trafficking mechanisms of the epidermal growth factor receptor family. Cell Biosci 2012; 2:13; http://dx.doi.org/10.1186/2045-3701-2-13; PMID: 22520625
- Hagan CR, Faivre EJ, Lange CA. Scaffolding actions of membrane-associated progesterone receptors. Steroids 2009; 74:568 - 72; http://dx.doi.org/10.1016/j.steroids.2008.12.004; PMID: 19135465
- Stanisić V, Lonard DM, O’Malley BW. Modulation of steroid hormone receptor activity. Prog Brain Res 2010; 181:153 - 76; http://dx.doi.org/10.1016/S0079-6123(08)81009-6; PMID: 20478437